The Progress in Photoacoustic and Laser Ultrasonic Tomographic Imaging for Biomedicine and Industry: A Review
Abstract
:1. Introduction
2. The Methods of PAI and LUI
2.1. Applications of PAI and LUI—Image Formation Principles
2.2. Forward and Inverse Problems of PAT and LUT—Reconstruction Algorithms
2.2.1. Algorithms for PAT
2.2.2. Algorithms for LUT
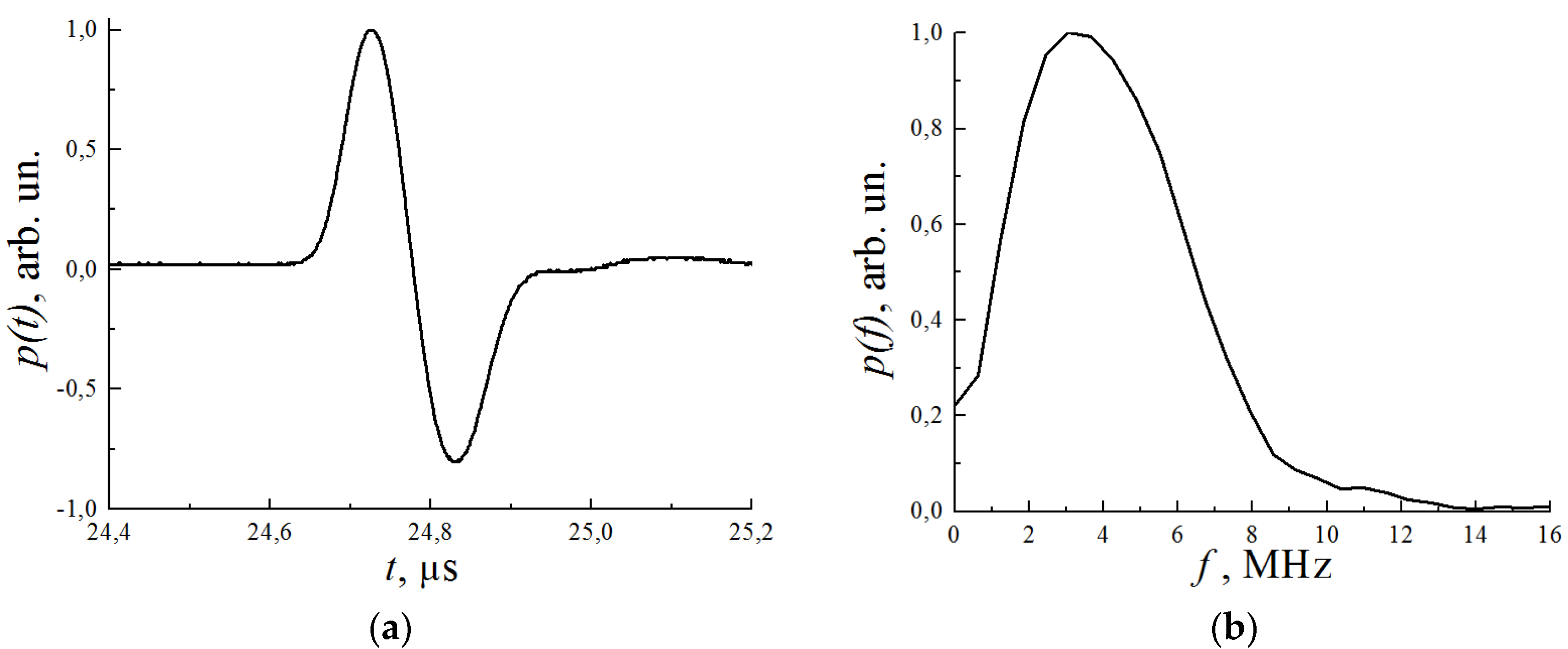
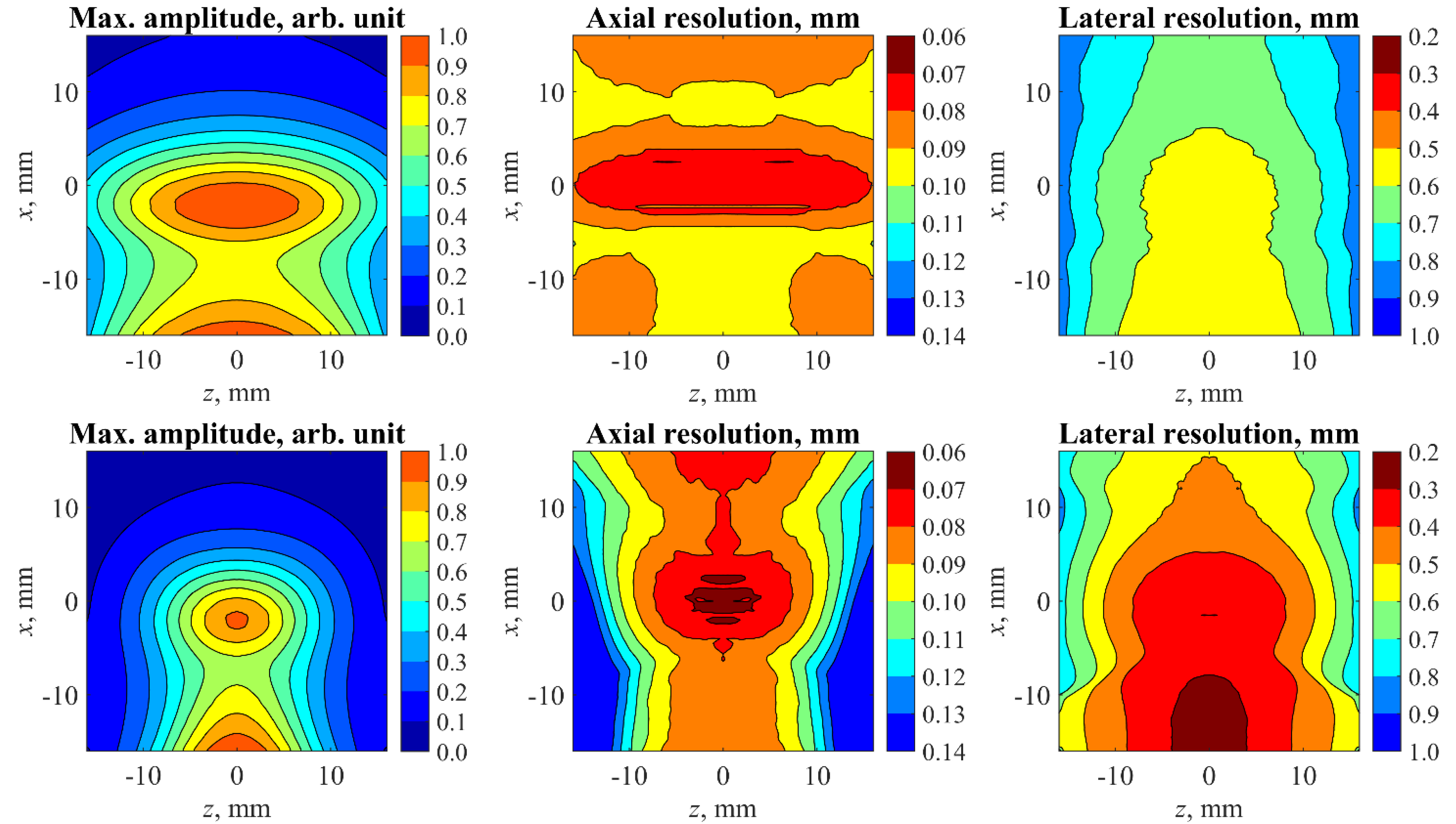
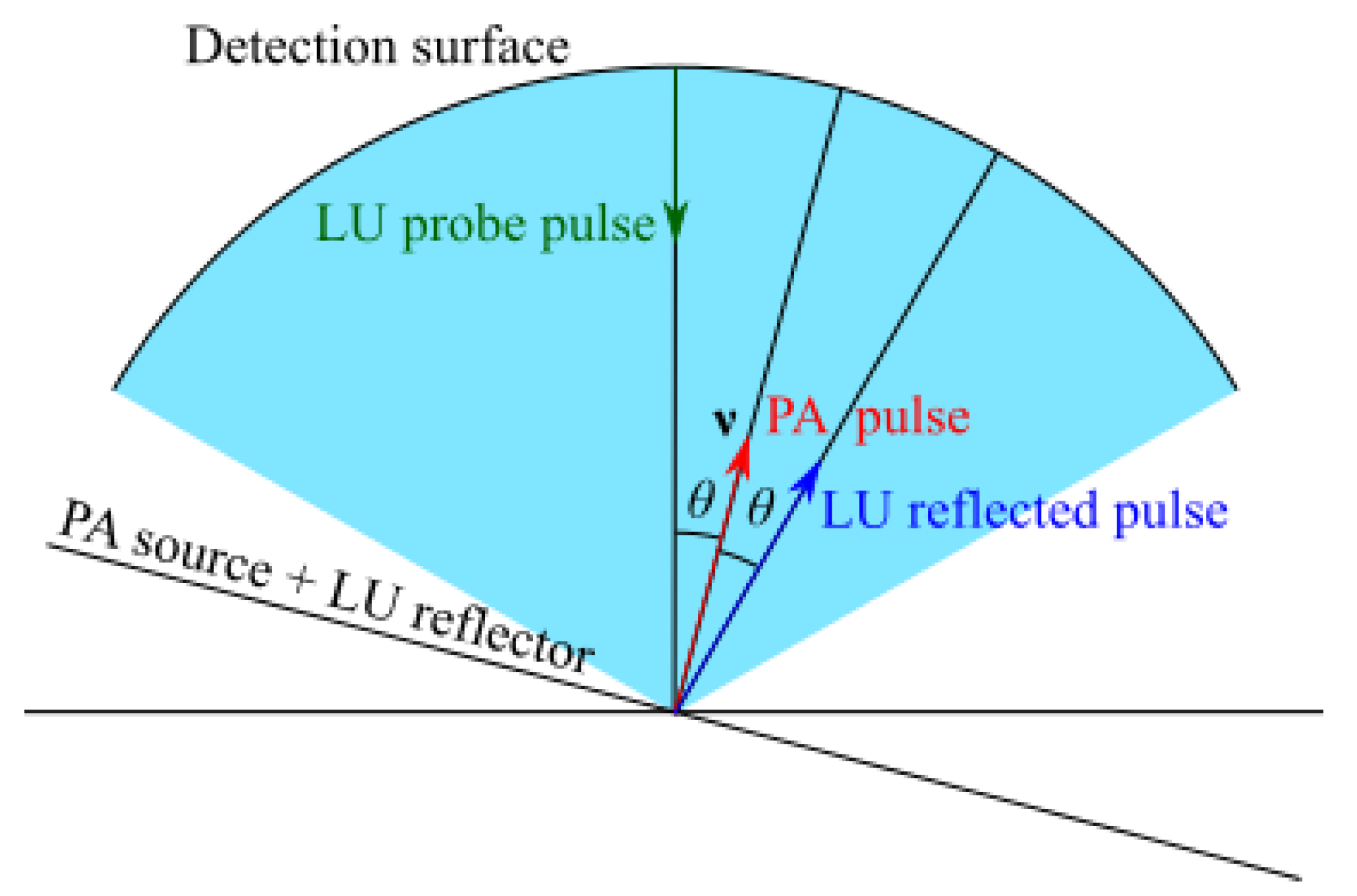
2.3. Spatial Resolution, Sampling, and Limited-View Issues
3. Combining PAI and LUI in Biomedicine
3.1. Hybrid Methods
3.2. Combined PA and LU Speed-of-Sound Imaging
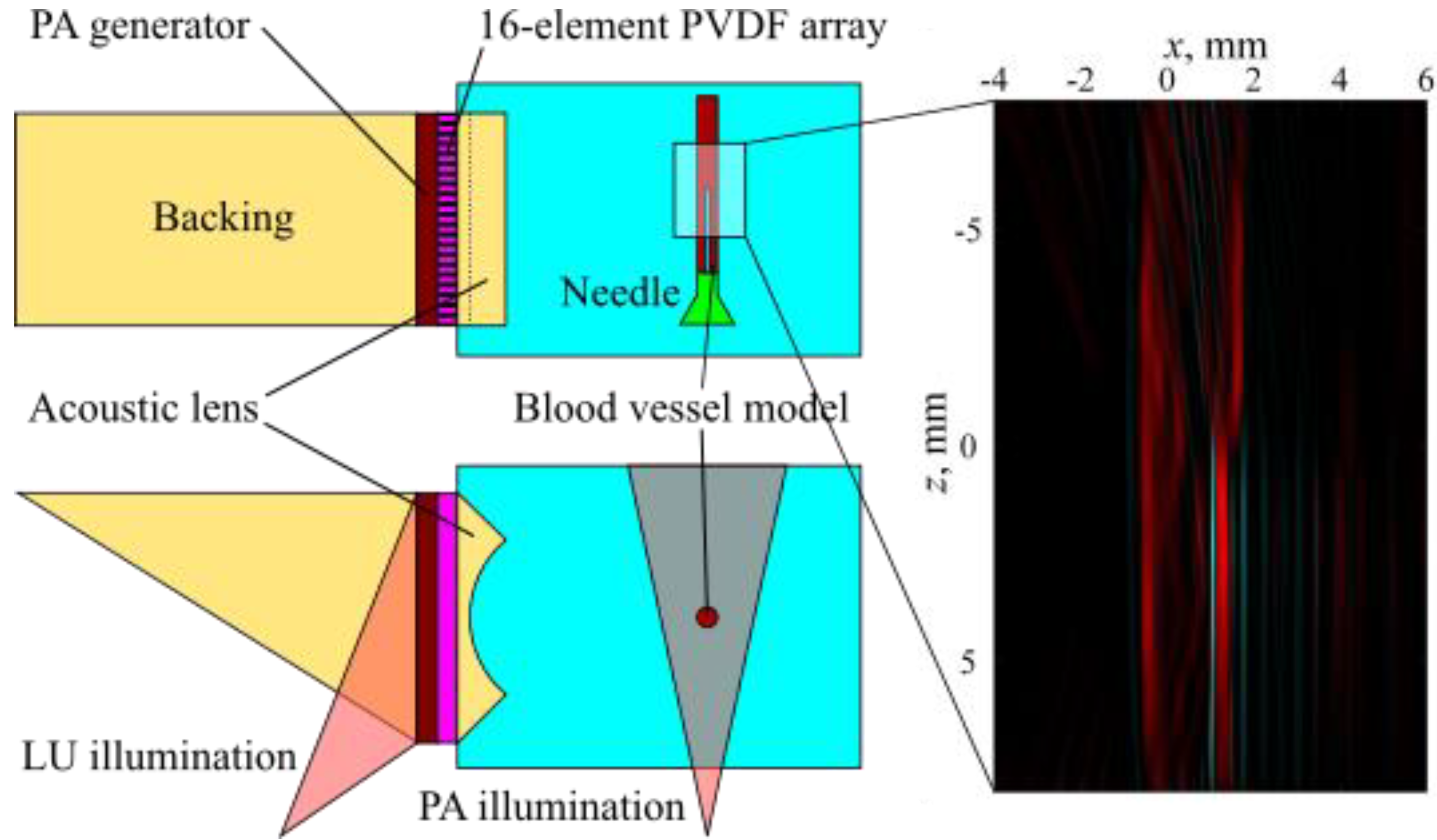
3.3. Combined PA and LU Pulse-Echo Imaging
4. Laser-Ultrasonic Imaging of Solid Bodies
4.1. LU Profilometry of Solids
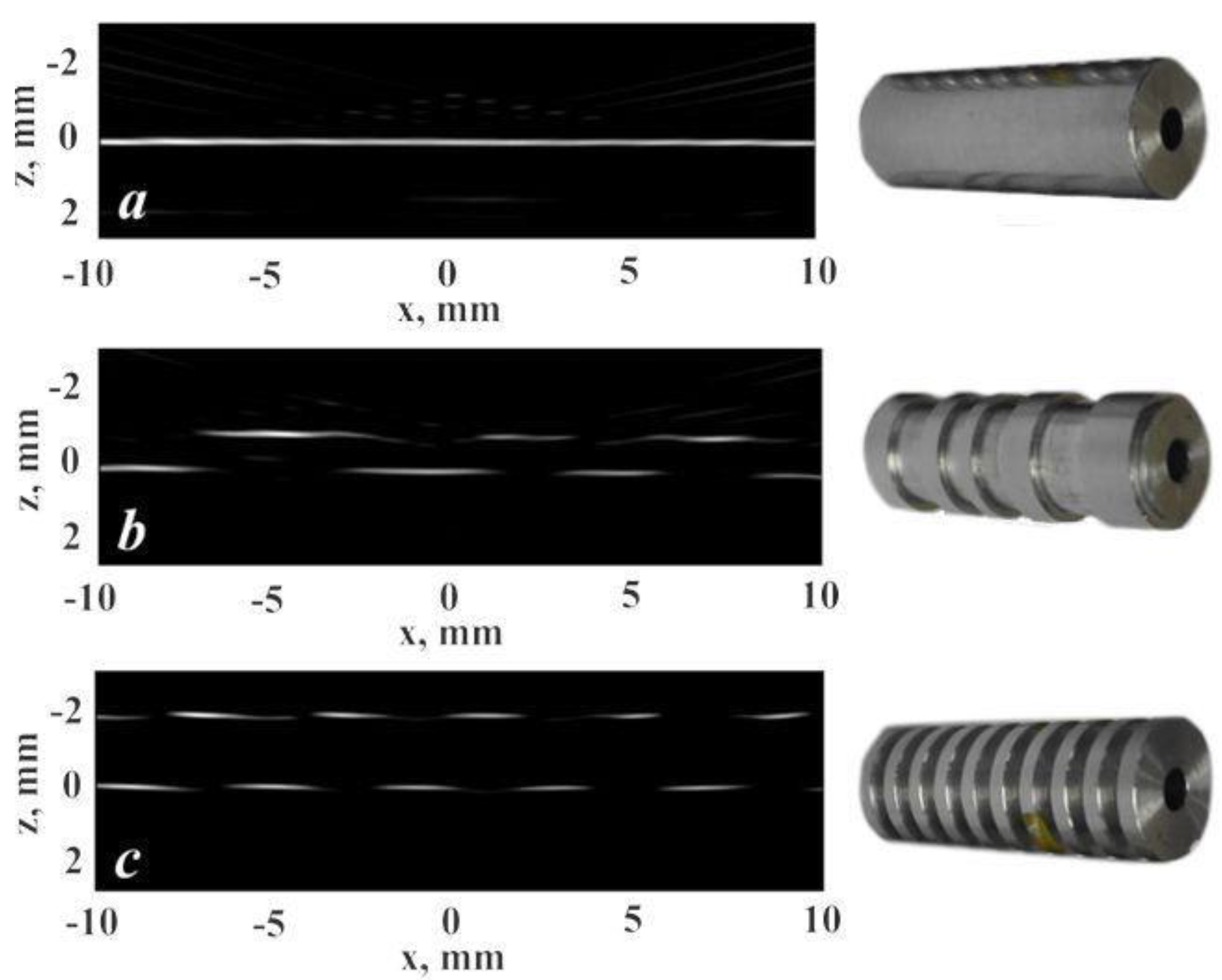
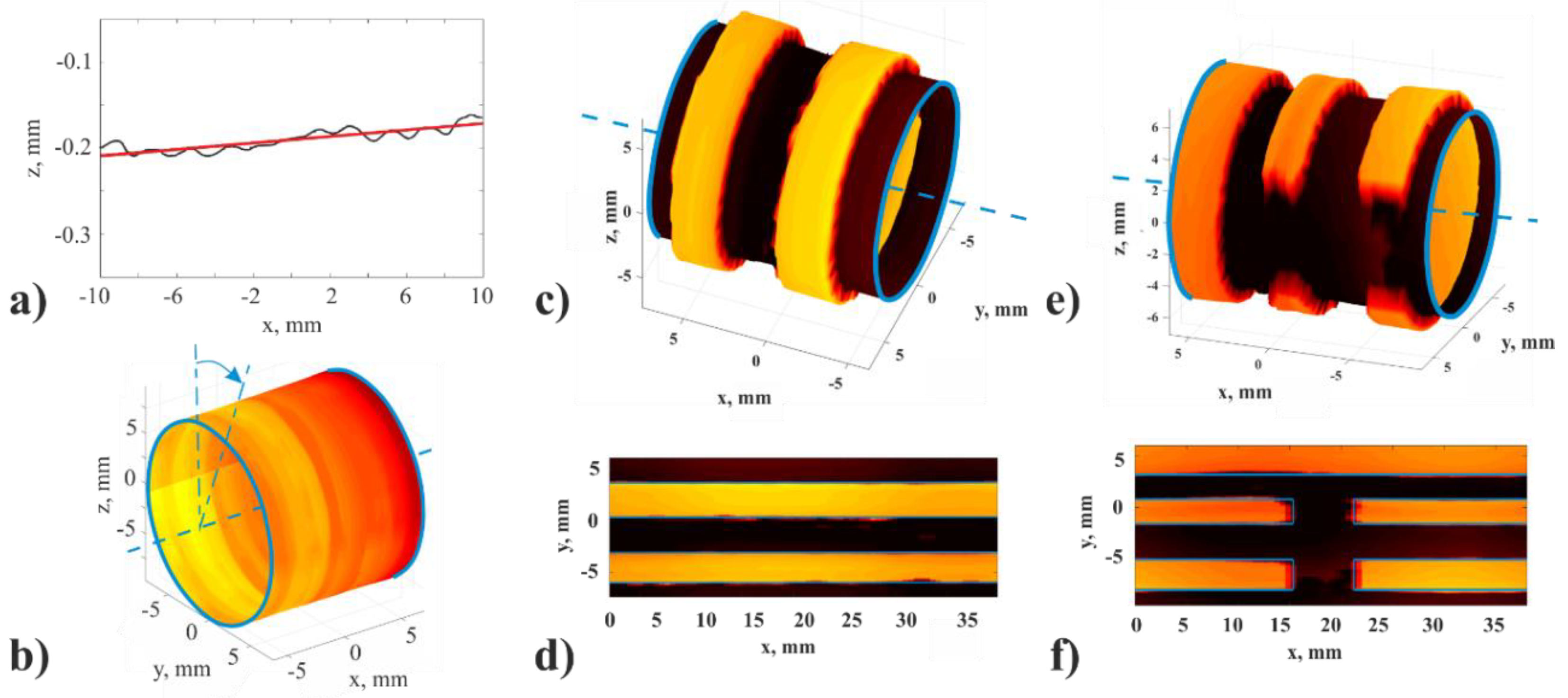
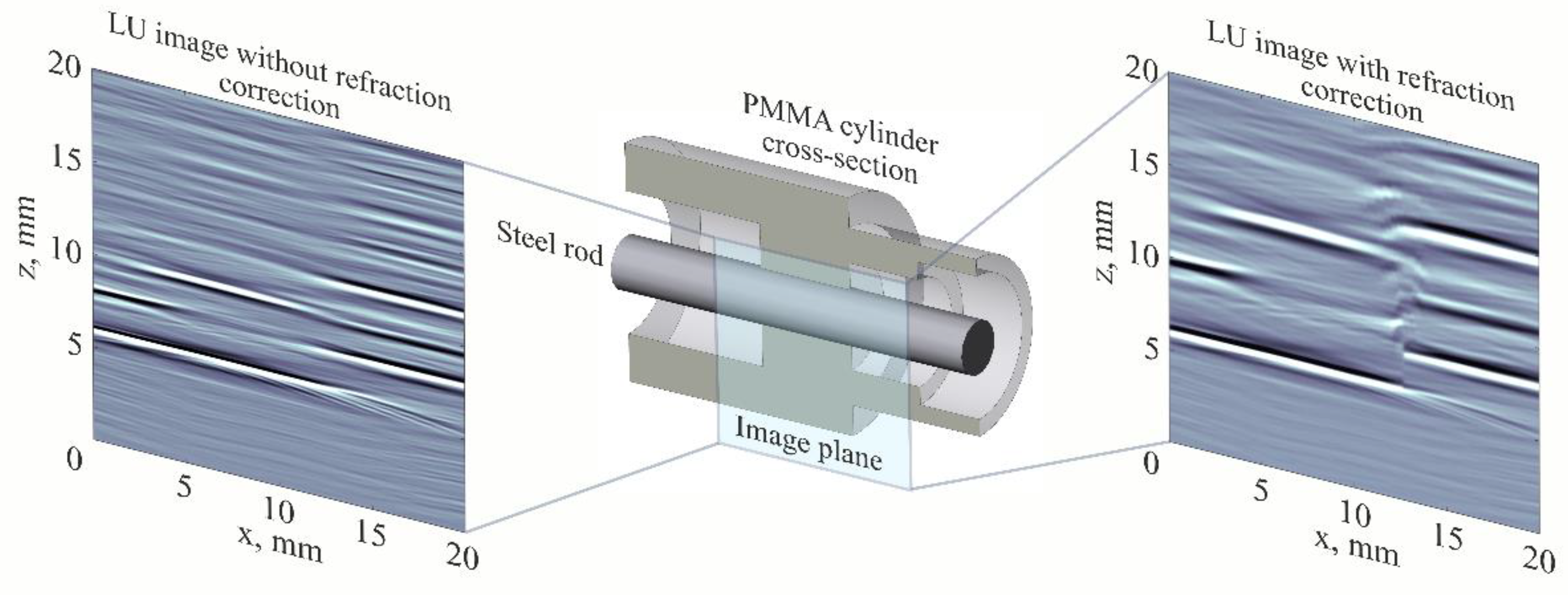
4.2. LU Measurements of Geometry of Solids with Complex Surface
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Symbology
References
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 3, 305–324. [Google Scholar] [CrossRef]
- Veingerov, M.L. New method of gas analysis based on optoacoustic Tyndall-Röntgen phenomena. Dokl. Akad. Nauk SSSR 1938, 19, 687–688. [Google Scholar]
- Gorelik, G.S. On a possible method of studying the energy exchange time between the different degrees of freedom of molecules in a gas. Dokl. Akad. Nauk SSSR 1946, 54, 779. [Google Scholar]
- Askar’yan, G.A.; Prokhorov, A.M.; Chanturiya, G.F.; Shipulo, G.P. The effects of a laser beam in a liquid. Sov. Phys. JETP 1963, 17, 1463–1465. [Google Scholar]
