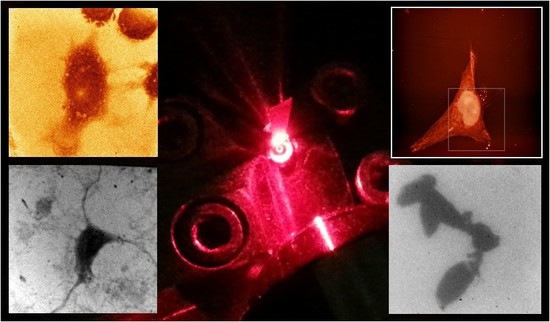
Bioimaging Using Full Field and Contact EUV and SXR Microscopes with Nanometer Spatial Resolution
Abstract
:1. Introduction and Previous Achievements
2. Materials and Methods
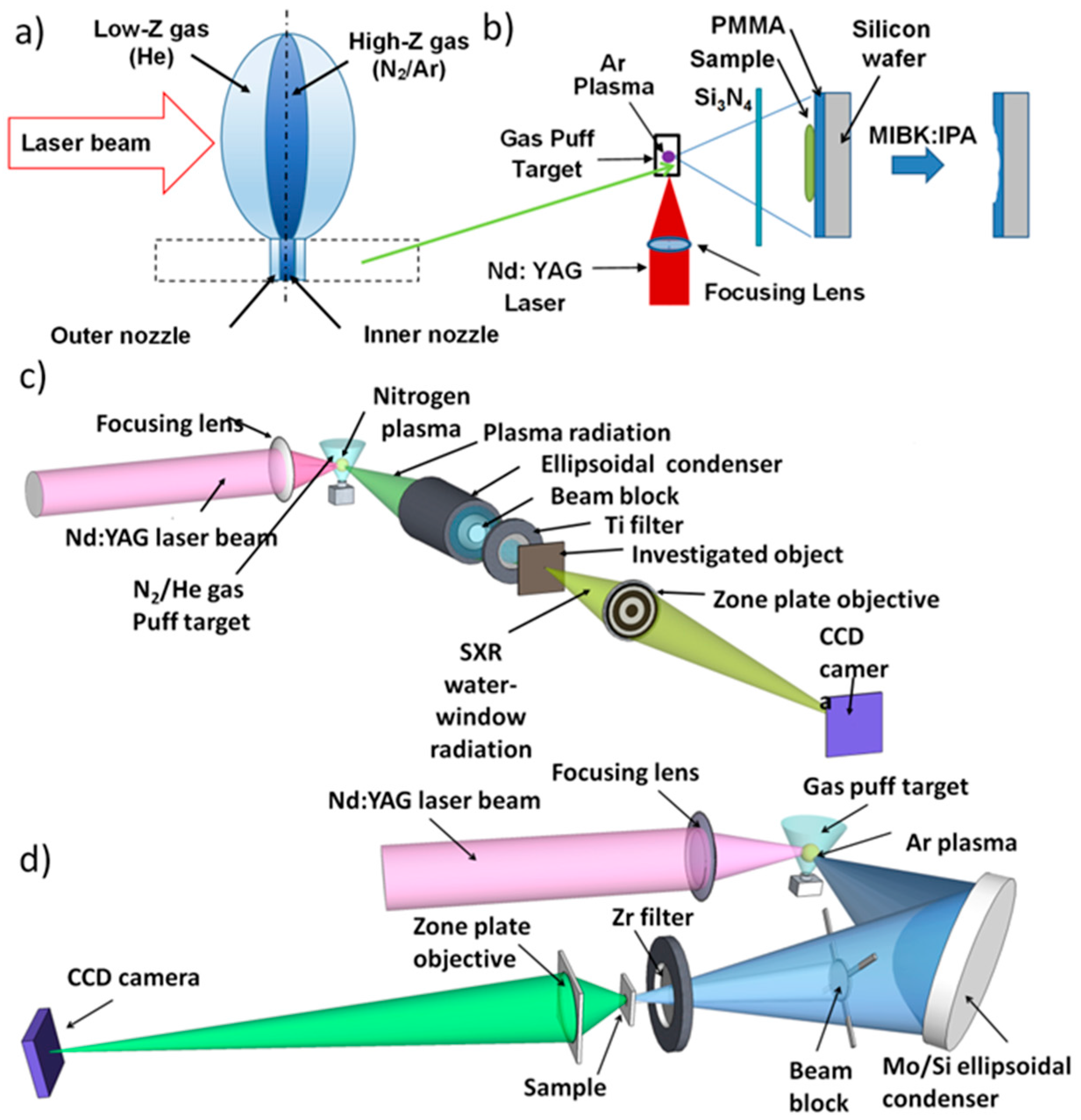
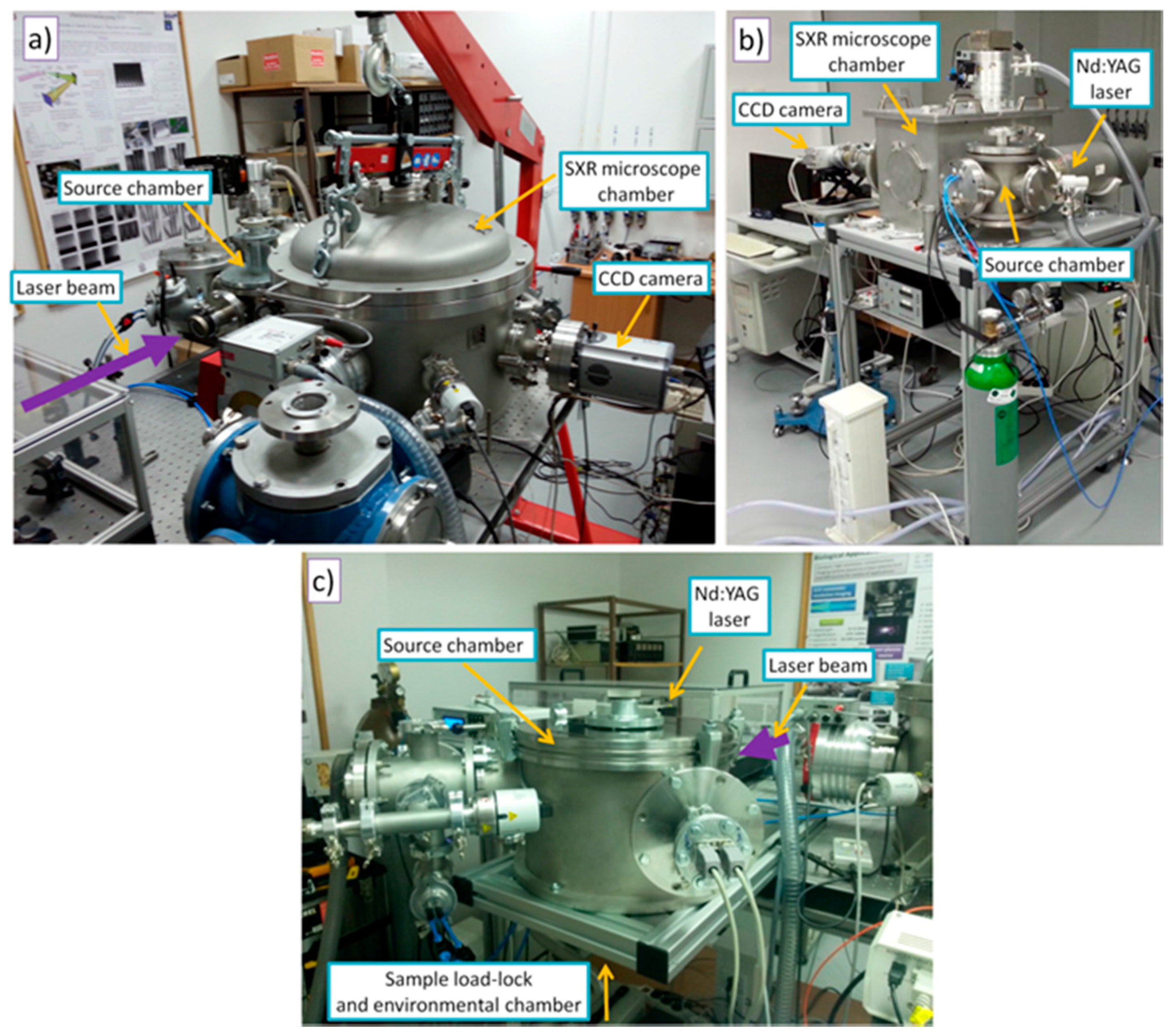
2.1. The EUV and SXR Microscopes
2.2. SXR Full-Field Microscope
2.3. EUV Full-Field Microscope
2.4. SXR Contact Microscope
3. Imaging with Compact EUV and SXR Microscopes Based on a Laser Plasma Sources
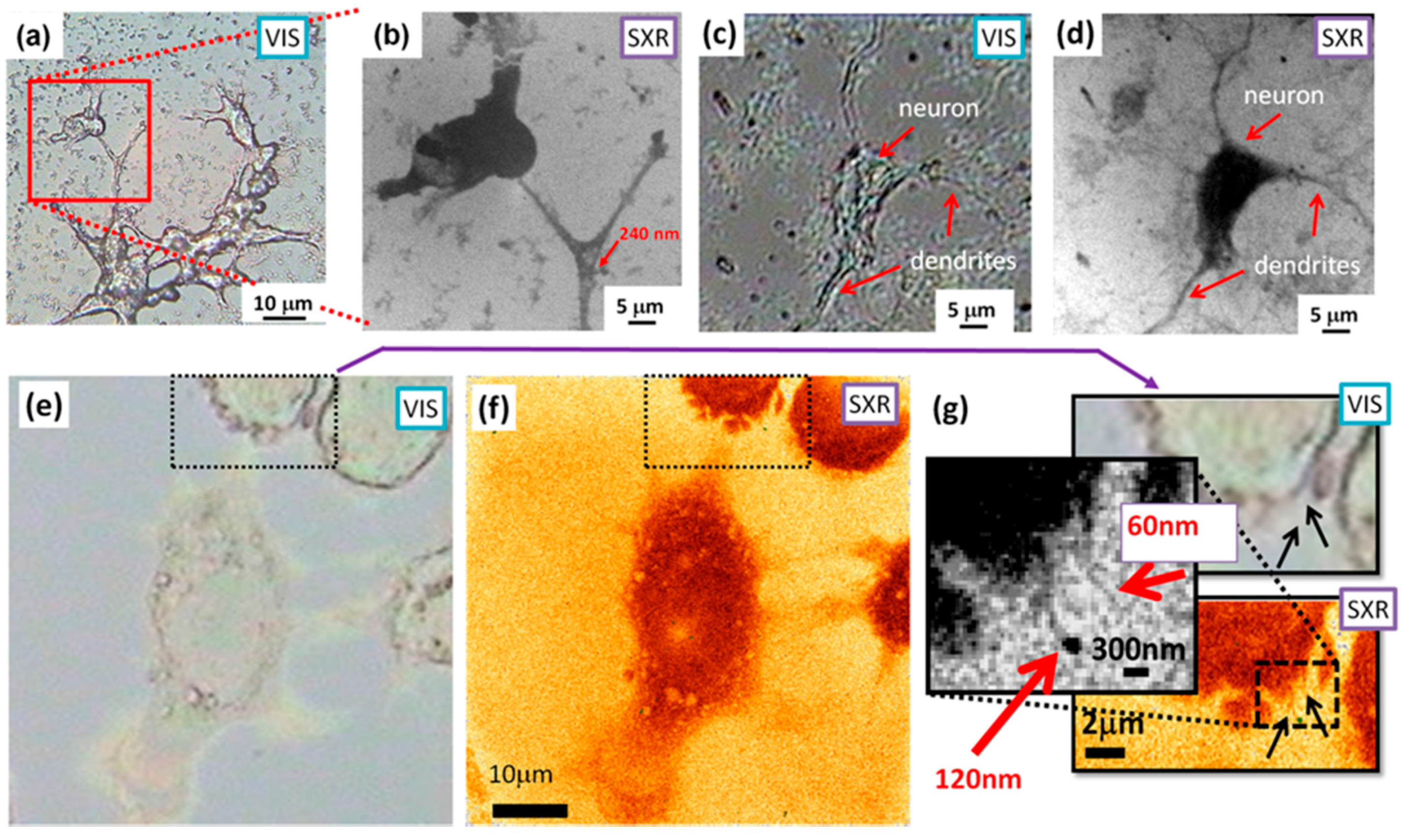
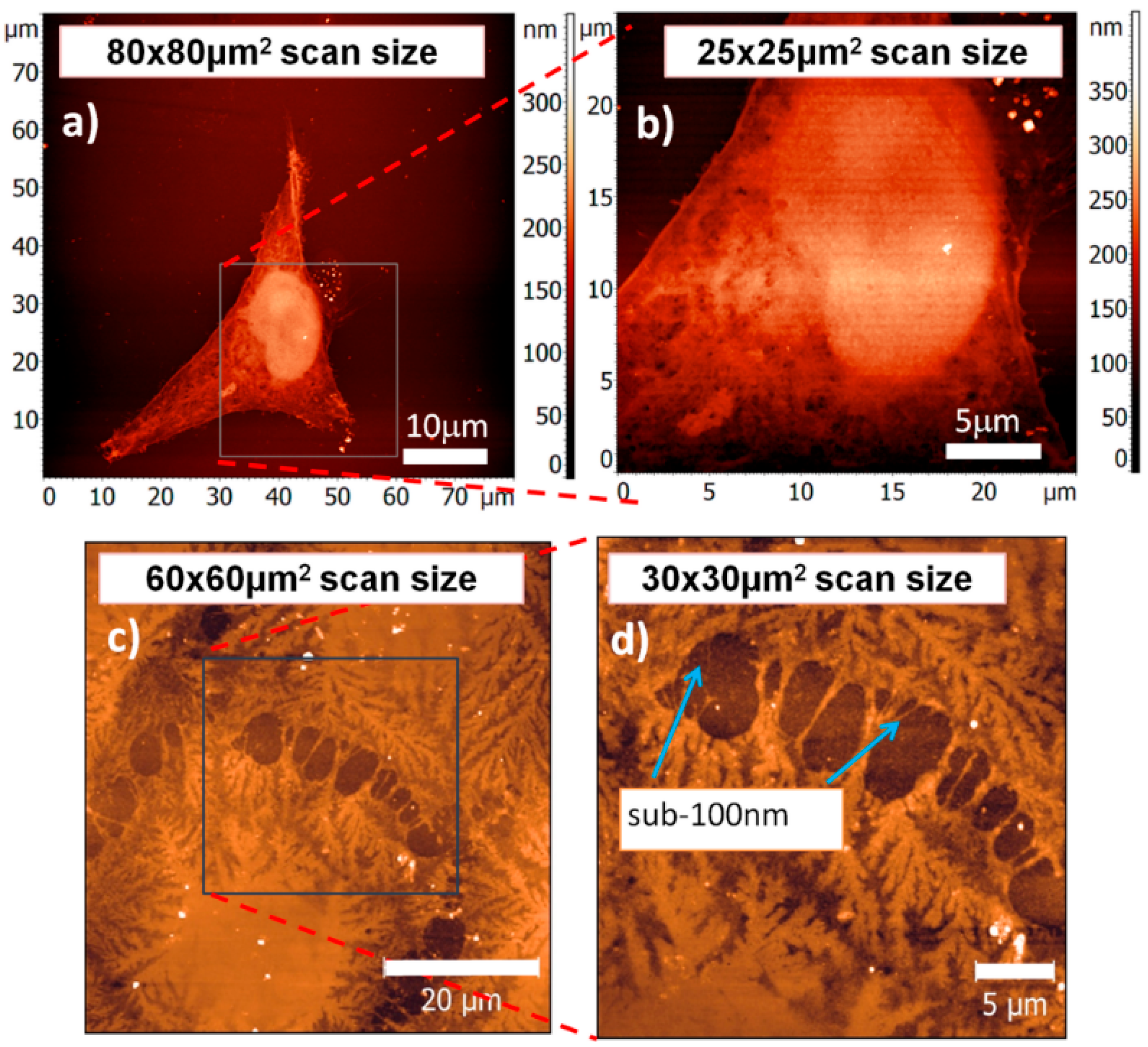
3.1. SXR Full-Field Imaging
3.2. EUV Full-Field Imaging
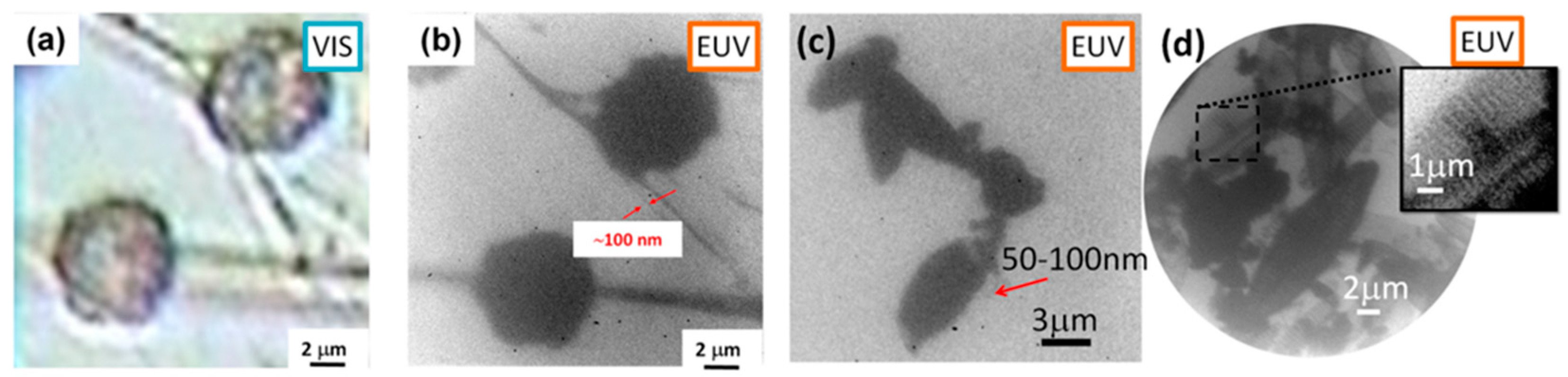
3.3. SXR Contact Microscopy Imaging
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yelin, D.; Silberg, Y. Laser scanning third-harmonic generation microscopy in biology. Opt. Express 1999, 5, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, C.E.; Bellouard, Y. A monolithic microtensile tester for the investigation of silicon dioxide micromechanics, fabricated and operated by a femtosecond laser. Micromachines 2015, 6, 1365–1386. [Google Scholar] [CrossRef]
- Technical Committee. ISO/TC20, Aircraft and space vehicles, Subcommittee SC14, Space systems and operations. In Definitions of Solar Irradiance Spectral Categories; ISO 21348; ISO: Geneva, Switzerland, 2007; pp. 6–7. [Google Scholar]
