Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review
Abstract
:1. Introduction
2. Biodiesel Production
2.1. Physical Methods
2.1.1. Direct Mixing Method
2.1.2. Microemulsion Method
2.2. Chemical Methods
2.2.1. Pyrolysis Method
2.2.2. Transesterification Method
3. Properties of Canola Oil Biodiesel
3.1. Density and Viscosity
3.2. Oxygen Content
3.3. Cetane Number
3.4. Calorific Value
3.5. Oxidation Stability
3.6. Iodine Index
3.7. Other Properties
4. Common Experimental Analysis Methods
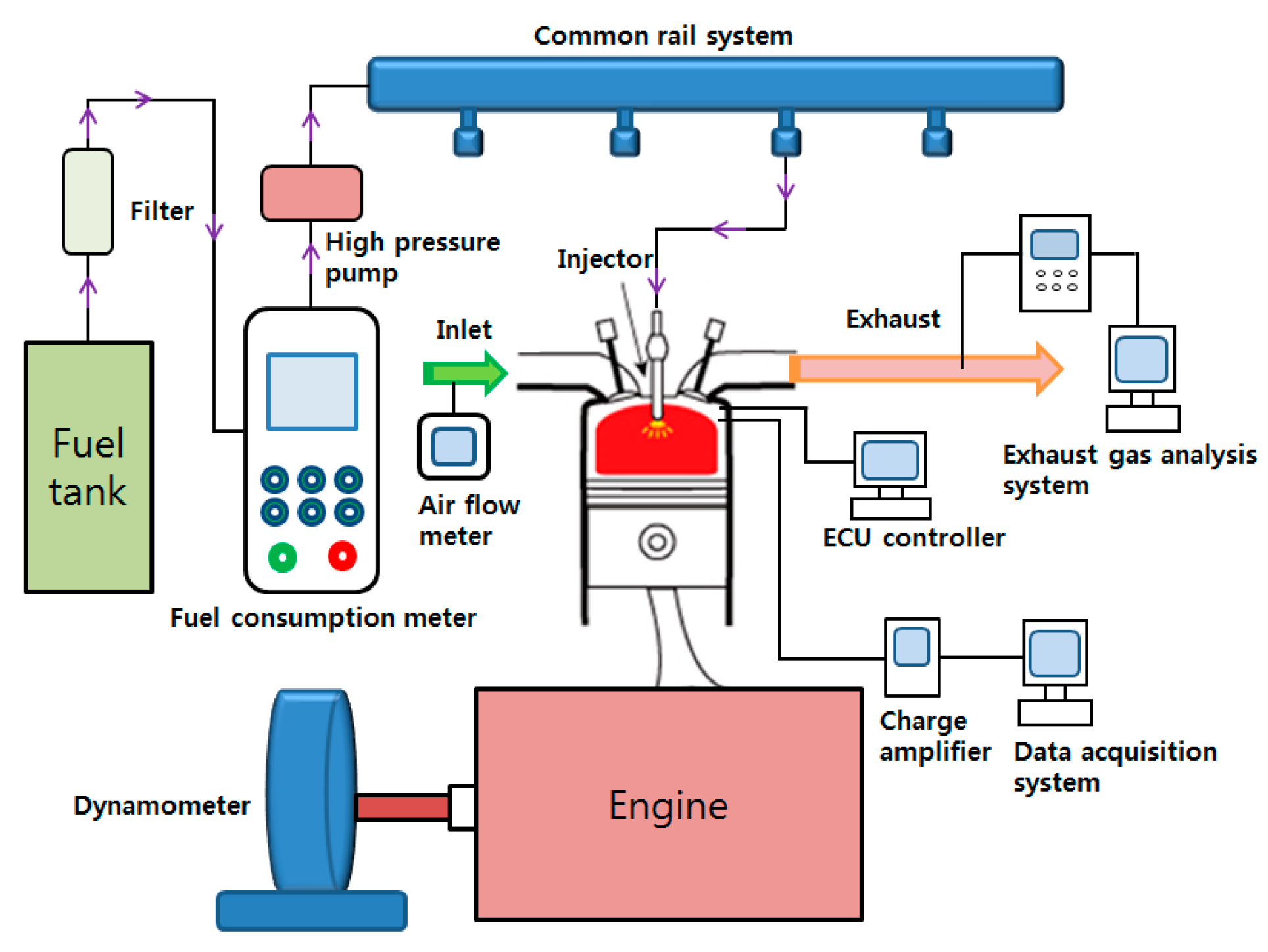
4.1. Experiment Settings and Methods
4.2. Traditional Analytical Formulas
4.2.1. The Rate of Heat Release
4.2.2. Exhaust Gas Recirculation
4.2.3. Brake Specific Fuel Consumption
4.2.4. Coefficient of Variation in Indicated Mean Effective Pressure
5. Results and Discussion
5.1. Spray Characteristics of Canola Oil Biodiesel
5.2. Combustion Characteristics
5.3. Ignition Delay and Coefficient of Variation in Indicated Mean Effective Pressure
5.4. Emission Characteristics
5.4.1. CO, HC, and PM Emissions
5.4.2. NOx Emission
6. Conclusions
- COB can be used as a good alternative fuel and can be used in diesel engines without engine modifications.