- White, R.M. Generation of elastic waves by transient surface heating. J. Appl. Phys. 1963, 34, 3559–3567. [Google Scholar] [CrossRef]
- Carome, E.F.; Clark, N.A.; Moeller, C.E. Generation of acoustic signals in liquids by ruby laser-induced thermal stress transient. Appl. Phys. Lett. 1964, 4, 95–97. [Google Scholar] [CrossRef]
- Cleary, S.F.; Hamrick, P.E. The generation of elastic plane wave by linear thermal transient. Va. J. Sci. 1968, 19, 228–231. [Google Scholar]
- Burmistrova, L.V.; Karabutov, A.A.; Portnyagin, A.I.; Rudenko, O.V.; Cherepetskaya, E.B. The method of transfer functions in problems of thermooptical sound generation. Sov. Phys. Acoust. 1978, 24, 655–663. [Google Scholar]
- Karabutov, A.A.; Rudenko, O.V.; Cherepetskaya, E.B. To the theory of thermooptical generation of non-stationary acoustic fields. Sov. Phys. Acoust. 1979, 25, 383–394. [Google Scholar]
- Karabutov, A.A.; Portnyagin, A.I.; Rudenko, O.V.; Cherepetskaya, E.B. Nonlinear transformation of thermooptically excited acoustic pulses. Sov. Phys. JETP Lett. 1979, 5, 369–373. [Google Scholar]
- Karabutov, A.A.; Omel’chuk, N.N.; Rudenko, O.V.; Chupryna, V.A. Quantitative study of the nonlinear transformation of sound pulses in liquid under thermooptical excitation. Moscow Univ. Bull. Ser. 1985, 40, 72–77. [Google Scholar]