- Le Gros, M.A.; McDermott, G.; Cinquin, B.P. Biological soft X-ray tomography on beamline 2.1 at the Advanced Light Source. J. Synchrotron Radiat. 2014, 21, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.N.; Barty, A.; Bogan, M.; Sebastien, B.; Frank, M.; Hau-Riege, S.; Marchesini, S.; Woods, B.; Sasa, B.; Benner, H.; et al. Femtosecond diffractive imaging with a soft-X-ray free-electron laser. Nat. Phys. 2006, 2, 839–843. [Google Scholar] [CrossRef]
- Wilke, R.N.; Priebe, M.; Bartels, M.; Giewekemeyer, K.; Diaz, A.; Karvinen, P.; Salditt, T. Hard X-ray imaging of bacterial cells: Nano-diffraction and ptychographic reconstruction. Opt. Express 2012, 20, 19232–19254. [Google Scholar] [CrossRef] [PubMed]
- Martz, D.H.; Selin, M.; von Hofsten, O.; Fogelqvist, E.; Holmberg, A.; Vogt, U.; Legall, H.; Blobel, G.; Seim, C.; Stiel, H.; et al. High average brightness water window source for short-exposure cryomicroscopy. Opt. Lett. 2012, 37, 4425–4427. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Higashiguchi, T.; Otsuka, T.; Jiang, W.; Endo, A.; Dunne, P.; O’Sullivan, G. ‘water window’ sources: Selection based on the interplay of spectral properties and multilayer reflection bandwidth. Appl. Phys. Lett. 2013, 102, 1–5. [Google Scholar] [CrossRef]
- Marino, S.; Palanco, S.; Gabás, M.; Romero, R.; Ramos-Barrado, J.R. Laser nano- and micro-structuring of silicon using a laser-induced plasma for beam conditioning. Nanotechnology 2015, 26, 55303. [Google Scholar] [CrossRef] [PubMed]
- Van Malderen, S.J.M.; van Elteren, J.T.; Vanhaecke, F. Submicrometer imaging by laser ablation–Inductively coupled plasma mass spectrometry via signal and image deconvolution approaches. Anal. Chem. 2015, 87, 6125–6132. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G. Cryo X-ray microscopy with high spatial resolution in amplitude and phase contrast. Ultramicroscopy 1998, 75, 85–104. [Google Scholar] [CrossRef]
