1. Introduction
To tailor the properties of nanoparticles and composite materials comprising such nanoparticles, control of the particle size is of vital importance. Nanoparticles are used in products for a wide variety of applications ranging from performance polymers to pharmaceutics, food ingredients, cosmetics and coatings and paints [
1,
2,
3,
4,
5,
6]. Very often, such well-defined nanoparticles are produced in bottom-up liquid phase syntheses using e.g., redox or sol-gel type reactions. A detailed study of the nucleation and growth of such particles would provide valuable information on their mechanism of formation, and could furthermore be used for process optimization and scale up. For such a study, real-time measurements of the particle size are required. For production scale synthesis, such real-time measurements could also provide data that enable rational intervention in a production process, hence minimizing the number of off-spec batches and waste.
Dynamic Light Scattering (DLS) is the standard technique for determining the size of nanoparticles in the range of a few nanometers to a few microns dispersed in liquids [
7,
8]. It makes use of a laser to study the time dependent scattering of small particles in liquid dispersions. Since such particles undergo Brownian motion, the distance between the scattering particles in the liquid constantly changes, which results in a fluctuating scattering intensity [
9]. Analysis of these fluctuations yields information about the average size and size distribution of the nanoparticles. Since in conventional DLS experiments no external flow is imposed on the dispersion, the fluctuating scattering intensity solely correlates to the diffusion of particles, and does not comprise translational information. It can thus be used to determine the diffusion coefficients of the particles, and from that the particle size distribution through use of the Stokes-Einstein equation. Consequently, such conventional DLS techniques do not allow for real-time measurements on flowing or moving dispersions.
DLS measurements rely on single scattering, which means that each photon should only be scattered once in the dispersion sample before it reaches the detector. Since this requires low particle concentrations, dispersion samples typically require dilution with additional solvent which is inconvenient and may even influence the state of the dispersed colloids. To overcome this, a significant amount of research was performed in the past decade focusing on suppressing multiple scattering. It showed that multiple scattering suppression in DLS measurements can e.g., be accomplished by using two lasers operating at different wavelengths, and two detectors having distinct bandpass filters to capture scattering information from each single laser. This technique is generally referred to as two-color DLS [
10]. Another more simple and robust arrangement of the beam-detector pairs is possible by displacing them symmetrically in a third dimension above and below the scattering plane, which is known as the three-dimensional (3D) cross-correlation geometry [
11]. Using this geometry, two scattering experiments are performed at the same time and on the same scattering volume using two beams and two detectors. The signals seen by the detectors are cross-correlated, and only photons that are scattered once will produce correlated intensity fluctuations on both detectors. In that way, the information on single scattered photons can be extracted, and used to determine that particle size distribution in turbid dispersions. However, an important drawback of this 3D technique is that each photon detector not only receives the desired information from its correspondingly positioned laser, but also undesired information from the second laser. Thus, cross-talk occurs between the two light scattering experiments. This drawback is overcome through temporal isolation of the two beam-detector pairs in modulated 3D cross-correlation DLS.
Real-time DLS measurements moreover are challenging since a flow is imposed on the dispersion in laboratory and pilot-scale reactors, e.g., through stirring or pumping. Accurate particle sizing using DLS usually necessitates a resting fluid in the measured sample [
12]. In actively mixed fluids, the diffusion is overlaid by a turbulent convection which prohibits diffusion measurements.
Here, we demonstrate a set-up based on modulated 3D cross-correlation DLS, which is capable of separating the scattering information resulting from diffusional and translational motion of particles, and consequently allows flow cell coupled measurements on flowing nanoparticle dispersions [
13]. In this setup, we pump a particle dispersion from the reactor through an analysis loop comprising a flow cell, and back into the reactor.
We successfully validated the set-up presented in this article through monitoring of the size of spherical silica (SiO2) nanoparticles during Stöber synthesis starting from the molecular precursor tetraethyl orthosilicate (TEOS). Conventional off-line DLS on non-moving liquid dispersions using a state of art DLS instrument, and transmission electron microscopy (TEM) are used to validate the particle size information obtained with our newly developed flow cell coupled DLS instrument.