- Cross, F.W.; Al-Dhahir, R.K.; Dyer, P.E.; MacRobert, A.J. Time-resolved photoacoustic studies of vascular tissue ablation at three laser wavelengths. Appl. Phys. Lett. 1987, 50, 1019–1021. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Esenaliev, R.O.; Jacques, S.L.; Tittel, F.K. Laser optoacoustic tomography for medical diagnostics principles. Proc. SPIE 1996, 2676, 22–32. [Google Scholar] [CrossRef]
- Brochu, F.; Brunker, J.; Joseph, J.; Tomaszewski, M.R.; Morscher, S.; Bohndiek, S.E. Towards quantitative evaluation of tissue absorption coefficients using light fluence correction in optoacoustic tomography. IEEE Trans. Med. Imaging 2017, 36, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Hupple, C.W.; Morscher, S.; Burton, N.C.; Pagel, M.D.; McNally, L.R.; Cárdenas-Rodríguez, J. A light-fluence-independent method for the quantitative analysis of dynamic contrast-enhanced multispectral optoacoustic tomography (DCE MSOT). Photoacoustics 2018, 10, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.; Masthoff, M.; Claussen, J. Multispectral optoacoustic tomography of the human breast: Characterisation of healthy tissue and malignant lesions using a hybrid ultrasound-optoacoustic approach. Eur. Radiol. 2018, 28, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Murfin, A.S.; Soden, R.A.J.; Hatrick, D.; Dewhurst, R.J. Laser-ultrasound detection systems: A comparative study with Rayleigh waves. Meas. Sci. Technol. 2000, 11, 1208–1219. [Google Scholar] [CrossRef]
- Lutzweiler, C.; Razansky, D. Optoacoustic imaging and tomography: Reconstruction approaches and outstanding challenges in image performance and quantification. Sensors 2013, 13, 7345–7384. [Google Scholar] [CrossRef] [PubMed]