- Chao, W.; Fischer, P.; Tyliszczak, T.; Rekawa, S.; Anderson, E.; Naulleau, P. Real space soft X-ray imaging at 10 nm spatial resolution. Opt. Express 2012, 20, 9777–9783. [Google Scholar] [CrossRef] [PubMed]
- Simons, H.; King, A.; Ludwig, W.; Detlefs, C.; Pantleon, W.; Schmidt, S.; Snigireva, I.; Snigirev, A.; Poulsen, H.F. Dark-field X-ray microscopy for multiscale structural characterization. Nat. Commun. 2015, 6, 6098. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.C.; Meirer, F.; Liu, Y.; Mester, Z.; Pianetta, P. Transmission X-ray microscopy for full-field nano imaging of biomaterials. Microsc. Res. Tech. 2011, 74, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Kwon, Y.; Nam, K.; Lim, J.; Kim, K.W.; Chon, K.; Kim, B.; Kim, D.E.; Kim, J.; Ahn, B.N.; et al. Compact soft X-ray transmission microscopy with sub-50 nm spatial resolution. Phys. Med. Biol. 2006, 51, N99–N107. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Kim, I.J.; Kim, C.M.; Yu, T.J.; Lee, S.K.; Sung, J.H.; Yoon, J.W.; Yun, H.; Jeong, T.M.; Choi, I.W.; et al. Single-shot nanometer-scale Fourier transform hologram using Ni-like Ag X-ray laser. In Springer Proceedings in Physics; Springer: New York, NY, USA, 2010; pp. 323–328. [Google Scholar]
