Inoculation with Glomus mosseae Improves the Growth and Salvianolic Acid B Accumulation of Continuously Cropped Salvia miltiorrhiza
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Mycorrhizal Inocula
2.3. Plant Material
2.4. Root Staining for Evaluation of AMF Root Colonization
2.5. Assessment of Plant Disease and Growth
2.6. Plant Nutrient Analysis
2.7. Quantitative Analysis of Phenolic Acids
2.8. Statistical Analysis
3. Results
3.1. AMF Root Colonization
3.2. Effects of AMF on Field Diseases and S. miltiorrhiza Growth
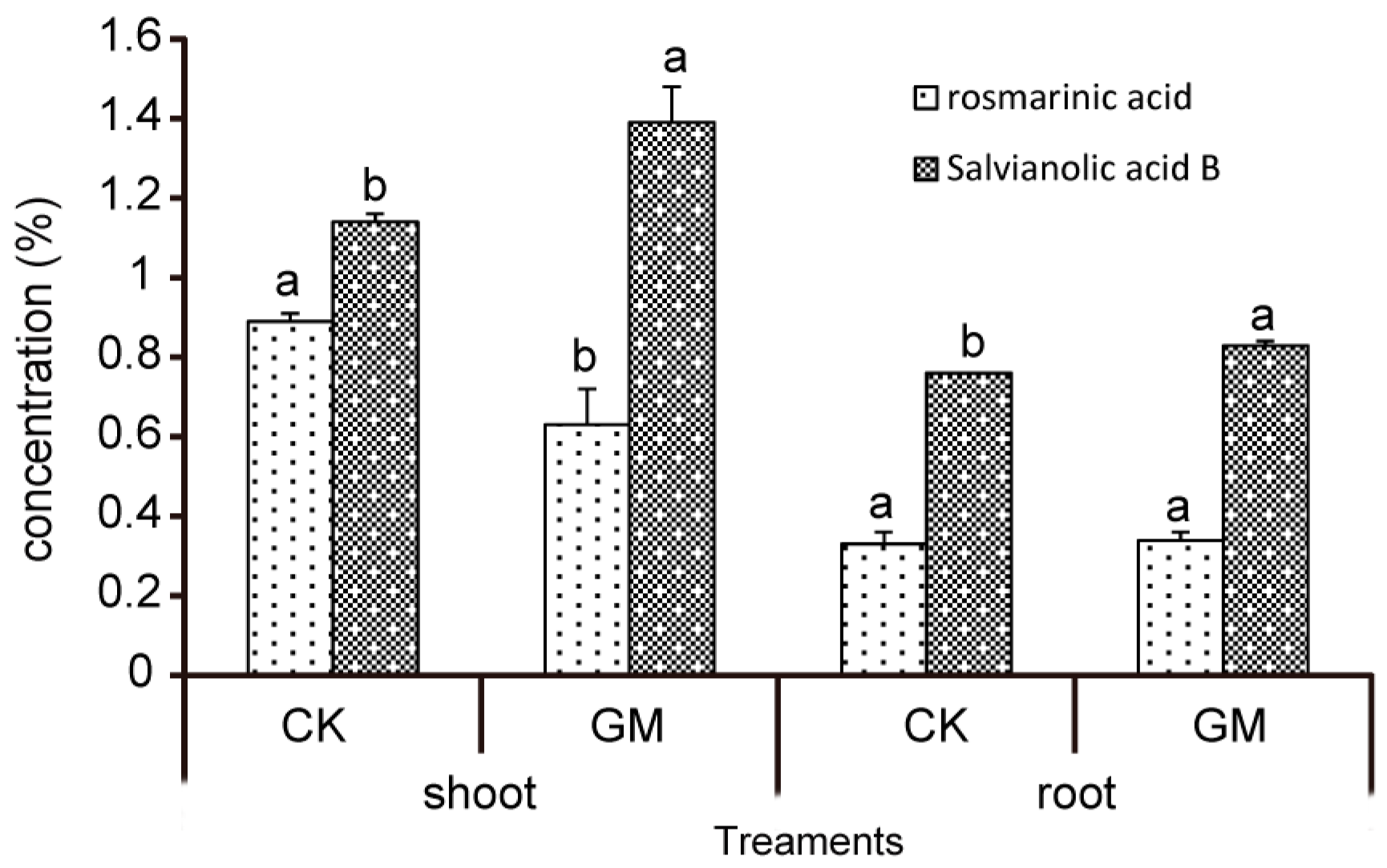
3.3. Effect of G. mosseae on Phenolic Acids in S. miltiorrhiza
3.4. Effect of AMF on Plant Mineral Nutrient Status
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, L.L.; Ma, R.F.; Liu, C.Y.; Liu, H.X.; Zhu, R.Y.; Guo, S.Z.; Tang, M.K.; Li, Y.; Niu, J.Z.; Fu, M.; et al. Salvia miltiorrhiza: A potential red light to the development of cardiovascular diseases. Curr. Pharm. Des. 2017, 23, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; An, S.J.; Fu, J.Q.; Liu, P.; Shao, T.M.; Lie, M.L.; Li, X.; Jiao, Z.; Chai, X.Q. Mixed aqueous extract of Salvia miltiorrhiza reduces blood pressure through inhibition of vascular remodelling and oxidative stress in spontaneously hypertensive rats. Cell. Physiol. Biochem. 2016, 40, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lee, Y.C.; Lin, C.C.; Chang, C.H.; Chiu, C.D.; Chou, L.W.; Sun, M.F.; Yen, H.R. Characteristics of Traditional Chinese Medicine usage in patients with stroke in Taiwan: A nationwide population-based study. J. Ethnopharmacol. 2016, 186, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.P.; Guo, J.J.; Bao, J.L.; Lu, J.J.; Wang, Y.T. The anticancer properties of Salvia miltiorrhiza Bunge (Danshen): A systematic review. Med. Res. Rev. 2014, 34, 768–794. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Son, W.C.; Ryu, J.E.; Koo, B.A.; Kim, Y.S. Standardized salvia miltiorrhiza extract suppresses hepatic stellate cell activation and attenuates steatohepatitis induced by a methionine-choline deficient diet in mice. Molecules 2014, 19, 8189–8211. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Ming, Q.L.; Rahman, K.; Han, T.; Qin, L.P. Salvia miltiorrhiza: Traditional medicinal uses, chemistry, and pharmacology. Chin. J. Nat. Med. 2015, 13, 163–182. [Google Scholar] [CrossRef]