- Karabutov, A.A.; Podymova, N.B.; Letokhov, V.S. Time-resolved laser optoacoustic tomography of inhomogeneous media. Appl. Phys. B 1996, 63, 545–563. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Esenaliev, R.O.; Jacques, S.L.; Thomsen, S.; Tittel, F.K. Lateral and z-axial resolution in laser optoacoustic imaging with ultrasonic transducers. Proc. SPIE 1995, 2389, 198–208. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shan, Q. Optical remote measurement of ultrasound. Meas. Sci. Technol. 1999, 10, R139. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esenaliev, R.O.; Karabutov, A.A.; Oraevsky, A.A. Sensitivity of laser opto-acoustic imaging in detection of small deeply embedded tumors. IEEE J. Sel. Top. Quant. Electr. 1999, 5, 981–988. [Google Scholar] [CrossRef]
- Pelivanov, I.; Buma, T.; Xia, J.; Wei, C.-W.; O’Donnell, M. A new fiber-optic non-contact compact laser-ultrasound scanner for fast non-destructive testing and evaluation of aircraft composites. J. Appl. Phys. 2014, 115, 113105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oraevsky, A.; Karabutov, A. Ultimate sensitivity of time-resolved opto-acoustic detection. Proc. SPIE 2000, 3916, 1–12. [Google Scholar]
- Upputuri, P.K.; Pramanik, M. Recent advances toward preclinical and clinical translation of photoacoustic tomography: A review. J. Biomed. Opt. 2016, 22, 041006. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, L.; Zhu, L.; Xia, J.; Wang, L.V. Multiview Hilbert transformation for full-view photoacoustic computed tomography using a linear array. J. Biomed. Opt. 2015, 20, 066010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean-Ben, X.L.; Razansky, D. Portable spherical array probe for volumetric real-time optoacoustic imaging at centimeter-scale depths. Opt. Express 2013, 21, 28062–28071. [Google Scholar] [CrossRef] [PubMed]