- Park, J.J.J.; Kim, D.S.; Jeon, S.C.; Park, J.J.J.; Lee, K.H.; Lee, J.; Kim, K.N.; Yoo, J.J.; Nam, C.H. Table-top soft X-ray microscope adopting a PMMA phase-reversal zone plate. Opt. Lett. 2009, 2, 5–6. [Google Scholar]
- Dierolf, M.; Thibault, P.; Menzel, A.; Kewish, C.M.; Jefimovs, K.; Schlichting, U.; Von König, K.; Bunk, O.; Pfeiffer, F. Ptychographic coherent diffractive imaging of weakly scattering specimens. New J. Phys. 2010, 12, 35017. [Google Scholar] [CrossRef]
- Marconi, M.C.; Wachulak, P.W. Extreme ultraviolet lithography with table top lasers. Prog. Quantum Electron. 2010, 34, 173–190. [Google Scholar] [CrossRef]
- Wachulak, P.W.; Wegrzynski, L.; Bartnik, A.; Fok, T.; Jarocki, R.; Kostecki, J.; Szczurek, M.; Fiedorowicz, H. Characterization of a dual-gas multi-jet gas puff target for high-order harmonic generation using extreme ultraviolet shadowgraphy. Laser Part. Beams 2013, 31, 219–227. [Google Scholar] [CrossRef]
- Brewer, C.A.; Brizuela, F.; Wachulak, P.; Martz, D.H.; Chao, W.; Anderson, E.H.; Attwood, D.T.; Vinogradov, A.V.; Artyukov, I.A.; Ponomareko, A.G.; et al. Single-shot extreme ultraviolet laser imaging of nanostructures with wavelength resolution. Opt. Lett. 2008, 33, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Wachulak, P.W.; Brewer, C.A.; Brizuela, F.; Menoni, C.S.; Chao, W.; Anderson, E.H.; Bartels, R.A.; Rocca, J.J.; Marconi, M.C. Analysis of extreme ultraviolet microscopy images of patterned nanostructures based on a correlation method. J. Opt. Soc. Am. 2008, 25, B20–B26. [Google Scholar] [CrossRef]