- Based on engine combustion performance and exhaust emission characteristics, BD20 is a qualified alternative fuel compared with other blended COB fuels.
- The oxygen atoms in COB play a major role in reducing CO, HC, and PM emissions. However, their presence promote combustion, increase combustion temperature, and increase NOx emissions. EGR technology can significantly reduce NOx emissions.
- The optimum conditions for the direct injection of high common rail diesel engine fueled with COB are obtained based on a large amount of experimental data related to engine combustion performance and exhaust emission characteristics. The optimal conditions are 2000 rpm engine speed, 10% EGR rate, and 10 degree pilot injection timing.
- This paper reviewed the research findings of various blended ratios of COB fuels in a variety of complex experimental conditions. The optimum mixing ratio of biodiesel and optimum engine parameters were obtained. This paper will serve as a valuable reference for the development and application of COB to diesel engines and the design of engines.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Asif, F.; Christopher, S.W.; Michael, P.W. Air Pollution from Motor Vehicles: Standards and Technologies for Controlling Emissions; World Bank Publications: Washington, DC, USA, 1996. [Google Scholar]
- Aparna Seetharam, K. AUTOMOBILE EXHAUST POLLUTION. Available online: http://jchps.com/specialissues/Special%20issue3/14%20jchps%20si3%20K.%20Aparna%20Seetharam%2073-74.pdf (accessed on 27 August 2017).
- Bhandarkar, S. Vehicular Pollution, Their Effect on Human Heatlh and Mitigation Measures. Veh. Eng. 2013, 1, 33–40. [Google Scholar]
- Sharaf, J. Exhaust Emissions and Its Control Technology for an Internal Combustion Engine. Int. J. Eng. Res. Appl. 2013, 3, 947–960. [Google Scholar]
- Brugge, D.; Durant, J.L.; Rioux, C. Near-highway pollutants in motor vehicle exhaust: A review of epidemiologic evidence of cardiac and pulmonary health risks. Environ. Health 2007, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.C.; Choi, N.J. Fabrication of Functional Polyurethane/Rare Earth Nanocomposite Membranes by Electrospinning and Its VOCs Absorption Capacity from Air. Nanomaterials 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wu, P.; Liu, Y.; Song, Y. Environmental and Dynamic Conditions for the Occurrence of Persistent Haze Events in North China. Engineering 2017, 3, 266–271. [Google Scholar] [CrossRef]
- Fu, H.; Chen, J. Formation, features and controlling strategies of severe haze-fog pollutions in China. Sci. Total Environ. 2017, 578, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Guttikunda, S.K.; Carmichael, G.R.; Wang, Y.; Liu, Z.; Stanier, C.O.; Saide, P.E.; Yu, M. Health impacts and economic losses assessment of the 2013 severe haze event in Beijing area. Sci. Total Environ. 2015, 511, 553–561. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Abdul Adam, A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B.K. A comprehensive review of biodiesel as an alternative fuel for compression ignition engine. Renew. Sustain. Energy Rev. 2016, 57, 799–821. [Google Scholar] [CrossRef]
