Na-Doping Effects on Thermoelectric Properties of Cu2−xSe Nanoplates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cu2−xSe Nanoplate and Nanowire
2.3. Synthesis of Na-Doped Cu2−xSe Nanoplate
2.4. Characterization of Materials
2.5. Characterization of the Thermoelectric Properties
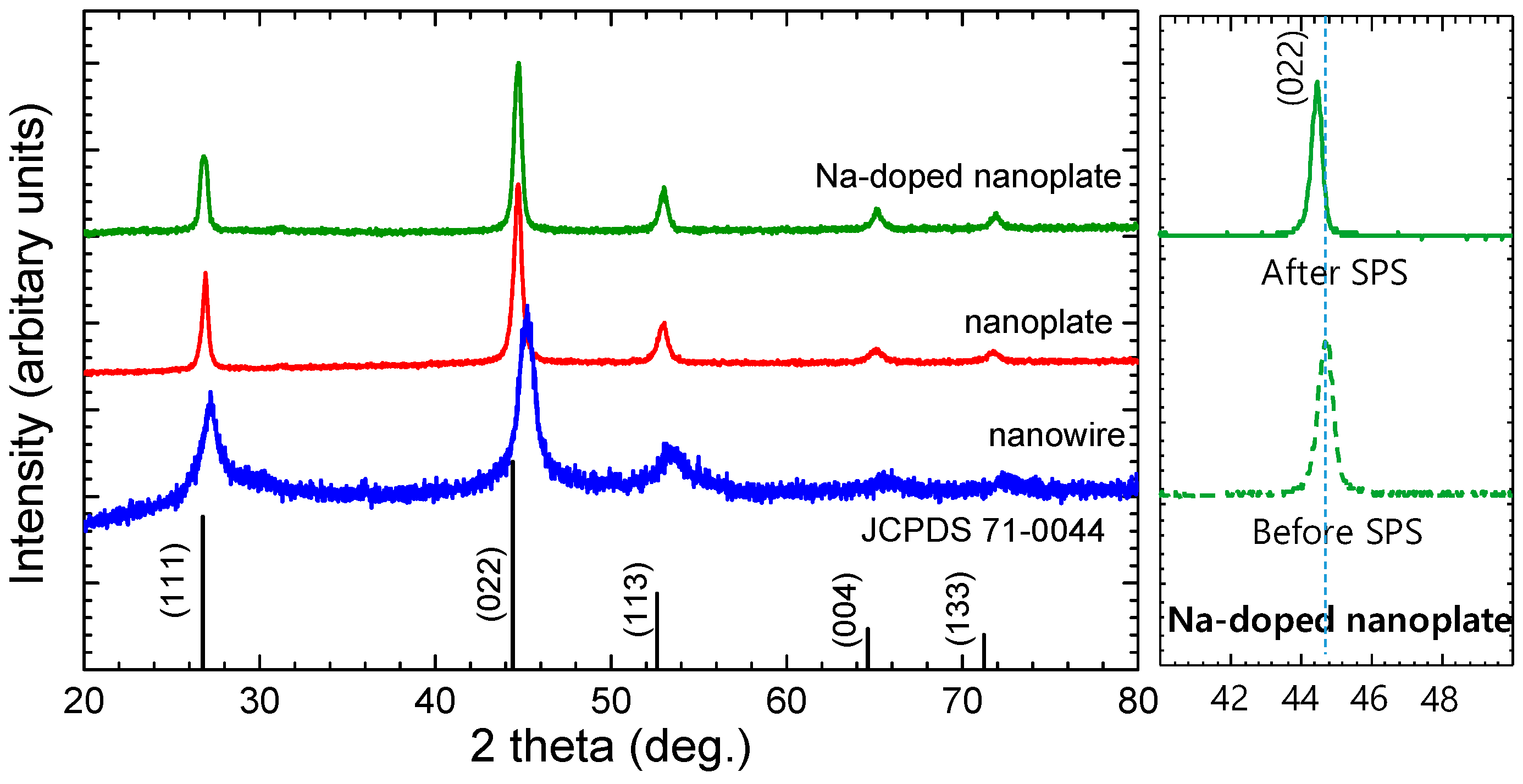
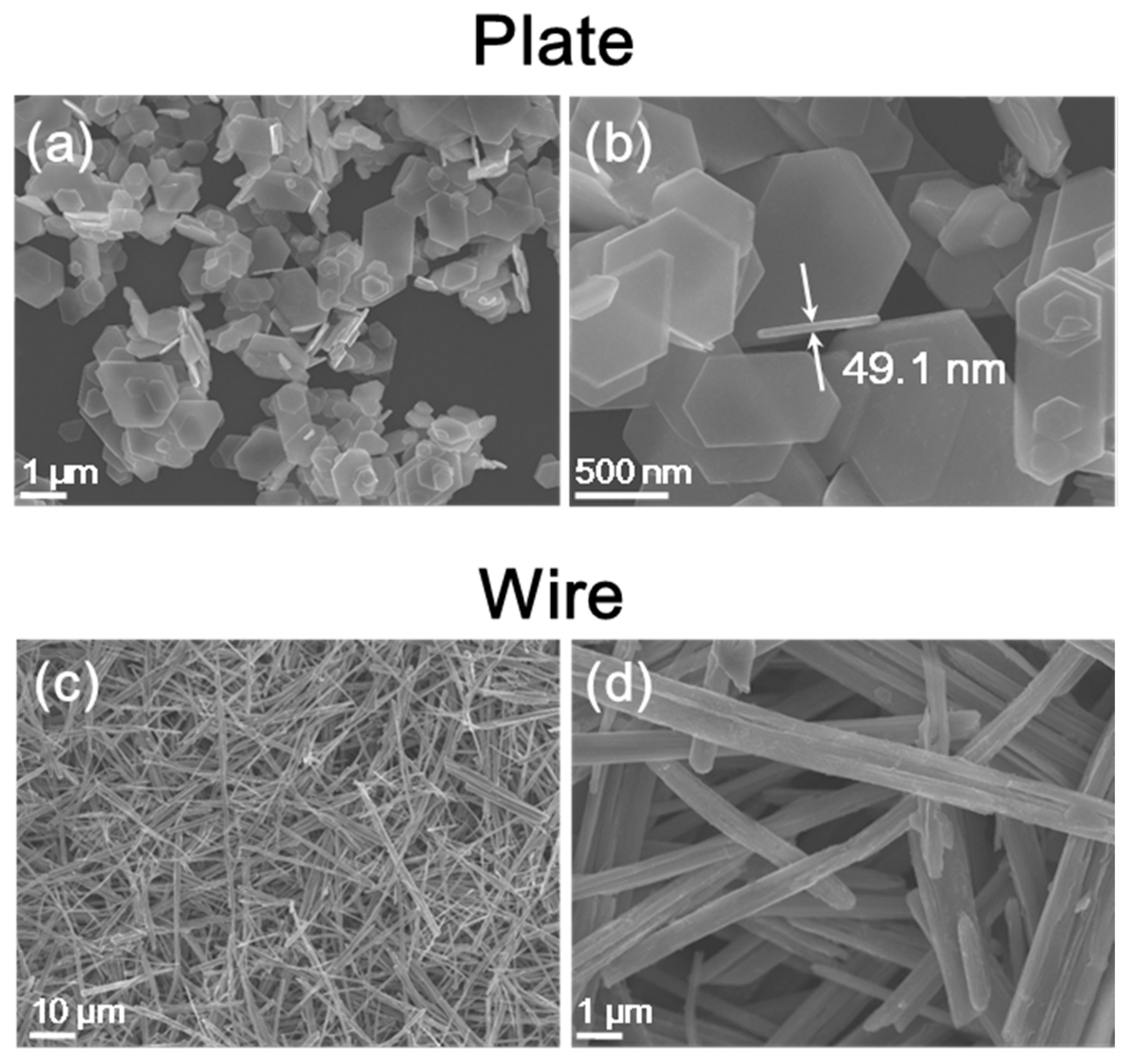
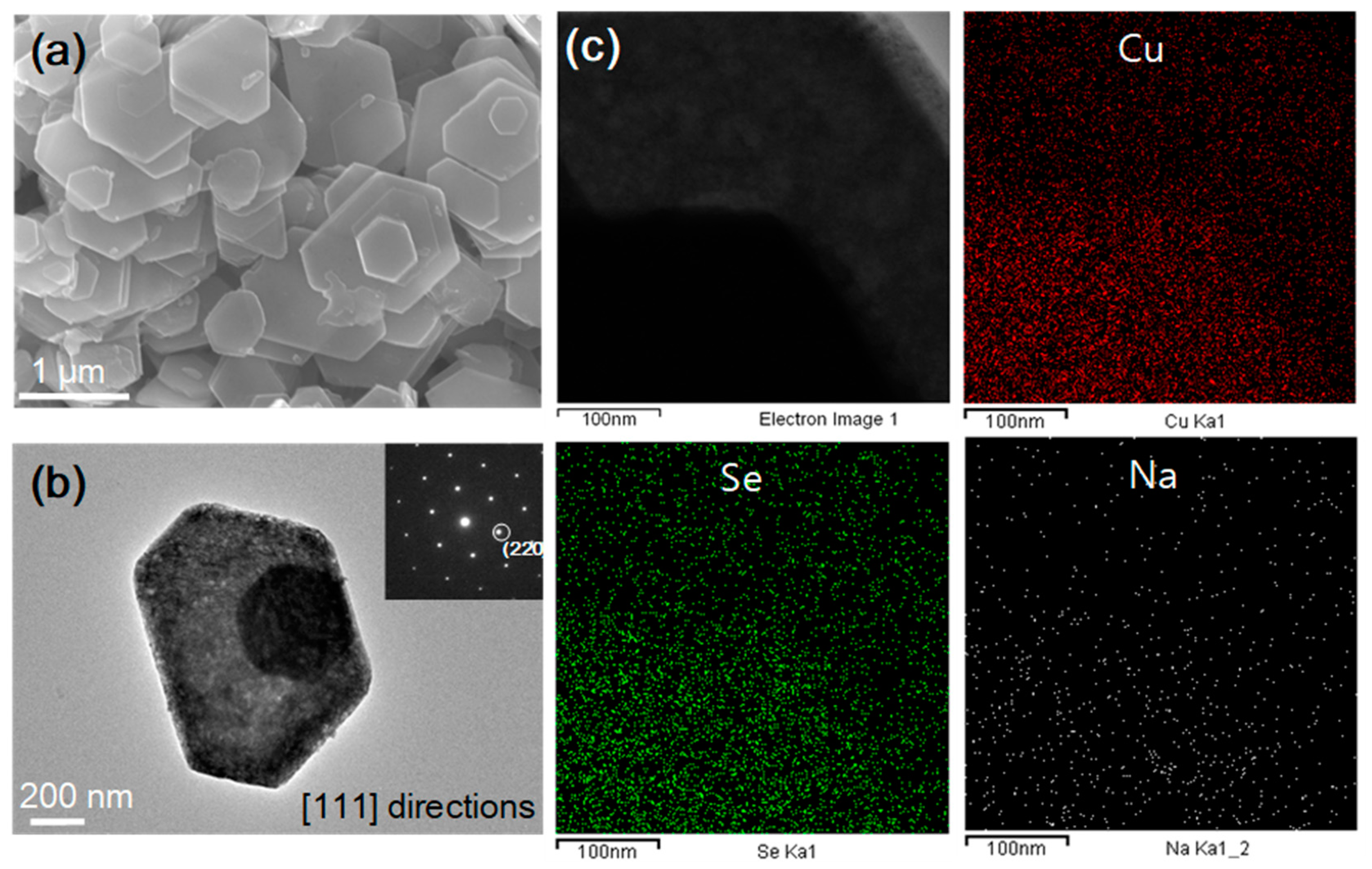
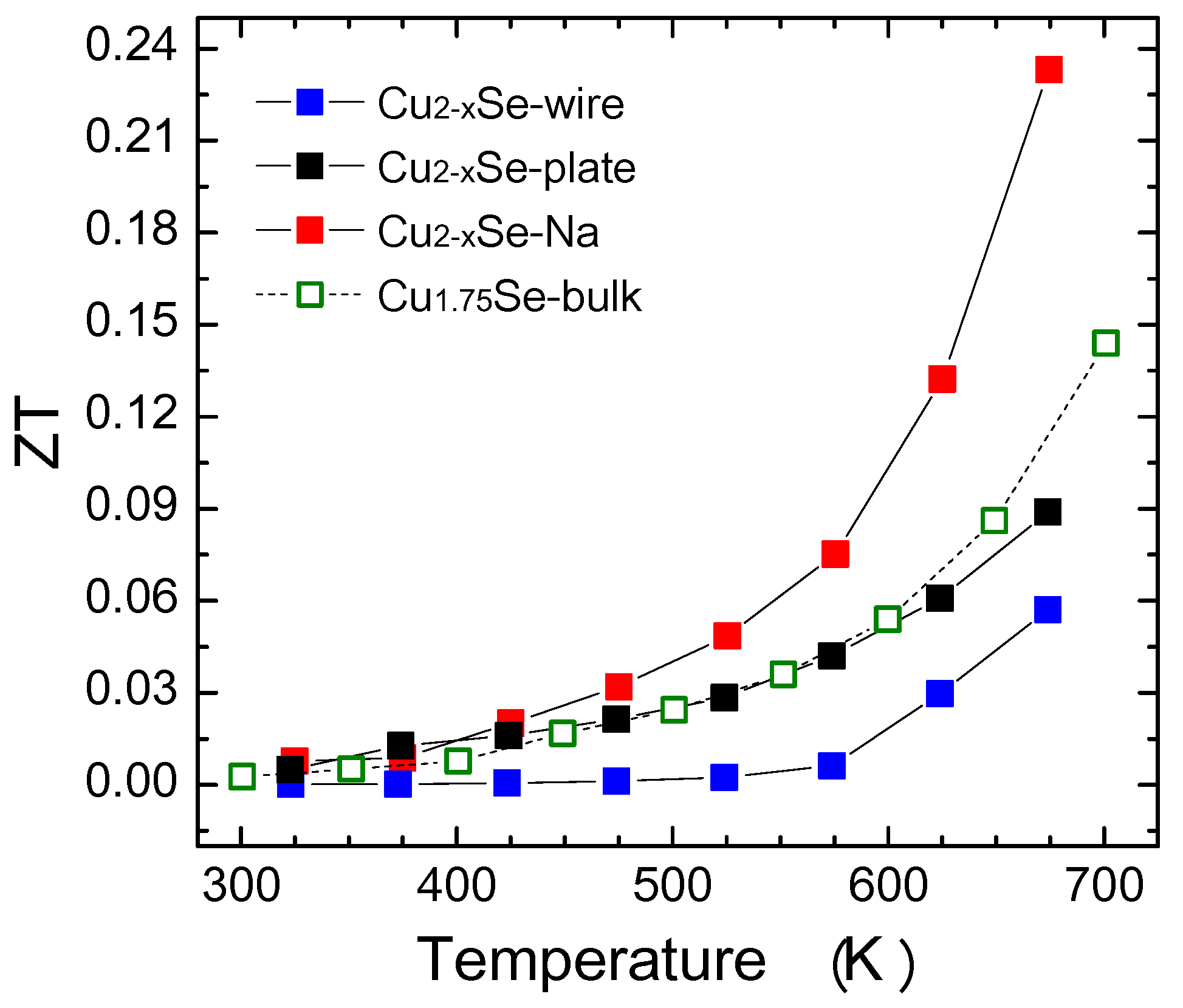
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.M. CRC Handbook of Thermoelectrics; CRC Press: New York, NY, USA, 1995. [Google Scholar]
- Yang, L.; Chen, Z.-G.; Dargusch, M.S.; Zou, J. High performance thermoelectric materials: Progress and their applications. Adv. Energy Mater. 2017, 1701797. [Google Scholar] [CrossRef]
- Bell, L.E. Cooling, heating, generating power, and recovering waste heat with thermoelectric systems. Science 2008, 321, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Moshwan, R.; Yang, L.; Zou, J.; Chen, Z.-G. Eco-friendly SnTe thermoelectric materials: Progress and future challenges. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Zhang, Q.; Liao, B.L.; Lan, Y.C.; Lukas, K.; Liu, W.S.; Esfarjani, K.; Opeil, C.; Broido, D.; Chen, G.; Ren, Z.F. High thermoelectric performance by resonant dopant indium in nanostructured SnTe. Proc. Natl. Acad. Sci. USA 2013, 110, 13261–13266. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tan, X.J.; Yin, K.; Liu, H.J.; Tang, X.F.; Shi, J.; Zhang, Q.J.; Uher, C. Convergence of Conduction Bands as a Means of Enhancing Thermoelectric Performance of n-Type Mg2Si1−xSnx Solid Solutions. Phys. Rev. Lett. 2012, 108. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gibbs, Z.M.; Takagiwa, Y.; Snyder, G.J. Tuning bands of PbSe for better thermoelectric efficiency. Energy Environ. Sci. 2014, 7, 804–811. [Google Scholar] [CrossRef]
- Girard, S.N.; He, J.Q.; Zhou, X.Y.; Shoemaker, D.; Jaworski, C.M.; Uher, C.; Dravid, V.P.; Heremans, J.P.; Kanatzidis, M.G. High Performance Na-doped PbTe-PbS Thermoelectric Materials: Electronic Density of States Modification and Shape-Controlled Nanostructures. J. Am. Chem. Soc. 2011, 133, 16588–16597. [Google Scholar] [CrossRef] [PubMed]
- Rhyee, J.S.; Lee, K.H.; Lee, S.M.; Cho, E.; Kim, S.I.; Lee, E.; Kwon, Y.S.; Shim, J.H.; Kotliar, G. Peierls distortion as a route to high thermoelectric performance in In4Se3-delta crystals. Nature 2009, 459, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-G.; Han, G.; Yang, L.; Cheng, L.; Zou, J. Nanostructured thermoelectric materials: Current research and future challenge. Prog. Nat. Sci. 2012, 22, 535–549. [Google Scholar] [CrossRef]
- He, Y.; Day, T.; Zhang, T.S.; Liu, H.L.; Shi, X.; Chen, L.D.; Snyder, G.J. High thermoelectric performance in non-toxic earth-abundant copper sulfide. Adv. Mater. 2014, 26, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.L.; Wang, X.L.; Fei, F.Y.; Wang, J.Y.; Cheng, Z.X.; Dou, S.X.; Wang, J.; Snyder, G.J. High thermoelectric and mechanical performance in highly dense Cu2−xS bulks prepared by a melt-solidification technique. J. Mater. Chem. A 2015, 3, 9432–9437. [Google Scholar] [CrossRef]