- Wachulak, P.W.; Marconi, M.C.; Bartels, R.A.; Menoni, C.S.; Rocca, J.J. Soft X-ray laser holography with wavelength resolution. J. Opt. Soc. Am. 2008, 25, 1811–1814. [Google Scholar] [CrossRef]
- Vaschenko, G.; Brizuela, F.; Brewer, C.; Grisham, M.; Mancini, H.; Menoni, C.S.; Marconi, M.C.; Rocca, J.J.; Chao, W.; Liddle, J.; et al. Nanoimaging with a compact extreme-ultraviolet laser. Opt. Lett. 2005, 30, 2095–2097. [Google Scholar] [CrossRef] [PubMed]
- Juschkin, L.; Freiberger, R.; Bergmann, K. EUV microscopy for defect inspection by dark-field mapping and zone plate zooming. J. Phys. Conf. Ser. 2009, 186, 12030. [Google Scholar] [CrossRef]
- Legall, H.; Blobel, G.; Stiel, H.; Sandner, W.; Seim, C.; Takman, P.; Martz, D.H.; Selin, M.; Vogt, U.; Hertz, H.M.; et al. Compact X-ray microscope for the water window based on a high brightness laser plasma source. Opt. Express 2012, 20, 18362–18369. [Google Scholar] [CrossRef] [PubMed]
- Kirz, J.; Jacobsen, C.; Howells, M. Soft X-ray microscopes and their biological applications. Q. Rev. Biophys. 1995, 28, 33–130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.C.; Feder, R.; Shinozaki, D.M.; Tan, K.H.; Eason, R.W.; Michette, A.; Rosser, R.J. Soft X-ray contact microscopy. Nucl. Instrum. Methods Phys. Res. A 1986, 246, 668–674. [Google Scholar] [CrossRef]
- Ford, T.W.; Stead, A.D.; Cotton, R.A. Soft X-ray contact microscopy of biological materials. Electron Microsc. Rev. 1991, 4, 269–292. [Google Scholar] [CrossRef]