- Razon, L.F.; Knothe, G. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar]
- Othman, M.F.; Adam, A.; Najafi, G.; Mamat, R. Green fuel as alternative fuel for diesel engine: A review. Renew. Sustain. Energy Rev. 2017, 80, 694–709. [Google Scholar] [CrossRef]
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Hu, N.; Tan, J.; Wang, X.; Zhang, X.; Yu, P. Volatile organic compound emissions from an engine fueled with an ethanol-biodiesel-diesel blend. J. Energy Inst. 2017, 90, 101–109. [Google Scholar] [CrossRef]
- Dwivedi, G.; Jain, S.; Sharma, M.P. Diesel engine performance and emission analysis using biodiesel from various oil sources—Review. J. Mater. Environ. Sci. 2013, 4, 434–447. [Google Scholar]
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw Hill: New York, NY, USA, 1988. [Google Scholar]
- Kegl, B.; Kegal, M.; Pehan, S. Green Diesel Engines-Biodiesel Usage in Diesel Engines; Springer: London, UK, 2013. [Google Scholar]
- Abdullah Hil Baky, M.; Mustafizur Rahman, M.; Sadrul Islam, A.K.M. Development of renewable energy sector in Bangladesh: Current status and future potentials. Renew. Sustain. Energy Rev. 2017, 73, 1184–1197. [Google Scholar] [CrossRef]
- Biswas, S.; Katiyar, R.; Gurjar, B.R.; Pruthi, V. Biofuels and Their Production through Different Catalytic Routes. Chem. Biochem. Eng. Q. 2017, 31, 47–62. [Google Scholar] [CrossRef]
- Permpool, N.; Gheewala, S.H. Environmental and energy assessment of alternative fuels for diesel in Thailand. J. Clean. Prod. 2017, 142, 1176–1182. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of CO2 concentration on algal growth: A review. Renew. Sustain. Energy Rev. 2014, 38, 172–179. [Google Scholar] [CrossRef]
- Sorate, K.A.; Bhale, P.V. Impact of Biodiesel on Fuel System Materials Durability. J. Sci. Ind. Res. 2013, 72, 48–57. [Google Scholar]
- Sharma, Y.C.; Singh, B.; Upadhyay, S.N. Advancements in Development and Characterization of Biodiesel: A Review. Fuel 2008, 87, 2355–2373. [Google Scholar] [CrossRef]
- Liaquat, A.M.; Masjuki, H.H.; Kalam, M.A.; Fazal, M.A.; Khan, A.F.; Fayaz, H.; Varman, M. Impact of palm biodiesel blend on injector deposit formation. Appl. Energy 2013, 111, 882–893. [Google Scholar] [CrossRef]
- Chaikool, P.; Intravised, K.; Patsin, P.; Laonapakul, T. A Study of Effect of Biodiesel on Common-Rail Injection Nozzle. SAE Int. J. Fuels Lubr. 2016, 9, 712–716. [Google Scholar] [CrossRef]
- Gadonneix, P.; Castro, F.B.D.; Medeiros, N.F.D.; Drouin, R.; Jain, C.P.; Kim, Y.D.; Ferioli, J.; Nadeau, M.J.; Sambo, A.; Teyssen, J.; et al. Biofuels: Policies, Standards and Technologies; World Energy Council Regency House: London, UK, 2010; ISBN 978-0-946121-03-8. [Google Scholar]
- Koizumi, T. Biofuel Programs in East Asia: Developments, Perspectives, and Sustainability. In Environmental Impact of Biofuels; InTech: Rijeka, Croatia, 2011; Chapter 11. [Google Scholar]