3. Results and Discussion
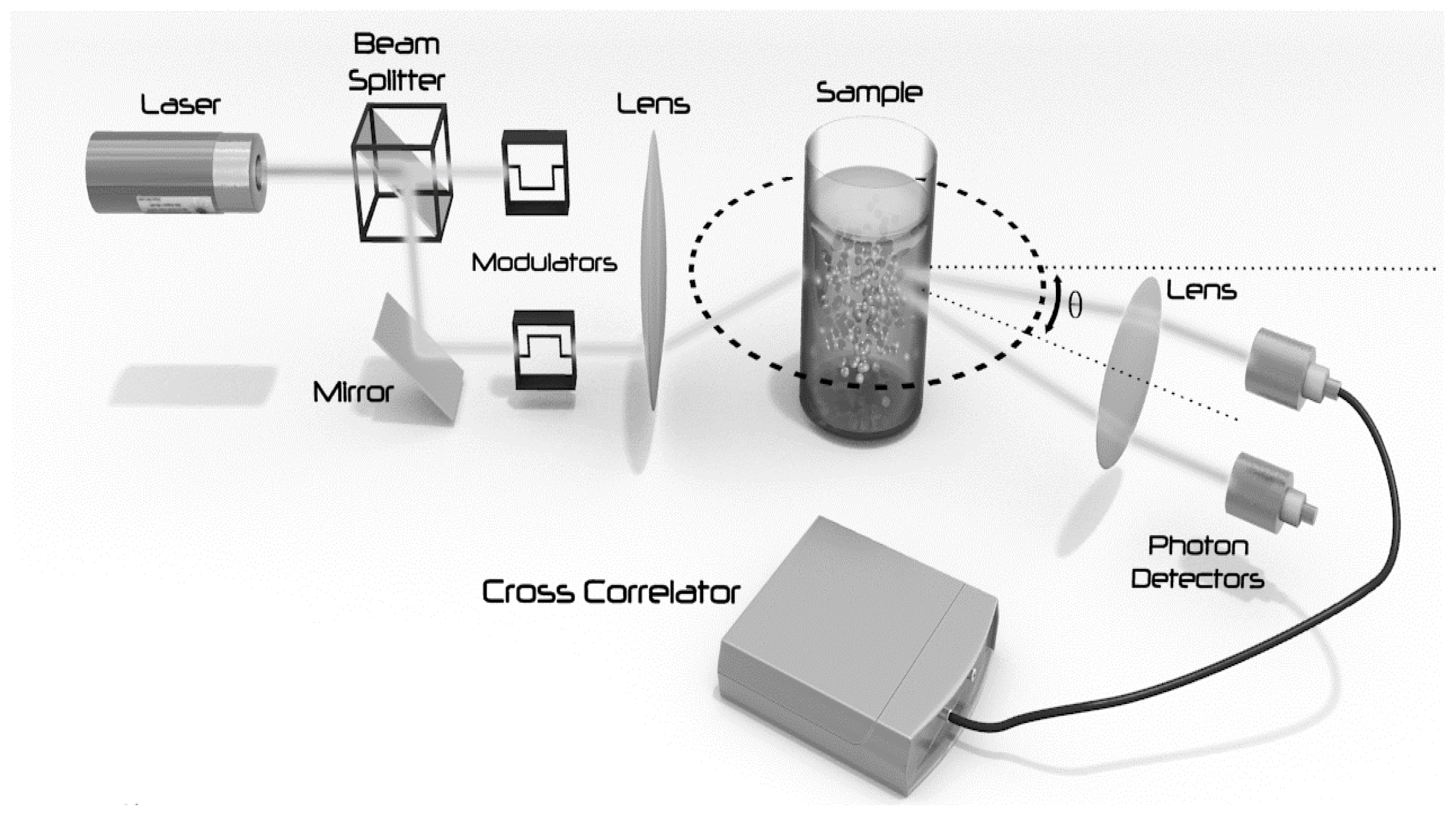
The aim of our work was to design, build and validate a set-up for flow cell coupled DLS measurements that enables real-time monitoring of the size of nanoparticles during liquid phase synthesis. For flow cell coupled real-time measurements of dispersions, we used a DLS unit equipped with the 3D cross correlation geometry (the unit is based on the commercially available NanoLab 3D, LS Instruments AG, Fribourg, Switzerland). The technology applied in this instrument is based on two simultaneous light scattering experiments performed at the same scattering vector on the same sample volume in order to extract only the single scattering information common to both (
Figure 1). It enables short measurement acquisition times (dark counts <250 s
−1).