- Zhao, K.P.; Blichfeld, A.B.; Chen, H.Y.; Song, Q.F.; Zhang, T.S.; Zhu, C.X.; Ren, D.D.; Hanus, R.; Qiu, P.F.; Iversen, B.B.; et al. Enhanced thermoelectric performance through tuning bonding energy in Cu2Se1−xSx liquid-like materials. Chem. Mater. 2017, 29, 6367–6377. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.G.; Han, G.; Hong, M.; Zou, Y.C.; Zou, J. High-performance thermoelectric Cu2Se nanoplates through nanostructure engineering. Nano Energy 2015, 16, 367–374. [Google Scholar] [CrossRef]
- Gahtori, B.; Bathula, S.; Tyagi, K.; Jayasimhadri, M.; Srivastava, A.K.; Singh, S.; Budhani, R.C.; Dhar, A. Giant enhancement in thermoelectric performance of copper selenide by incorporation of different nanoscale dimensional defect features. Nano Energy 2015, 13, 36–46. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.-G.; Han, G.; Hong, M.; Zou, J. Impacts of Cu deficiency on the thermoelectric properties of Cu2−xSe nanoplates. Acta Mater. 2016, 113, 140–146. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.S.; Shi, X.; Wei, S.H.; Chen, L.D. High thermoelectric performance in copper telluride. NPG Asia Mater. 2015, 7, e210. [Google Scholar] [CrossRef]
- Mallick, M.M.; Vitta, S. Realizing high figure-of-merit in Cu2Te using a combination of doping, hierarchical structure, and simple processing. J. Appl. Phys. 2017, 122. [Google Scholar] [CrossRef]