- Kang, S.; Selosse, S.; Maïzi, N. Strategy of bioenergy development in the largest energy consumers of Asia (China, India, Japan and South Korea). Energy Strateg. Rev. 2015, 8, 56–65. [Google Scholar] [CrossRef]
- Yan, J.; Lin, T. Biofuels in Asia. Appl. Energy 2009, 86, 1–10. [Google Scholar] [CrossRef]
- Van Gerpen, J.; Shanks, B.; Pruszko, R.; Clements, D.; Knothe, G. Biodiesel Production Technology; National Renewable Energy Laboratory 1617 Cole Boulevard: Golden, CO, USA, 2014. [Google Scholar]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Gerpen, J.V. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Use of vegetable oils as I.C. engine fuels—A review. Renew. Energy 2004, 29, 727–742. [Google Scholar] [CrossRef]
- Aransiola, E.F.; Ojumu, T.V.; Oyekola, O.O.; Madzimbamuto, T.F.; Ikhu-Omoregbe, D.I.O. A review of current technology for biodiesel production: State of the art. Biomass Bioenergy 2014, 61, 276–297. [Google Scholar] [CrossRef]
- Rajalingam, A.; Jani, S.P.; Senthil Kumar, A.; Adam Khan, M. Production methods of biodiesel. J. Chem. Pharm. Res. 2016, 8, 170–173. [Google Scholar]
- Kurnia, J.C.; Jangam, S.V.; Akhtar, S.; Sasmito, A.P.; Mujumdar, A.S. Advances in biofuel production from oil palm and palm oil processing wastes: A review. Biofuel Res. J. 2016, 9, 332–346. [Google Scholar] [CrossRef]
- Ito, T.; Sakurai, Y.; Kakuta, Y.; Sugano, M.; Hirano, K. Biodiesel production from waste animal fats using pyrolysis method. Fuel Process. Technol. 2012, 94, 47–52. [Google Scholar] [CrossRef]
- Santos, A.L.F.; Martins, D.U.; Iha, O.K.; Ribeiro, R.A.M.; Quirino, R.L.; Suarez, P.A.Z. Agro-industrial residues as low-price feedstock for diesel-like fuel production by thermal cracking. Bioresour. Technol. 2010, 101, 6157–6162. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Convers. Manag. 2002, 43, 2349–2356. [Google Scholar] [CrossRef]
- Demirbas, A. Comparison of transesterification methods for production of biodiesel from vegetable oils and fats. Energy Convers. Manag. 2008, 49, 125–130. [Google Scholar] [CrossRef]
- Al-Zuhair, S. Production of biodiesel: Possibilities and challenges. Biofuels Bioprod. Biorefin. 2007, 1, 57–66. [Google Scholar] [CrossRef]
- Dizge, N.; Aydiner, C.; Imer, D.Y.; Bayramoglu, M.; Tanriseven, A.; Keskinler, B. Biodiesel production from sunflower, soybean, and waste cooking oils by transesterification using lipase immobilized onto a novel microporous polymer. Bioresour. Technol. 2009, 100, 1983–1991. [Google Scholar] [CrossRef]
- Meher, L.C.; Vidya Sagar, D.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Fukuda, H.; Kondo, A.; Noda, H. Biodiesel Fuel Production by Transesterification of Oils. J Biosci. Bioeng. 2001, 92, 405–416. [Google Scholar] [CrossRef]
- Sani, Y.M.; Daud, W.M.A.W.; Azia, A.A. Activity of solid acid catalysts for biodiesel production: A critical review. Appl. Catal. A Gen. 2014, 470, 140–161. [Google Scholar] [CrossRef]