- Esenaliev, R.; Petrov, I.; Petrov, Y.; Guptarak, J.; Boone, D.R.; Mocciaro, E.; Weisz, H.; Parsley, M.A.; Sell, S.L.; Hellmich, H.; et al. Nano-pulsed laser therapy is neuroprotective in a rat model of blast-induced neurotrauma. J. Neurotrauma 2018, 35, 1510–1522. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Time-domain reconstruction for thermoacoustic tomography in a spherical geometry. IEEE Trans. Med. Imaging 2002, 21, 814–822. [Google Scholar] [PubMed] [Green Version]
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Marshall, M.V.; Sevick-Muraca, E.M.; Lanza, G.; Kotyk, J.; Culver, J.; Wang, L.V.; Uddin, J.; Crews, B.C.; Marnett, L.J.; et al. Seeing it through: Translational validation of new medical imaging modalities. Biomed. Opt. Express 2012, 3, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, J.; Wang, L.V. Tutorial on photoacoustic tomography. J. Biomed. Opt. 2016, 21, 061007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.V.; Gao, L. Photoacoustic microscopy and computed tomography: From bench to bedside. Annu. Rev. Biomed. Eng. 2014, 16, 155–185. [Google Scholar] [CrossRef] [PubMed]
- Brunker, J.; Yao, J.; Laufer, J.; Bohndiek, S.E. Photoacoustic imaging using genetically encoded reporters: A review. J. Biomed. Opt. 2017, 22, 070901. [Google Scholar] [CrossRef] [PubMed]