- Liu, X.B.; Xavier, C.; Jann, J.; Wu, H.L. Salvianolic acid B (Sal B) protects retinal pigment epithelial cells from oxidative stress-induced cell death by activating glutaredoxin 1 (Grx1). Int. J. Mol. Sci. 2016, 17, 1835. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, W.B.; Chao, X.D.; Zhang, L.; Qu, Y.; Huo, J.L.; Fei, Z. Salvianolic acid B attenuates brain damage and inflammation after traumatic brain injury in mice. Brain Res. Bull. 2011, 84, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Fei, W.Q.; Zhou, J.C.; Zhang, L.M.; Chen, L.X.; Zhang, X.M.; Liang, X.; Xie, J.S.; Fang, Y.; Sui, X.B.; et al. Salvianolic acid B, a novel autophagy inducer, exerts antitumor activity as a single agent in colorectal cancer cells. Oncotarget 2016, 7, 61509–61519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Xuan, L.J. An achievement on the modernization of Chinese traditional medicine: Research and development of depsides salts from Salvia miltiorrhiza and its preparations. Bull. Chin. Acad. Sci. 2005, 20, 377–380. [Google Scholar]
- Zeng, M.; Di, X.H.; Wang, J.P. Effectiveness and safety of salvianolate injection on acute cerebral infarction. Chin. J. Exp. Tradit. Med. Formulae 2015, 21, 225–229. [Google Scholar]
- Crookston, R.; Kurle, J.; Copeland, P.; Ford, J.; Lueschen, W. Rotational cropping sequence affects yield of corn and soybean. Agron. J. 1991, 83, 108–113. [Google Scholar] [CrossRef]
- Kelley, K.; Long, J.J.; Todd, T. Long-term crop rotations affect soybean yield, seed weight, and soil chemical properties. Field Crop Res. 2003, 83, 41–50. [Google Scholar] [CrossRef]
- Kirkegaard, J.; Christen, O.; Krupinsky, J.; Layzell, D. Break crop benefits in temperate wheat production. Field Crop Res. 2008, 107, 185–195. [Google Scholar] [CrossRef]
- Zhang, C.L.; Sun, Q.; Ye, Q. Obstacle effect of continuous cropping on Salvia miltiorrhiza growth. Acta Bot. Boreali-Occident. Sin. 2005, 25, 1029–1034. [Google Scholar]
- Yang, L.; Miao, Z.Q.; Yang, G.; Shao, A.J.; Huang, L.Q.; Shen, Y.; Wang, X.; Chen, M.L. Research on wilt disease of Salvia miltiorrhiza and its pathogen. Chin. J. Chin. Mater. Med. 2013, 38, 4040–4043. [Google Scholar]
- Yu, J.Q.; Shou, S.Y.; Qian, Y.R.; Zhu, Z.Z.; Hu, W.H. Autotoxic potential of cucurbit crops. Plant Soil 2000, 223, 147–151. [Google Scholar] [CrossRef]
- Huang, H.C.; Chou, C.H.; Erickson, R.S. Soil sickness and its control. Allelopath. J. 2006, 18, 1–21. [Google Scholar]
- Yang, M.L.; Yang, J.; Chang, Q. Effect of different straw reapplication on yields of cucumber and soil fertility. North. Hortic. 2011, 6, 46–48. [Google Scholar]