- Kurosaki, K.; Goto, K.; Kosuga, A.; Muta, H.; Yamanaka, S. Thermoelectric and thermophysical characteristics of Cu2Te-Tl2Te pseudo binary system. Mater. Trans. 2006, 47, 1432–1435. [Google Scholar] [CrossRef]
- Ballikaya, S.; Chi, H.; Salvador, J.R.; Uher, C. Thermoelectric properties of Ag-doped Cu2Se and Cu2Te. J. Mater. Chem. A 2013, 1, 12478–12484. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wang, X.L.; Yun, F.F.; Wang, J.Y.; Cheng, Z.X.; Dou, S.X.; Wang, J.; Snyder, G.J. The effects of Te2− and I− substitutions on the electronic structures, thermoelectric performance, and hardness in melt-quenched highly dense Cu2−xSe. Adv. Electron. Mater. 2015, 1. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.-G.; Han, G.; Hong, M.; Huang, L.; Zou, J. Te-doped Cu2Se nanoplates with a high average thermoelectric figure of merit. J. Mater. Chem. A 2016, 4, 9213–9219. [Google Scholar] [CrossRef]
- Liu, H.L.; Yuan, X.; Lu, P.; Shi, X.; Xu, F.F.; He, Y.; Tang, Y.S.; Bai, S.Q.; Zhang, W.Q.; Chen, L.D.; et al. Ultrahigh thermoelectric performance by electron and phonon critical scattering in Cu2Se1−xIx. Adv. Mater. 2013, 25, 6607–6612. [Google Scholar] [CrossRef] [PubMed]
- Balapanov, M.K.; Ishembetov, R.K.; Kuterbekov, K.A.; Kubenova, M.M.; Almukhametov, R.F.; Yakshibaev, R.A. Transport phenomena in superionic NaxCu2−xS (x = 0.05; 0.1; 0.15; 0.2) compounds. Ionics 2017. [Google Scholar] [CrossRef]