- Kado, M.; Kishimoto, M.; Tamotsu, S.; Yasuda, K.; Aoyama, M.; Shinohara, K. Imaging of fine structures of cellular organelles in hydrated biological cells by a soft X-ray microscope combined with a fluorescence microscope. In Proceedings of the Conference of X-ray Lasers and Coherent X-ray Sources: Development and Applications X, San Diego, CA, USA, 25 August 2013. [Google Scholar]
- Fiedorowicz, H.; Bartnik, A.; Jarocki, R.; Kostecki, J.; Krzywinski, J.; Mikołajczyk, J.; Rakowski, R.; Szczurek, A.; Szczurek, M. Compact laser plasma EUV source based on a gas puff target for metrology applications. J. Alloys Compd. 2005, 401, 99–103. [Google Scholar] [CrossRef]
- Ul Ahad, I.; Bartnik, A.; Fiedorowicz, H.; Kostecki, J.; Korczyc, B.; Ciach, T.; Brabazon, D. Surface modification of polymers for biocompatibility via exposure to extreme ultraviolet radiation. J. Biomed. Mater. Res. A 2014, 102, 3298–3310. [Google Scholar] [CrossRef] [PubMed]
- Adjei, D.; Wiechec, A.; Wachulak, P.; Ayele, M.; Lekki, J.; Kwiatek, W.; Bartnik, A.; Davídková, M.; Vyšín, L.; Juha, L.; et al. DNA strand breaks induced by soft X-ray pulses from a compact laser plasma source. Radiat. Phys. Chem. 2016, 120, 17–25. [Google Scholar] [CrossRef]
- Wachulak, P.; Bartnik, A.; Skorupka, M.; Kostecki, J.; Jarocki, R.; Szczurek, M.; Wegrzynski, L.; Fok, T.; Fiedorowicz, H. Water-window microscopy using a compact, laser-plasma SXR source based on a double-stream gas-puff target. Appl. Phys. B Lasers Opt. 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Heck, J.; Attwood, D.; Berkeley, E.; Meyer-ilse, W.; Anderson, E. Resolution determination in X-ray microscopy: An analysis of the effects of partial coherence and illumination spectrum. J. X-ray Sci. Technol. 1998, 8, 95–104. [Google Scholar] [PubMed]