- Fester, T.; Sawers, R. Progress and challenges in agricultural applications of arbuscular mycorrhizal fungi. Crit. Rev. Plant Sci. 2011, 30, 459–470. [Google Scholar] [CrossRef]
- Weisany, W.; Raei, Y.; Salmasi, S.Z.; Sohrabi, Y.; Ghassemi-Golezani, K. Arbuscular mycorrhizal fungi induced changes in rhizosphere, essential oil and mineral nutrients uptake in dill/common bean intercropping system. Ann. Appl. Biol. 2016, 169, 384–397. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.; Schouteden, N.; van Tuinen, D.; Chatagnier, O.; Elsen, A.; De Waele, D.; Panis, B.; Gianinazzi-Pearson, V. Mycorrhiza-induced resistance against the rooteknot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 2013, 60, 45–54. [Google Scholar] [CrossRef]
- Van der Heijden, M.G.; Streitwolf-Engel, R.; Riedl, R.; Siegrist, S.; Neudecker, A.; Ineichen, K.; Boller, T.; Wiemken, A.; Sanders, I.R. The mycorrhizal contribution to plant productivity, plant nutrition and soil structure in experimental grassland. New Phytol. 2006, 172, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Eun, J.C.; Do, J.L.; Chi, D.W.; Hong, L.K.; Yong, H.C.; Ju, S.C. Effects of AMF inoculation on growth of Panax ginseng C.A. Meyer seedlings and on soil structures in mycorrhizosphere. Sci. Hortic. 2009, 122, 633–637. [Google Scholar]
- Feddermann, N.; Finlay, R.; Thomas, B. Functional diversity in arbuscular mycorrhiza—The role of gene expression, phosphorous nutrition and symbiotic efficiency. Fungal Ecol. 2010, 3, 1–8. [Google Scholar] [CrossRef]
- Urcoviche, R.C.; Gazim, Z.C.; Dragunski, D.G.; Barcellos, F.G.; Albertona, O. Plant growth and essential oil content of Mentha crispa inoculated with arbuscular mycorrhizal fungi under different levels of phosphorus. Ind. Crop Prod. 2015, 67, 103–107. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Nell, M.; Lamien-Meda, A.; Steinkellner, S.; Wawrosch, C.; Kopp, B.; Zitterl, W.; Vierheilig, H.; Novak, J. Effects of root colonization by symbiotic arbuscular mycorrhizal fungi on the yield of pharmacologically active compounds in Angelica archangelica L. Acta Physiol. Plant. 2015, 37, 21–31. [Google Scholar] [CrossRef]
- Džafić, E.; Pongrac, P.; Likar, M.; Regvar, M.; Vogel-Mikuš, K. The arbuscular mycorrhizal fungus Glomus mosseae alleviates autotoxic effects in maize (Zea mays L.). Eur. J. Soil Biol. 2013, 58, 59–65. [Google Scholar] [CrossRef]