- Oraevsky, A.A.; Andreev, V.G.; Karabutov, A.A.; Esenaliev, R.O. Two-dimensional optoacoustic tomography: Array transducers and image reconstruction algorithm. Proc. SPIE 1999, 3601, 256–267. [Google Scholar]
- Chen, S.-L.; Guo, L.J.; Wang, X. All-optical photoacoustic microscopy. Photoacoustics 2015, 3, 143–150. [Google Scholar] [CrossRef]
- Zhang, H.F.; Maslov, K.; Stoica, G.; Wang, L.V. Functional photoacoustic microscopy for high resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006, 24, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Li, G.; Xia, D.; Li, G.; Xia, D.; Li, Z.; Guo, Z.; Chen, S.L. Synthetic aperture focusing technique for photoacoustic endoscopy. Opt. Express 2017, 25, 20162–20171. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 2352. [Google Scholar] [CrossRef] [PubMed]
- Simonova, V.A.; Savateeva, E.V.; Karabutov, A.A. Novel combined optoacoustic and laser ultrasound transducer array system. MSU Phys. Bull. B 2009, 64, 394–396. [Google Scholar] [CrossRef]
- Bychkov, A.S.; Cherepetskaya, E.B.; Karabutov, A.A.; Makarov, V.A. Laser optoacoustic tomography for the study of femtosecond laser filaments in air. Laser Phys. Lett. 2016, 13, 085401. [Google Scholar] [CrossRef]
- Uryupina, D.S.; Bychkov, A.S.; Pushkarev, D.V.; Mitina, E.V.; Savel’ev, A.B.; Kosareva, O.G.; Panov, N.A.; Karabutov, A.A.; Cherepetskaya, E.B. Laser optoacoustic diagnostics of femtosecond filaments in air using wideband piezoelectric transducers. Laser Phys. Lett. 2016, 13, 095401. [Google Scholar] [CrossRef]