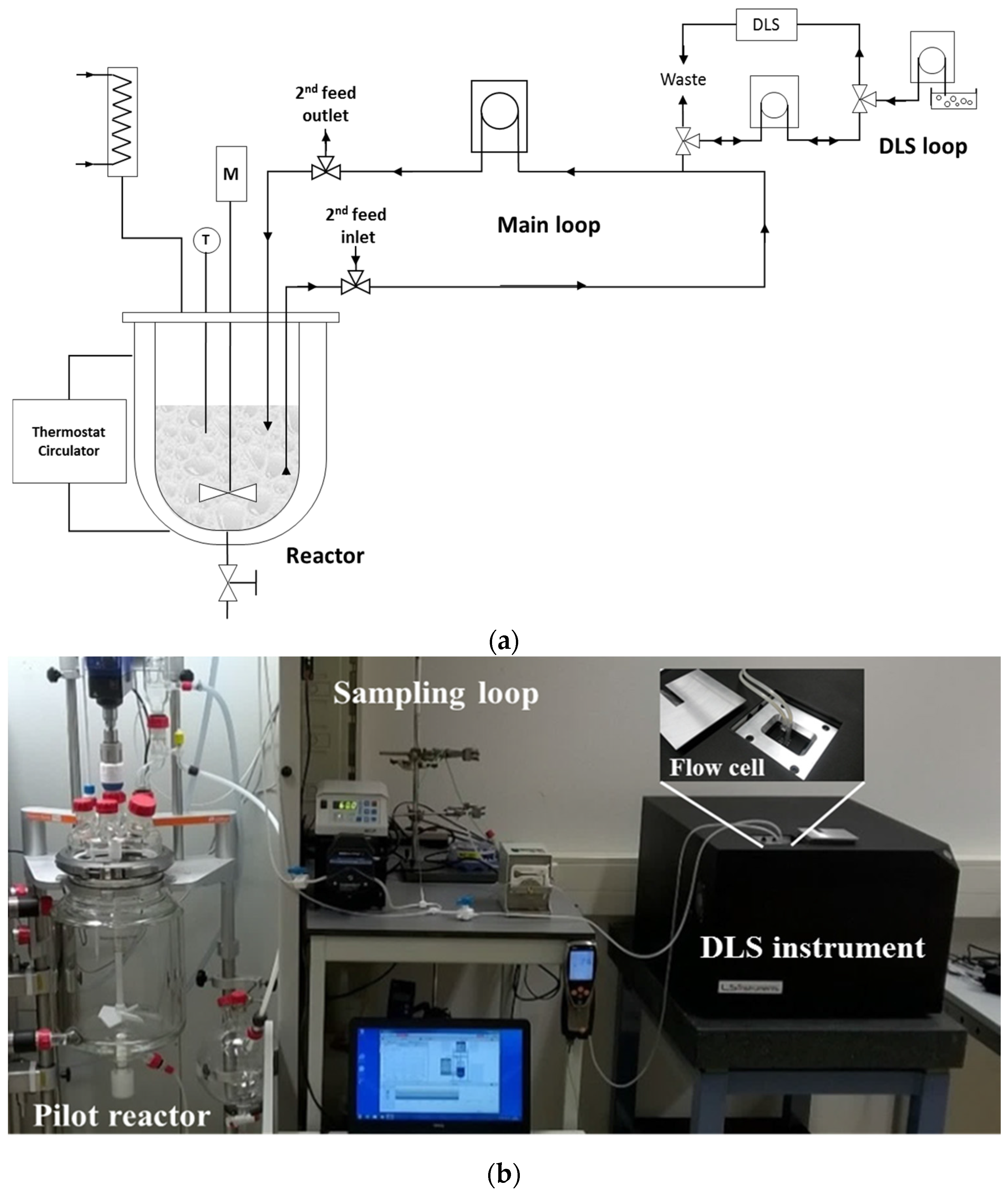
To perform measurements in stirred fluids, a custom designed analysis loop was used to couple the DLS instrument to a stirred 2 L double-walled tank reactor. The dispersion is lead to the measurement module by means of an analysis loop (
Figure 2).
In the analysis loop, the reaction mixture is circulated continuously using a Watson pump with flexible tubing. The pump is placed after the cuvette to circumvent contamination of the sample with agglomerated particles due to scaling of the pump. After measurement, the DLS loop is rinsed by demineralized water to clean it before the next sample enters the measuring zone.
To validate the set-up, we started monitoring the growth of silica nanoparticles in a Stöber synthesis starting from the molecular precursor TEOS in a mixture of water, ethanol and ammonia. This is a prototypical liquid phase synthesis of nanoparticles. In this sol-gel synthesis, TEOS partially hydrolyses, and through subsequent polycondensation silica nanoparticles are formed as shown in the reaction Equations (1)–(3) [
16].
This process proceeds according to an aggregation-based kinetic model [
17,
18]. The size of the silica nanoparticles resulting from this synthesis depends amongst others on the concentration of ammonia and TEOS. The average particle size increases with increasing concentration of ammonia. The initial concentration of TEOS is inversely proportional to the size of the resulting silica nanoparticles.