- Olvera, A.A.; Moroz, N.A.; Sahoo, P.; Ren, P.; Bailey, T.P.; Page, A.A.; Uher, C.; Poudeu, P.F.P. Partial indium solubility induces chemical stability and colossal thermoelectric figure of merit in Cu2Se. Energy Environ. Sci. 2017, 10, 1668–1676. [Google Scholar] [CrossRef]
- Bailey, T.P.; Hui, S.; Xie, H.Y.; Olvera, A.; Poudeu, P.F.P.; Tang, X.F.; Uher, C. Enhanced ZT and attempts to chemically stabilize Cu2Se via Sn doping. J. Mater. Chem. A 2016, 4, 17225–17235. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.X.; Yang, Z.H.; Ding, S.X.; Zeng, C.Y.; Chen, L.L.; Wang, Q.; Yang, S.H. Large-scale synthesis of long crystalline Cu2−xSe nanowire bundles by water-evaporation-induced self-assembly and their application in gas sensing. Adv. Funct. Mater. 2009, 19, 1759–1766. [Google Scholar] [CrossRef]
- Ge, Z.H.; Liu, X.Y.; Feng, D.; Lin, J.Y.; He, J.Q. High-performance thermoelectricity in nanostructured earth-abundant copper sulfides bulk materials. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Jiang, Y.; Wu, Y.; Xie, B.; Zhang, S.Y.; Qian, Y.T. Room temperature preparation of novel Cu2−xSe nanotubes in organic solvent. Nanotechnology 2004, 15, 283–286. [Google Scholar] [CrossRef]
- Poulston, S.; Parlett, P.M.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Teeter, G. X-ray and ultraviolet photoelectron spectroscopy measurements of Cu-doped CdTe(111)-B: Observation of temperature-reversible CuxTe precipitation and effect on ionization potential. J. Appl. Phys. 2007, 102. [Google Scholar] [CrossRef]