- Potemkin, F.V.; Mareev, E.I.; Rumiantsev, B.V.; Bychkov, A.S.; Karabutov, A.A.; Cherepetskaya, E.B.; Makarov, V.A. Two-dimensional photoacoustic imaging of femtosecond filament in water. Laser Phys. Lett. 2018, 15, 075403. [Google Scholar] [CrossRef]
- Bychkov, A.S.; Zarubin, V.P.; Karabutov, A.A.; Simonova, V.A.; Cherepetskaya, E.B. On the use of an optoacoustic and laser ultrasonic imaging system for assessing peripheral intravenous access. Photoacoustics 2017, 5, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, L.V. Photoacoustic tomography and sensing in biomedicine. Phys. Med. Biol. 2009, 54, 59–97. [Google Scholar] [CrossRef] [PubMed]
- Karabutov, A.A.; Podymova, N.B.; Cherepetskaya, E.B. Determination of uniaxial stresses in steel structures by the laser-ultrasonic method. J. Appl. Mech. Tech. Phys. 2017, 58, 503–510. [Google Scholar] [CrossRef]
- Cherepetskaya, E.B.; Karabutov, A.A.; Mironova, E.A.; Podymova, N.B.; Zharinov, A.N. Contact laser-ultrasonic evaluation of residual stress. Appl. Mech. Mater. 2016, 843, 118–124. [Google Scholar] [CrossRef]
- Podymova, N.B.; Karabutov, A.A. Combined effects of reinforcement fraction and porosity on ultrasonic velocity in SiC particulate aluminum alloy matrix composites. Compos. Part B Eng. 2017, 113, 138–143. [Google Scholar] [CrossRef]
- Karabutov, A.A.; Cherepetskaya, E.B.; Podymova, N.B. Laser-Ultrasonic Measurement of Local Elastic Moduli. In Proceedings of the VIIIth International Workshop NDT in Progress, Prague, Czech Republic, 12–14 October 2015; pp. 75–78. Available online: https://www.ndt.net/events/NDTP2015/app/content/Paper/26_Cherepetskaya.pdf (accessed on 12 October 2018).