- Wachulak, P.; Torrisi, A.; Nawaz, M.; Bartnik, A.; Adjei, D.; Vondrová, Š.; Turňová, J.; Jančarek, A.; Limpouch, J.; Vrbová, M.; et al. A Compact “water window” microscope with 60 nm spatial resolution for applications in biology and nanotechnology. Microsc. Microanal. 2015, 21, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Wachulak, P.; Torrisi, A.; Bartnik, A.; Adjei, D.; Kostecki, J.; Wegrzynski, L.; Jarocki, R.; Szczurek, M.; Fiedorowicz, H. Desktop water window microscope using a double-stream gas puff target source. Appl. Phys. B 2015, 118, 573–578. [Google Scholar] [CrossRef]
- Wachulak, P.; Torrisi, A.; Bartnik, A.; Węgrzyński, Ł.; Fok, T.; Fiedorowicz, H. A desktop extreme ultraviolet microscope based on a compact laser-plasma light source. Appl. Phys. B 2016, 123, 1–5. [Google Scholar] [CrossRef]
- Torrisi, A.; Wachulak, P.; Węgrzyński, Ł.; Fok, T.; Bartnik, A.; Parkman, T.; Vondrová, Š.; Turňová, J.; Jankiewicz, B. J.; Bartosewicz, B.; et al. A stand-alone compact EUV microscope based on gas-puff target source. J. Microsc. 2017, 265, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ayele, M.; Czwartos, J.; Adjei, D.; Wachulak, P.; Ahad, I.U.; Bartnik, A.; Wegrzynski, Ł.; Szczurek, M.; Jarocki, R.; Fiedorowicz, H.; et al. Contact microscopy using a compact laser produced plasma soft X-ray source. Acta Phys. Pol. A 2016, 129, 237–240. [Google Scholar] [CrossRef]