- Schellenberger, A.; Schlaf, R.; Pettenkofer, C.; Jaegermann, W. XPS and SXPS studies on in-situ prepared Na/InSe insertion compounds. Solid State Ion. 1993, 66, 307–312. [Google Scholar] [CrossRef]
- Yu, J.L.; Zhao, K.P.; Qiu, P.F.; Shi, X.; Chen, L.D. Thermoelectric properties of copper-deficient Cu2−xSe (0.05 ≤ x ≤ 0.25) binary compounds. Ceram. Int. 2017, 43, 11142–11148. [Google Scholar] [CrossRef]
- Xiao, X.; Xie, W.; Tang, X.; Zhang, Q. Phase transition and high temperature thermoelectric properties of copper selenide Cu2−xSe (0 ≤ x ≤ 0.25). Chin. Phys. B 2011, 20, 087201. [Google Scholar] [CrossRef]


| Sample | Na | Cu | Se | Composition |
|---|---|---|---|---|
| Cu2−xSe-wire | - | 0.613 | 0.397 | Cu1.59Se |
| Cu2−xSe-plate | - | 0.637 | 0.363 | Cu1.75Se |
| Na-Cu2−xSe-plate | 0.051 | 0.636 | 0.313 | Na0.162Cu2.03Se |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Han, M.-K.; Kim, S.-J. Na-Doping Effects on Thermoelectric Properties of Cu2−xSe Nanoplates. Appl. Sci. 2018, 8, 12. https://doi.org/10.3390/app8010012
Jin Y, Han M-K, Kim S-J. Na-Doping Effects on Thermoelectric Properties of Cu2−xSe Nanoplates. Applied Sciences. 2018; 8(1):12. https://doi.org/10.3390/app8010012
Chicago/Turabian StyleJin, Yingshi, Mi-Kyung Han, and Sung-Jin Kim. 2018. "Na-Doping Effects on Thermoelectric Properties of Cu2−xSe Nanoplates" Applied Sciences 8, no. 1: 12. https://doi.org/10.3390/app8010012