- Thanh, L.T.; Okitsu, K.; Boi, L.V.; Maeda, Y. Catalytic Technologies for Biodiesel Fuel Production and Utilization of Glycerol: A Review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Labarta, J.A.R.; Valdes, F.J. New Heterogeneous Catalytic Transesterification of Vegetable and Used Frying Oil. Ind. Eng. Chem. Res. 2010, 49, 9068–9076. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Kinetics of transesteri® cation in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Ilgen, O.; Dincer, I.; Yildiz, M.; Alptekin, E.; Boz, N.; Canakci, M.; Akin, A.N. Investigation of Biodiesel Production from Canola Oil using Mg-Al Hydrotalcite Catalysts. Turk. J. Chem. 2007, 31, 509–514. [Google Scholar]
- Yadava, D.K.; Vasudev, S.; Singh, N.; Mohapatra, T.; Prabhu, K.V. Breeding Major Oil Crops: Present Status and Future Research Needs. In Technological Innovations in Major World Oil Crops, Volume 1: Breeding; Springer: New York, NY, USA, 2012; Chapter 2; Volume XIII, 405p, ISBN 978-1-4614-0355-5. [Google Scholar]
- Issariyakul, T.; Kulkarni, M.G.; Meher, L.C.; Dalai, A.K.; Bakhshi, N.N. Biodiesel production from mixtures of canola oil and used cooking oil. Chem. Eng. J. 2008, 140, 77–85. [Google Scholar] [CrossRef]
- Yoon, S.K.; Kim, M.S.; Kim, H.J.; Choi, N.J. Effects of canola oil biodiesel fuel blends on combustion, performance, and emissions reduction in a common rail diesel engine. Energies 2014, 7, 8132–8149. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef]
- Suh, H.K.; Lee, C.S. A review on atomization and exhaust emissions of a biodiesel-fueled compression ignition engine. Renew. Sustain. Energy Rev. 2016, 58, 1601–1620. [Google Scholar] [CrossRef]
- Sivaramakrishnan, K.; Ravikumar, P. Determination of higher heating value of biodiesels. Int. J. Eng. Sci. Technol. 2011, 3, 7981–7987. [Google Scholar]
- Al-Hamamre, Z.; Al-Salaymeh, A. Physical properties of (jojoba oil + biodiesel), (jojoba oil + diesel) and (biodiesel + diesel) blends. Fuel 2014, 123, 175–188. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Watts, K.C. Viscosities of canola, jatropha and soapnut biodiesel at elevated temperatures and pressures. Fuel 2012, 102, 789–794. [Google Scholar] [CrossRef]
- Macedo, T.O.; Pereira, R.G.; Pardal, J.M.; Soares, A.S.; Lameira, V.J. Viscosity of Vegetable Oils and Biodiesel and Energy Generation. World Acad. Sci. Eng. Technol. 2013, 7, 161–167. [Google Scholar]
- Verduzco, L.F.R. Density and viscosity of biodiesel as a function of temperature: Empirical models. Renew. Sustain. Energy Rev. 2013, 19, 652–665. [Google Scholar] [CrossRef]
- Aworanti, O.A.; Agarry, S.E.; Ajani, A.O. A Laboratory Study of the Effect of Temperature on Densities and Viscosities of Binary and Ternary Blends of Soybean Oil, Soy Biodiesel and Petroleum Diesel Oil. Adv. Chem. Eng. Sci. 2012, 2, 444–452. [Google Scholar] [CrossRef]
- Fasina, O.O.; Colley, Z. Viscosity and Specific Heat of Vegetable Oils as a Function of Temperature: 35 °C to 180 °C. Int. J. Food Prop. 2008, 11, 738–746. [Google Scholar] [CrossRef]