- Hage-Ahmed, K.; Moyses, A.; Voglgruber, A.; Hadacek, F.; Steinkellner, S. Alterations in root exudation of intercropped tomato mediated by the arbuscular mycorrhizal fungus Glomus mosseae and the soilborne pathogen Fusarium oxysporum f.sp. lycopersici. J. Phytopathol. 2013, 161, 763–773. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, Q.Z.; Bu, Q.L. Analysis on climatic change of Laiwu City in recent 52 years. Acta Agric. Jiangxi 2011, 23, 163–166. [Google Scholar]
- Sun, H.T. Genus Salvia. In Flora Reipublicae Popularis Sinicae; Wu, C.Y., Li, H.W., Eds.; Science Press: Beijing, China, 1977; Volume 66, pp. 146–148. [Google Scholar]
- Liu, W.; Zhang, L.; Zhou, J.; Shi, G.Y.; Liu, J.H.; Wang, X. Comparison of active constituents at different growth stages in Salvia miltiorrhiza Bge. and Salvia miltiorrhiza Bge. f. alba. Plant Sci. J. 2013, 31, 596–602. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R.G. Quantifying vesicular-arbuscular mycorrhizae, a proposed method towards soil standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agrochemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000; pp. 25–109. [Google Scholar]
- Fornes, F.; Jaramillo, C.X.; García-de-la-Fuente, R.; Belda, R.M.; Lidón, A. Composted organic wastes from the pharmaceutical and agro-food industries induce soil bioactivity and nodulation in alfalfa. J. Sci. Food Agric. 2014, 94, 3030–3037. [Google Scholar] [CrossRef] [PubMed]
- Helgason, T.; Merryweather, J.W.; Denison, J.; Wilson, P.; Young, J.P.W.; Fitter, A.H. Selectivity and functional diversity in arbuscular mycorrhizas of co-occurring fungi and plants from a temperate deciduous woodland. J. Ecol. 2002, 90, 371–384. [Google Scholar] [CrossRef]
- Trotta, A.; Varese, G.C.; Gnavi, E.; Fusconi, A.; Sampb, S.; Berta, G. Interactions between the soilborne root pathogen Phytophthora nicotiana var. parasitica and the arbuscular mycorrhizal fungus Glomus mosseae in tomato plants. Plant Soil 1996, 185, 199–209. [Google Scholar] [CrossRef]
- Berta, G.; Sampo, S.; Gamalero, E.; Massa, N.; Lemanceau, P. Suppression of Rhizoctonia root-rot of tomato by Glomus mossae BEG12 and Pseudomonas fluorescens A6RI is associated with their effect on the pathogen growth and on the root morphogenesis. Eur. J. Plant Pathol. 2005, 111, 279–288. [Google Scholar] [CrossRef]