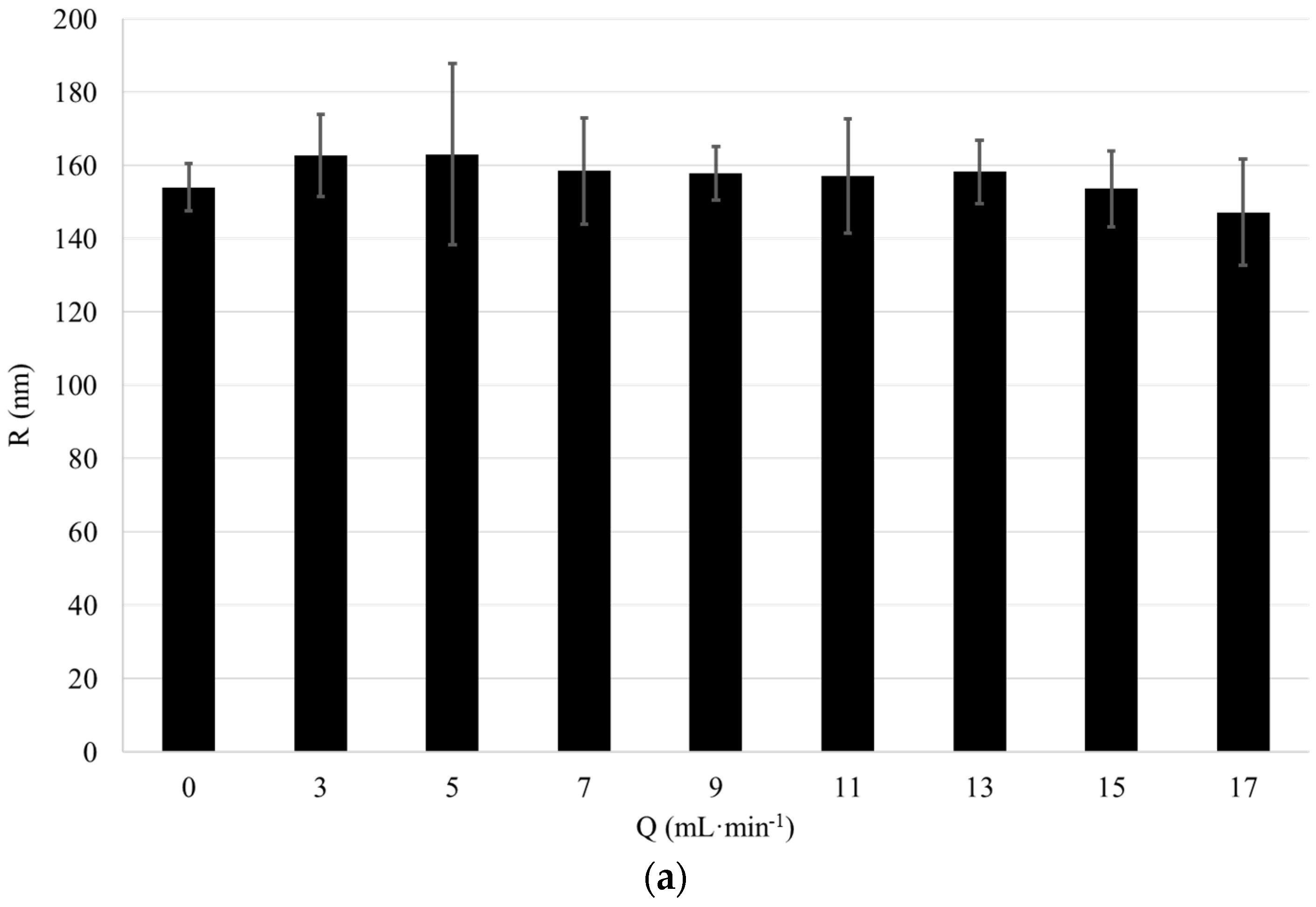
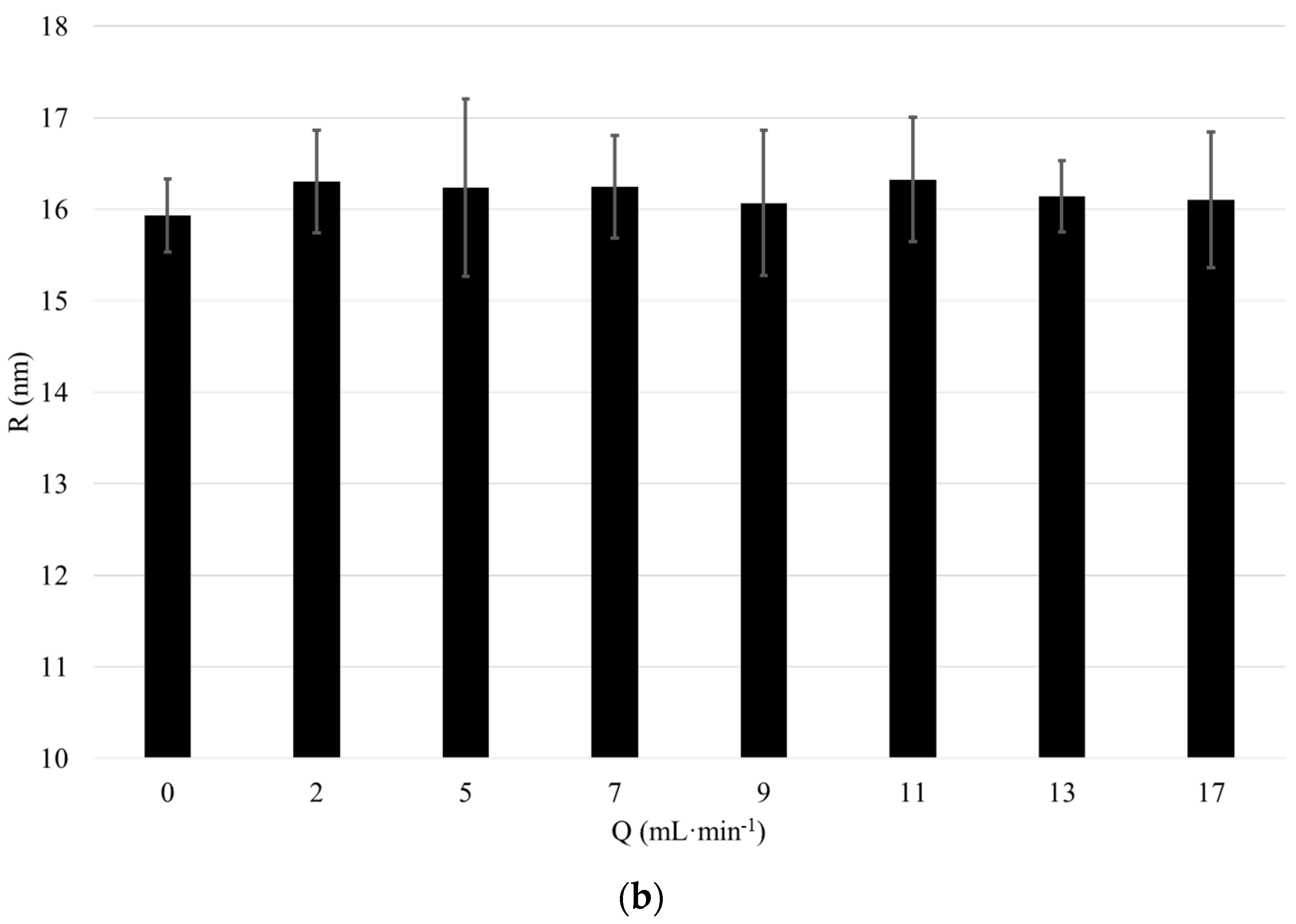
We started validating the effect of flow rate on the particle size measured in the flow-cell coupled DLS set-up with 3D cross correlation geometry. Therefore, a 1 wt-% dispersion of “large” and “small” silica particles of a radius of 157 nm and 17.6 nm, respectively, were circulated in the analysis loop comprising the DLS at flow rates
Q between 0 and 17 mL·min
−1. The particle radii were determined using conventional DLS. The displayed particle size is measured with the 3D cross correlation DLS whilst the dispersion was pumped through the flow cell at a defined flow rate
Q between 0 and 17 mL·min
−1. For each flow rate, the presented value for the particle size represents an average of ten DLS measurements (
Figure 3, error bars represent standard deviation).
The average particle radius measured using conventional DLS is 157 nm for the large particles and 17.6 nm for the small particles. The average particle size measured using flow cell coupled DLS with 3D modulation is 157 nm for the large particles and 16.2 nm for the small particles. Thus, the measurement results obtained with conventional DLS and DLS with 3D cross correlation geometry performed at Q = 0 mL·min−1 are in excellent agreement. Furthermore, both for the large and small particles the particle size measured using DLS with 3D cross correlation geometry remains nearly constant for flow rates up to 17 mL·min−1. Thus, through the use of a modulated 3D cross correlation DLS instrument, we were able to accurately analyze dispersions comprising differently sized silica nanoparticles at flow rates up to 17 mL·min−1.