- Basha, S.A.; Gopal, K.R.; Jebaraj, S. A review on biodiesel production, combustion, emissions and performance. Renew. Sustain. Energy Rev. 2009, 13, 1628–1634. [Google Scholar] [CrossRef]
- Song, H.; Quinton, K.S.; Peng, Z.; Zhao, H.; Ladommatos, N. Effects of Oxygen Content of Fuels on Combustion and Emissions of Diesel Engines. Energies 2016, 9, 28. [Google Scholar] [CrossRef]
- Nakano, M.; Okawa, K. Study of oxygen-containing hydrocarbons in exhaust emission from a spark ignition combustion engine. Int. J. Engine Res. 2014, 15, 572–580. [Google Scholar] [CrossRef]
- Mwang, J.K.; Lee, W.J.; Chang, Y.C.; Chen, C.Y.; Wang, L.C. An overview: Energy saving and pollution reduction by using green fuel blends in diesel engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]
- Lin, B.F.; Huang, J.H.; Huang, D.Y. Effects of Biodiesel from Palm Kernel Oil on the Engine Performance, Exhaust Emissions, and Combustion Characteristics of a Direct Injection Diesel Engine. Energy Fuels 2008, 22, 4229–4234. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Juneja, M.; Singh, K.; Singh, S. Investigating the effect of fuel cetane number, oxygen content, fuel density, and engine operating variables on NOx emissions of a heavy duty diesel engine. Environ. Prog. Sustain. Energy 2017, 36, 214–221. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion Efficiency Impacts of Biofuels. Energy Sources Part A 2009, 31, 602–609. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rahman, M.M. Performance and emission characteristics of biodiesel-diesel blend and environmental and economic impacts of biodiesel production: A review. Renew. Sustain. Energy Rev. 2017, 74, 938–948. [Google Scholar] [CrossRef]
- Yaakob, Z.; Narayanan, B.; Padikkaparambil, S.; Unni, K.S.; Akbar, P.M. A review on the oxidation stability of biodiesel. Renew. Sustain. Energy Rev. 2014, 35, 136–153. [Google Scholar] [CrossRef]
- García, M.; Botella, L.; Gil-Lalaguna, N.; Arauzo, J.; Gonzalo, A.; Sánchez, J.L. Antioxidants for biodiesel: Additives prepared from extracted fractions of bio-oil. Fuel Process. Technol. 2017, 156, 407–414. [Google Scholar] [CrossRef]
- Focke, W.W.; Mashele, R.P.; Nhlapo, N.S. Stabilization of low-density polyethylene films containing metal stearates as photodegradants. J. Vinyl Addit. Technol. 2011, 17, 21–27. [Google Scholar] [CrossRef]
- Barabás, I.; Todoruț, I.A. Biodiesel quality, standards and properties. In Biodiesel-Quality, Emissions and By-Products; Montero, G., Ed.; InTech E-Publishing: Rijeka, Croatia, 2011; pp. 3–28. ISBN 978-953-307-784-0. [Google Scholar]
- Daun, J.K.; Eskin, N.A.M.; Hickling, D. Canola: Chemistry, Production, Processing, and Utilization; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Borugadda, V.B.; Somidi, A.K.R.; Dalai, A.K. Chemical/Structural Modification of Canola Oil and Canola Biodiesel: Kinetic Studies and Biodegradability of the Alkoxides. Lubricants 2017, 5, 11. [Google Scholar] [CrossRef]
- Ge, J.C.; Yoon, S.K.; Kim, M.S.; Choi, N.J. Application of Canola Oil Biodiesel/Diesel Blends in a Common Rail Diesel Engine. Appl. Sci. 2017, 7, 34. [Google Scholar] [CrossRef]