- Ayele, M.; Wachulak, P.; Czwartos, J.; Adjei, D.; Bartnik, A.; Wegrzynski, Ł.; Szczurek, M.; Pina, L.; Fiedorowicz, H. Development and characterization of a laser-plasma soft X-ray source for contact microscopy. Nucl. Instr. Meth. B 2017, in press. [Google Scholar]
- Otomo, K.; Hibi, T.; Kozawa, Y. STED microscopy—Super-resolution bio-imaging utilizing a stimulated emission depletion. Microscopy 2015, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Farahani, J.; Schibler, M.; Bentolila, L. Stimulated emission depletion (STED) microscopy: From theory to practice. Microsc. Sci. Technol. Appl. Educ. 2010, 2, 1539–1547. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wachulak, P.; Torrisi, A.; Ayele, M.; Czwartos, J.; Bartnik, A.; Węgrzyński, Ł.; Fok, T.; Parkman, T.; Salačová, Š.; Turňová, J.; et al. Bioimaging Using Full Field and Contact EUV and SXR Microscopes with Nanometer Spatial Resolution. Appl. Sci. 2017, 7, 548. https://doi.org/10.3390/app7060548
Wachulak P, Torrisi A, Ayele M, Czwartos J, Bartnik A, Węgrzyński Ł, Fok T, Parkman T, Salačová Š, Turňová J, et al. Bioimaging Using Full Field and Contact EUV and SXR Microscopes with Nanometer Spatial Resolution. Applied Sciences. 2017; 7(6):548. https://doi.org/10.3390/app7060548
Chicago/Turabian StyleWachulak, Przemysław, Alfio Torrisi, Mesfin Ayele, Joanna Czwartos, Andrzej Bartnik, Łukasz Węgrzyński, Tomasz Fok, Tomáš Parkman, Šárka Salačová, Jana Turňová, and et al. 2017. "Bioimaging Using Full Field and Contact EUV and SXR Microscopes with Nanometer Spatial Resolution" Applied Sciences 7, no. 6: 548. https://doi.org/10.3390/app7060548