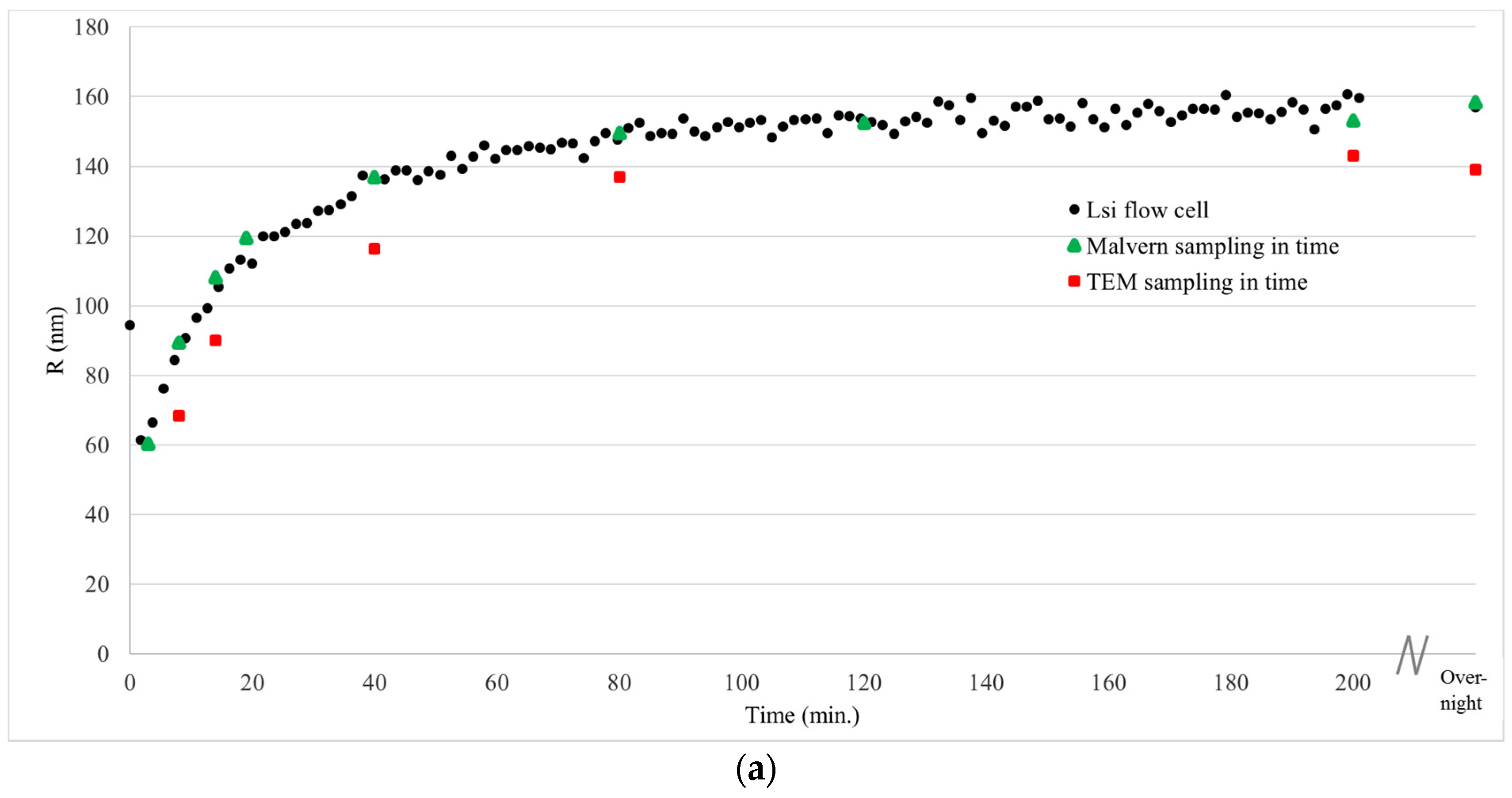
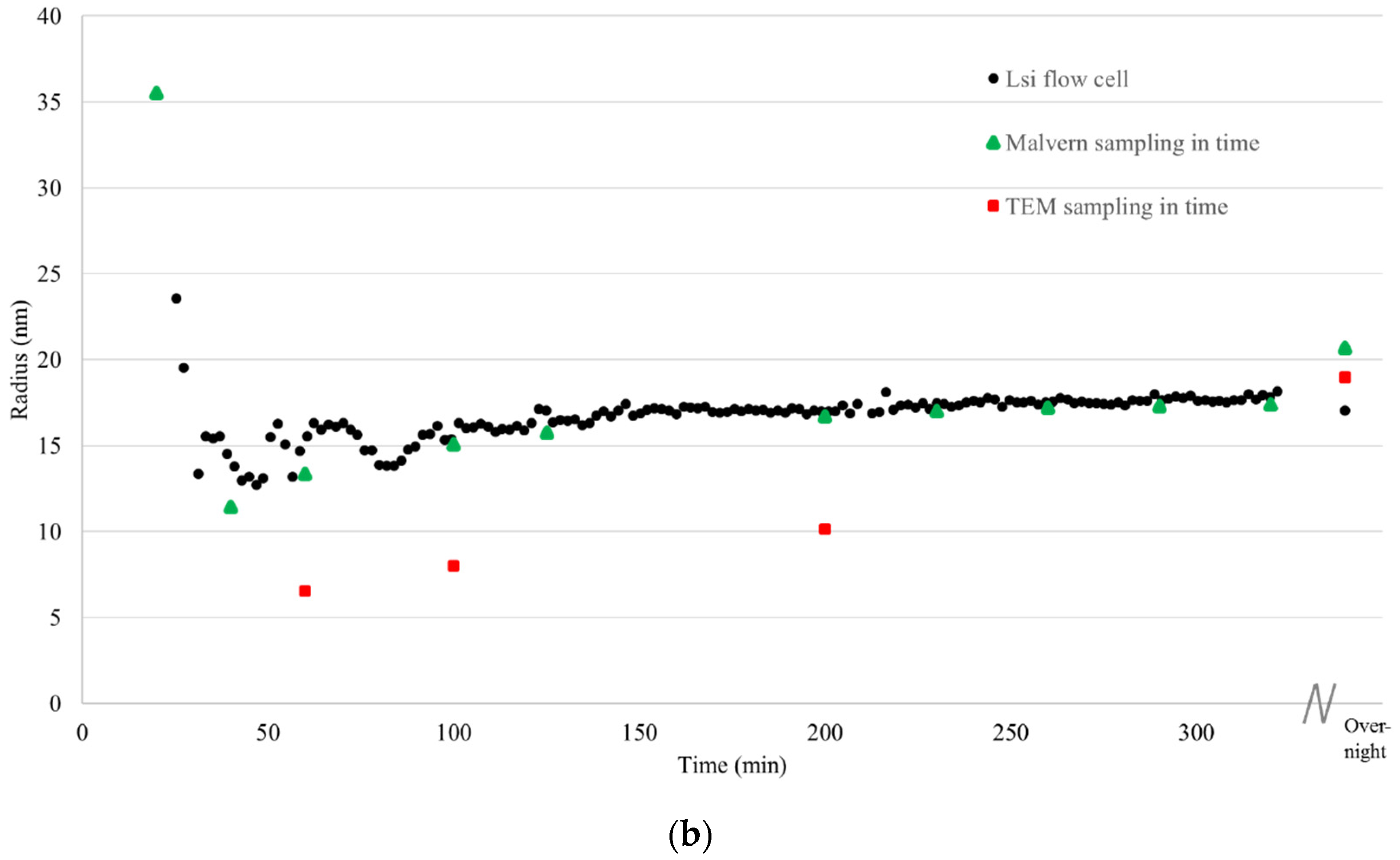
During the synthesis of the large and small silica nanoparticles, starting from the molecular precursor TEOS, the particle growth was monitored in real time using the analysis loop connected to the 3D cross correlation DLS unit (
Figure 4a,b).
For comparison, also off-line DLS measurements on non-moving dispersions were performed using a state-of-art DLS instrument (
Figure 4, green triangles). For that purpose, samples were taken during the reaction. Furthermore, samples were taken for TEM analysis, and immediately dried on a TEM grid after which the samples were analyzed (
Figure 4, red squares). The deviation in particle size obtained using DLS between the off-line measured samples and the samples measured in flow is maximum 7%. This small deviation in particle size measured with conventional DLS on non-moving dispersions and in flow with the 3D cross correlation geometry DLS during synthesis of silica nanoparticles demonstrates that particle growth can be accurately monitored in real-time using our newly developed flow cell coupled DLS setup connected to the reactor using the analysis loop.
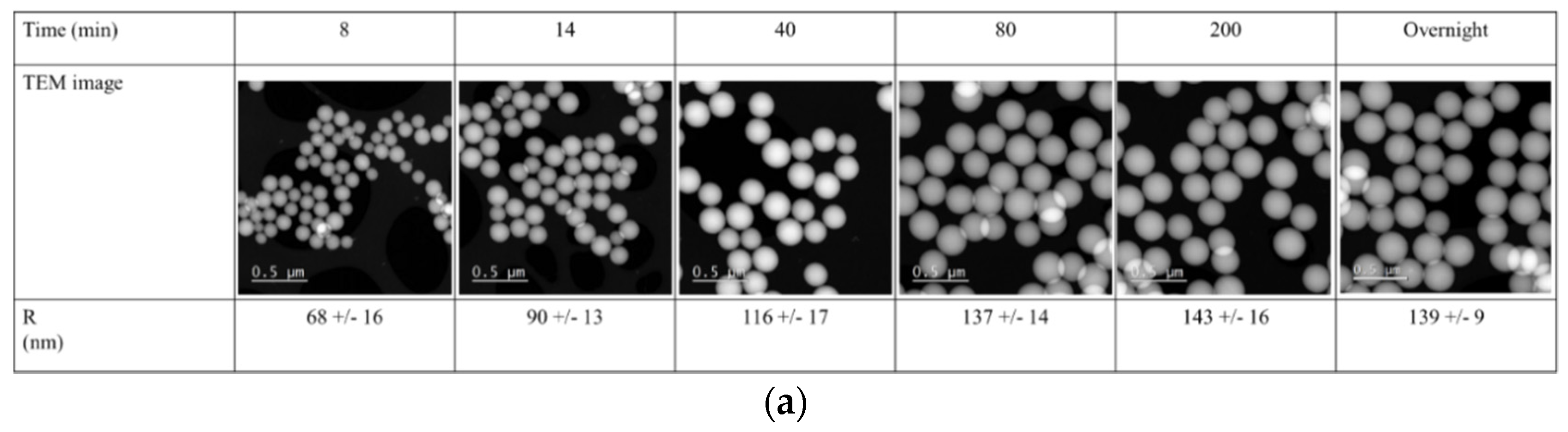
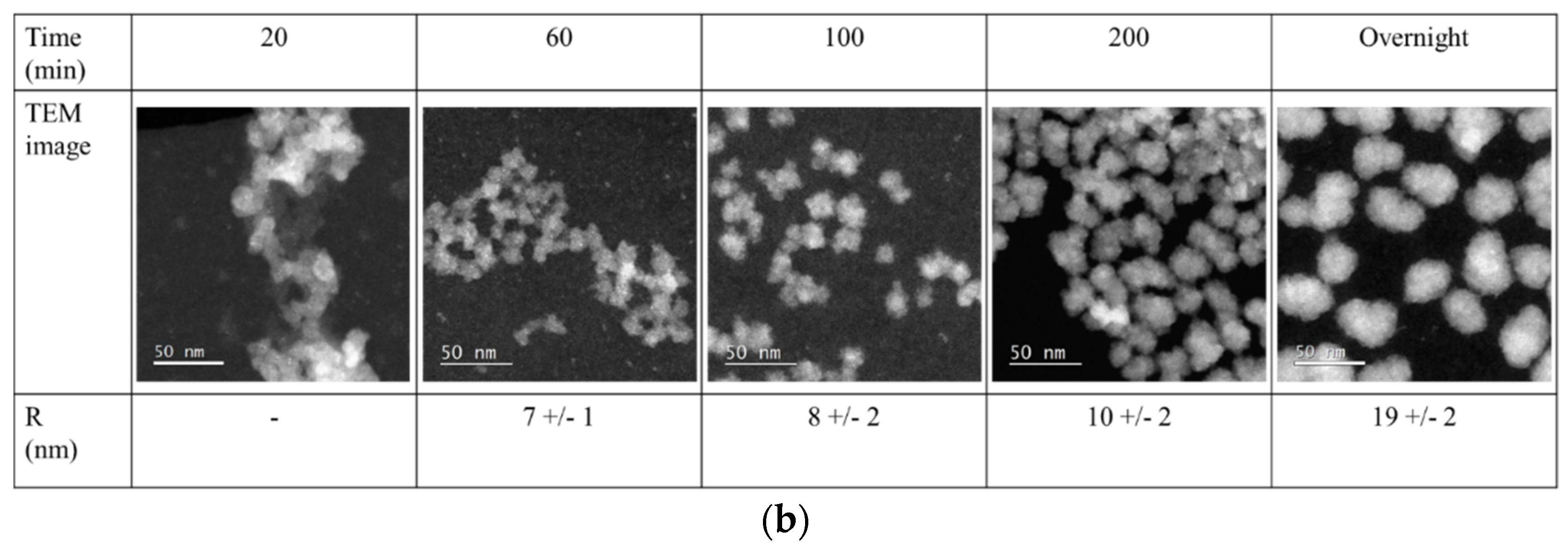