- Ge, J.C.; Kim, M.S.; Yoon, S.K.; Choi, N.J. Effects of Pilot Injection Timing and EGR on Combustion, Performance and Exhaust Emissions in a Common Rail Diesel Engine Fueled with a Canola Oil Biodiesel-Diesel Blend. Energies 2015, 8, 7312–7325. [Google Scholar] [CrossRef]
- Qi, D.H.; Chen, H.; Geng, L.M.; Bian, Y.Z.H.; Ren, X.C.H. Performance and combustion characteristics of biodiesel-diesel-methanol blend fuelled engine. Appl. Energy 2010, 87, 1679–1686. [Google Scholar] [CrossRef]
- Anbarasu, A.; Karthikeyan, A. Performance and Emission Characteristics of Direct Injection Diesel Engine Running on Canola Oil/Diesel Fuel Blend. Am. J. Eng. Res. 2014, 3, 202–207. [Google Scholar]
- Yasin, M.H.M.; Mamat, R.; Aziz, A.; Yusop, A.F.; Ali, M.H. Investigation on combustion parameters of palm biodiesel operating with a diesel engine. J. Mech. Eng. Sci. 2015, 9, 1714–1726. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, Z.; Wang, J.; Wang, B.; Ning, D.; Zhang, Y. Effect of Compression Ratio on Cycle-by-Cycle Variations in a Natural Gas Direct Injection Engine. Energy Fuels 2009, 23, 5357–5366. [Google Scholar] [CrossRef]
- Sayin, C.; Gumus, M.; Canakci, M. Effect of Fuel Injection Timing on the Emissions of a Direct-Injection (DI) Diesel Engine Fueled with Canola Oil Methyl Ester-Diesel Fuel Blends. Energy Fuels 2010, 24, 2675–2682. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Robbins, C. Review of the effects of biodiesel on NOx emissions. Fuel Process. Technol. 2012, 96, 237–249. [Google Scholar] [CrossRef]
- Lapuerta, M.; Armas, O.; Rodríguez-Fernández, J. Effect of biodiesel fuels on diesel engine emissions. Prog. Energy Combust. Sci. 2008, 34, 198–223. [Google Scholar] [CrossRef]





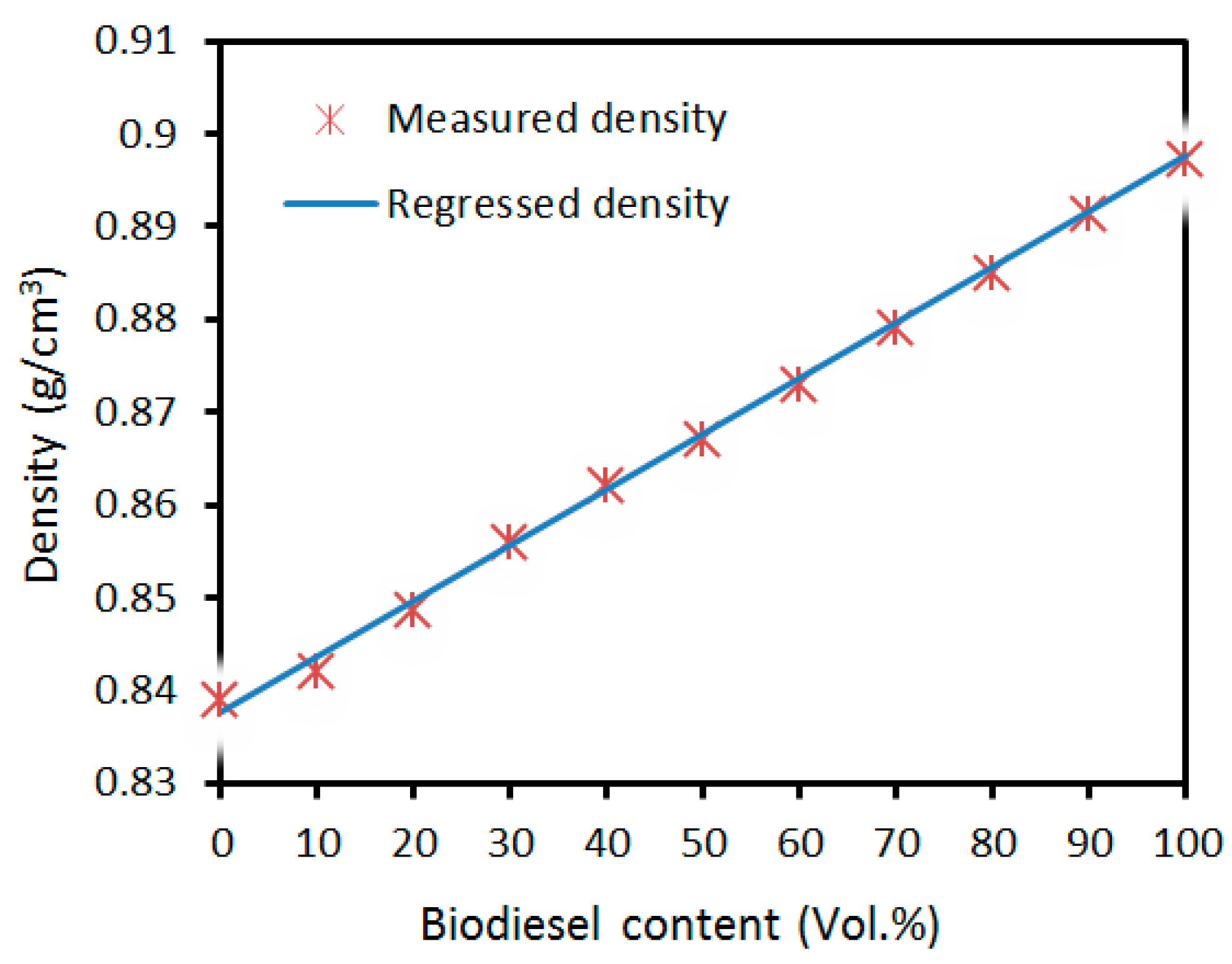











| Properties (Units) | Pure Diesel | BD 100 1 | BD 10 2 | BD 20 3 | BD 30 4 | Test Method |
|---|---|---|---|---|---|---|
| Density (kg/m3 at 15 °C) | 836.8 | 880 | 842 | 846 | 850 | ASTM D941 |
| Viscosity (mm2/s at 40 °C) | 2.719 | 4.290 | 2.818 | 2.991 | 3.172 | ASTM D445 |
| Calorific value (MJ/kg) | 43.96 | 39.49 | 43.29 | 42.71 | 42.12 | ASTM D4809 |
| Cetane index | 55.8 | 61.5 | - | - | - | ASTM D4737 |
| Flash point (°C) | 55 | 182 | - | - | - | ASTM D93 |
| Pour point (°C) | −21 | −8 | - | - | - | ASTM D97 |
| Oxidation stability (h/110 °C) | 25 | 15 | - | - | - | EN 14112 |
| Ester content (%) | - | 98.9 | - | - | - | EN 14103 |
| Oxygen (%) | 0 | 10.8 | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, J.C.; Yoon, S.K.; Choi, N.J. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Appl. Sci. 2017, 7, 881. https://doi.org/10.3390/app7090881
Ge JC, Yoon SK, Choi NJ. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Applied Sciences. 2017; 7(9):881. https://doi.org/10.3390/app7090881
Chicago/Turabian StyleGe, Jun Cong, Sam Ki Yoon, and Nag Jung Choi. 2017. "Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review" Applied Sciences 7, no. 9: 881. https://doi.org/10.3390/app7090881