- Zhang, L.D.; Zhang, L.J.; Christie, P.; Li, X.L. Pre-inoculation with arbuscular mycorrhizal fungi suppresses root knot nematode (Meloidogyne incognita) on cucumber (Cucumis sativus). Biol. Fertil. Soils 2008, 45, 205–211. [Google Scholar] [CrossRef]
- Krishna, H.; Das, B.; Attri, B.L.; Grover, M.; Ahmed, N. Suppression of Botryosphaeria canker of apple by arbuscular mycorrhizal fungi. Crop Prot. 2010, 29, 1049–1054. [Google Scholar] [CrossRef]
- Ismail, Y.; Mccormick, S.; Hijri, M. The arbuscular mycorrhizal fungus, Glomus irregulare, controls the mycotoxin production of Fusarium sambucinum in the pathogenesis of potato. FEMS Microbiol. Lett. 2013, 348, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.G.; Reddy, M.S. Aspergillus tubingensis improves the growth and native mycorrhizal colonization of bermudagrass in bauxite residue. Bioremediat. J. 2011, 3, 157–164. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Tsror Lahkim, L.; Zipori, I.; Hazanovsky, M.; Wininger, S.; Dag, A. Effect of AMF application on growth, productivity and susceptibility to Verticillium wilt of olives grown under desert conditions. Symbiosis 2010, 52, 103–111. [Google Scholar] [CrossRef]
- Jones, M.D.; Smith, S.E. Exploring functional definitions of mycorrhizas, are mycorrhizas always mutualisms? Can. J. Bot. 2004, 82, 1089–1109. [Google Scholar] [CrossRef]
- Zhong, S.; Mo, Y.; Guo, G.; Zeng, H.; Jin, Z. Effect of continuous cropping on soil chemical properties and crop yield in banana plantation. J. Agric. Sci. Technol. 2014, 16, 239–250. [Google Scholar]
- Bagyaraj, D.J. Ecology of arbuscular mycorrhizal fungi. In Microbial Diversity and Biotechnology in Food Security; Kharwar, R.N., Upadhyay, R.S., Dubey, N.K., Raghuwanshi, R., Eds.; Springer India: New Delhi, India, 2014; pp. 133–146. [Google Scholar]
- Vierheilig, H.; Gagnon, H.; Strack, D.; Maier, W. Accumulation of cyclohexenone derivatives in barley, wheat and maize roots in response to inoculation with different arbuscular mycorrhizal fungi. Mycorrhiza 2000, 9, 291–293. [Google Scholar] [CrossRef]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Glomus macrocarpum, a potential bioinoculant to improve essential oil quality and concentration in Dill (Anethum graveolens L.) and Carum (Trachyspermum ammi (Linn.) Sprague). World J. Microbiol. Biotechnol. 2002, 18, 459–463. [Google Scholar] [CrossRef]