The solvodynamic radius of the particles during synthesis, obtained through DLS, was compared to particle size obtained using transmission electron microcopy (
Figure 4, red squares,
Figure 5).
The average particle size obtained through TEM analyses was determined through measurement of at least 25 particles per samples. The particle sizes obtained using TEM analyses were smaller than those obtained using DLS. This can be explained by the fact that through DLS the solvodynamic radius of the particle is measured, and through TEM the radius of the dry particles [
19]. For the large silica particles, the radii determined through TEM were approximately 10% smaller than those obtained through DLS. For the small silica particles, this difference was significantly larger, due the strong deviation of these particles from ideal spheres (
Figure 5b). In contrast to the DLS techniques presented in this article, however, TEM not only provides information on the particle size but also on its shape and architecture. In addition to size, these are also important characteristics that influence the particle’s property.
4. Conclusions
We have successfully developed a flow cell coupled DLS set-up that allows to monitor the size of nanoparticles in real-time during bottom-up liquid phase synthesis. Through the use of a modulated 3D cross correlation DLS instrument, we were able to accurately analyze dispersions comprising silica nanoparticles in water/alcohol mixtures. Through Stöber synthesis, we prepared small and large silica nanoparticles with a radius of approx. 20 nm and 150 nm, respectively. Both dispersions could be accurately analyzed using our novel flow cell coupled 3D cross correlation DLS set-up at silica concentrations of approx. 2.25 wt-% and flow rates up to 17 mL·min−1. Furthermore, we demonstrated that the particle size could be accurately monitored in real-time during Stöber synthesis of the silica spheres starting from TEOS as molecular precursor. This was confirmed by conventional off-line DLS and TEM analyses. Thus, the newly developed flow cell coupled DLS set-up enables the study of nucleation and growth of nanoparticles in bottom-up liquid phase synthesis, which provides rational input for process optimization. Furthermore, when coupled to a production sized reactor, it can help to minimize the number of off spec batches since it provides real-time information that enables rational process intervention. We expect to validate the set-up for sol-gel syntheses of nanoparticles other than silica (e.g., titania, zirconia) and redox synthesis for the production of metallic nanoparticles starting from (complex) metal salts.