- Araim, G.; Saleem, A.; Arnason, J.T.; Charest, C. Root clonization by an arbuscular mycorrhizal (AM) fungus increases growth and secondary metabolism of purple coneflower, Echinacea purpurea (L.) Moench. J. Agric. Food Chem. 2009, 57, 2255–2258. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, M.; Alizadeh, M.; Mashayekhi, K.; Asghari, H.R. In vitro propagation of four Iranian grape varieties: Influence of genotype and pretreatment with arbuscular mycorrhiza. Vitis 2012, 51, 175–182. [Google Scholar]
- Singh, D.P.; Srivastava, J.S.; Bahadur, A.; Singh, U.P.; Singh, S.K. Arbuscular mycorrhizal fungi induced biochemical changes in pea (Pisum sativum) and their effect on powdery mildew (Erysiphe pisi). J. Plant Dis. Prot. 2004, 111, 266–272. [Google Scholar]
- Zhang, R.Q.; Zhu, H.H.; Zhao, H.Q.; Yao, Q. Arbuscular mycorrhizal fungal inoculation increases phenolic synthesis in clover roots via hydrogen peroxide, salicylic acid and nitric oxide signaling pathways. J. Plant Physiol. 2013, 170, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Ling, Y.M.; Liu, D.H. Effects of Mn on growth and tanshinones accumulation of cultivated Salvia miltiorrhiza Bunge. Soils 2011, 43, 95–100. [Google Scholar]
- Brennan, R.F. The role of manganese and nitrogen nutrition in the susceptibility of wheat plants to take-all in western Australia. Fertil. Res. 1992, 31, 35–41. [Google Scholar] [CrossRef]
- Heine, G.; Max, J.F.J.; Führs, H.; Moran-Puente, D.W.; Heintz, D.; Horst, W.J. Effect of manganese on the resistance of tomato to Pseudocercospora fuligena. J. Plant Nutr. Soil Sci. 2011, 174, 827–836. [Google Scholar] [CrossRef]
- Wang, M.Y.; Christie, P.; Xiao, Z.Y.; Qin, C.P.; Wang, P. Iron concentration by Poncirus trifoliata L. Raf and Citrus reticulata Blanco grown on sand medium under different PH. Biol. Fertil. Soils 2008, 45, 65–72. [Google Scholar] [CrossRef]
- Lemanceau, P.; Expert, D.; Gaymard, F.; Bakker, P.A.H.M.; Briat, J.F. Role of Iron in Plant-Microbe Interactions. Adv. Bot. Res. 2009, 51, 491–549. [Google Scholar]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol. Res. 2008, 163, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.P.; Yadav, J.; Tiwari, K.N. Enhancement of nodulation and yield of chickpea by co-inoculation of indigenous Mesorhizobium spp. and plant growth-promoting rhizobacteria in eastern Uttar Pradesh. Commun. Soil Sci. Plant Anal. 2012, 43, 605–621. [Google Scholar] [CrossRef]


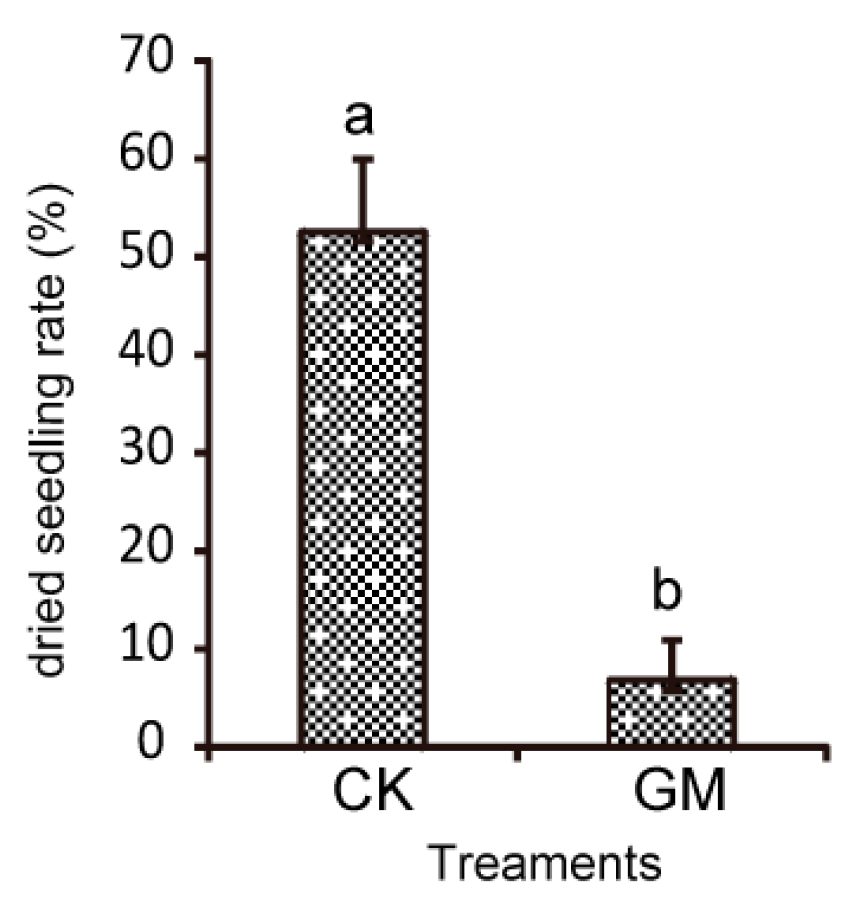
| Treatments | Biomass of Shoot (g) | Biomass of Root (g) | Leaf Number | Crown Width (cm) | Plant Height (cm) | Stem Diameter (cm) | Radical Number | Root | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Diameter (cm) | Number | ||||||||
| CK | 36.44 ± 1.70 b | 34.11 ± 3.19 b | 74.74 ± 11.99 a | 38.56 ± 1.62 b | 39.58 ± 2.67 b | 1.97 ± 0.09 b | 4.31 ± 1.89 a | 30.60 ± 0.79 b | 1.82 ± 0.16 a | 31.48 ± 2.47 a |
| GM | 53.97 ± 3.87 a | 47.47 ± 2.87 a | 84.75 ± 4.14 a | 42.66 ± 1.04 a | 49.86 ± 1.72 a | 2.33 ± 0.08 a | 7.70 ± .93 a | 36.64 ± 2.23 a | 1.99 ± 0.19 a | 30.82 ± 0.43 a |
| Treatments | Rosmarinic Acid (%) | Salvianolic Acid B (%) | |
|---|---|---|---|
| Shoot | CK | 0.89 ± 0.02 a | 1.14 ± 0.02 b |
| GM | 0.63 ± 0.09 b | 1.39 ± 0.09 a | |
| Root | CK | 0.33 ± 0.03 a | 0.76 ± 0.00 b |
| GM | 0.34 ± 0.02 a | 0.83 ± 0.01 a | |
| Treatments | N (g/kg) | P (g/kg) | K (g/kg) | Ca (g/kg) | Mg (g/kg) | Cu (mg/kg) | Zn (mg/kg) | Fe (g/kg) | Mn (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Shoot | CK | 16.40 ± 0.75 a | 4.31 ± 0.28 a | 26.08 ± 0.91 b | 2.37 ± 0.05 a | 5.03 ± 0.12 a | 16.41 ± 1.11 a | 75.03 ± 6.68 a | 1.48 ± 0.12 a | 65.93 ± 1.07 c |
| GM | 18.26 ± 1.07 a | 3.24 ± 0.11 b | 32.41 ± 0.24 a | 2.48 ± 0.06 a | 4.29 ± 0.24 a | 17.87 ± 0.38 a | 74.28 ± 3.04 a | 1.20 ± 0.12 b | 127.49 ± 10.2 a | |
| Root | CK | 15.21 ± 0.94 a | 2.87 ± 0.03 a | 10.35 ± 0.11 a | 0.53 ± 0.03 a | 2.67 ± 0.02 a | 18.01 ± 0.29 a | 44.01 ± 2.49 a | 0.76 ± 0.03 b | 33.8 ± 1.33 b |
| GM | 13.88 ± 0.67 a | 2.51 ± 0.04 b | 10.07 ± 0.26 a | 0.54 ± 0.01 a | 2.45 ± 0.07 b | 21.03 ± 1.80 a | 42.97 ± 2.40 a | 1.33 ± 0.11 a | 55.82 ± 5.94 a | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Yang, G.; Liu, D.; Li, M.; Qiu, H.; Guo, L.; Huang, L.; Chao, Z. Inoculation with Glomus mosseae Improves the Growth and Salvianolic Acid B Accumulation of Continuously Cropped Salvia miltiorrhiza. Appl. Sci. 2017, 7, 692. https://doi.org/10.3390/app7070692
Chen M, Yang G, Liu D, Li M, Qiu H, Guo L, Huang L, Chao Z. Inoculation with Glomus mosseae Improves the Growth and Salvianolic Acid B Accumulation of Continuously Cropped Salvia miltiorrhiza. Applied Sciences. 2017; 7(7):692. https://doi.org/10.3390/app7070692
Chicago/Turabian StyleChen, Meilan, Guang Yang, Dahui Liu, Minhui Li, Hongyan Qiu, Lanping Guo, Luqi Huang, and Zhi Chao. 2017. "Inoculation with Glomus mosseae Improves the Growth and Salvianolic Acid B Accumulation of Continuously Cropped Salvia miltiorrhiza" Applied Sciences 7, no. 7: 692. https://doi.org/10.3390/app7070692