- Manohar, S.; Willemink, R.G.H.; van der Heijden, F.; Slump, C.H.; van Leeuwen, T.G. Concomitant speed-of-sound tomography in photoacoustic imaging. Appl. Phys. Lett. 2007, 91, 131911. [Google Scholar] [CrossRef]
- Zarubin, V.; Bychkov, A.; Karabutov, A.; Simonova, V.; Cherepetskaya, E. Laser-induced ultrasonic imaging for measurements of solid surfaces in optically opaque liquids. Appl. Opt. 2018, 57, C70–C76. [Google Scholar] [CrossRef] [PubMed]
- Zarubin, V.; Bychkov, A.; Karabutov, A.; Simonova, V.; Cherepetskaya, E. A method of laser ultrasound tomography for solid surfaces mapping. MATEC Web Conf. 2018, 145, 05009. [Google Scholar] [CrossRef] [Green Version]
- Zarubin, V.; Bychkov, A.; Simonova, V.; Zhigarkov, V.; Karabutov, A.; Cherepetskaya, E. A refraction-corrected tomographic algorithm for immersion laser-ultrasonic imaging of solids with piecewise linear surface profile. Appl. Phys. Lett. 2018, 112, 214102. [Google Scholar] [CrossRef]
- Levesque, D.; Asaumi, Y.; Lord, M.; Bescond, C.; Hatanaka, H.; Tagami, M.; Monchalin, J.P. Inspection of thick welded joints using laser-ultrasonic SAFT. Ultrasonics 2016, 69, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Shibaev, I.A.; Cherepetskaya, E.B.; Bychkov, A.S.; Zarubin, V.P.; Ivanov, P.N. Evaluation of the internal structure of dolerite specimens using X-ray and laser ultrasonic tomography. IJCIET 2018, 9, 84–92. Available online: http://www.iaeme.com/ijciet/issues.asp?JType=IJCIET&VType=9&IType=9 (accessed on 14 October 2018).
- Wurzinger, G.; Nuster, R.; Paltauf, G. Combined photoacoustic, pulse-echo laser ultrasound, and speed-of-sound imaging using integrating optical detection. J. Biomed. Opt. 2016, 21, 086010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fehm, T.F.; Dean-Ben, X.L.; Razansky, D. Four dimensional hybrid ultrasound and optoacoustic imaging via passive element optical excitation in a hand-held probe. Appl. Phys. Lett. 2014, 105, 173505. [Google Scholar] [CrossRef]
- Rousseau, G.; Blouin, A.; Monchalin, J.-P. Non-contact photoacoustic tomography and ultrasonography for tissue imaging. Biomed. Opt. Express 2012, 3, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.-Y.; Wu, W.-S.; Hsieh, B.-Y.; Li, P.-C. Concurrent photoacoustic-ultrasound imaging using single-laser pulses. J. Biomed. Opt. 2015, 20, 086004. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Shragge, J.; van Wijk, K. Nonconfocal all-optical laser-ultrasound and photoacoustic imaging system for angle-dependent deep tissue imaging. J. Biomed. Opt. 2017, 22, 041014. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.B.E.; Chuah, S.Y.; Razansky, D.; Ho, C.J.H.; Malempati, P.; Dinish, U.S.; Bi, R.; Fu, C.Y.; Ford, S.J.; Lee, J.S.-S.; et al. Noninvasive real-time characterization of non-melanoma skin cancers with handheld optoacoustic probes. Photoacoustics 2017, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Varkentin, A.; Mazurenka, M.; Blumenröther, E. Trimodal system for in vivo skin cancer screening with combined optical coherence tomography-Raman and colocalized optoacoustic measurements. J. Biophotonics 2018, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hupple, C.; Burton, N.; Driessen, W. Dynamic imaging of chronic inflammation and tumour therapeutic response with multispectral optoacoustic tomography (MSOT). J. Nucl. Med. 2015, 56, 620. [Google Scholar]
- Karabutov, A.A.; Savateeva, E.V.; Podymova, N.B.; Oraevsky, A.A. Backward detection of laser-induced wide-band ultrasonic transients with optoacoustic transducer. J. Appl. Phys. 2000, 87, 2003–2014. [Google Scholar] [CrossRef]
- Suslov, O.A.; Novikov, A.A.; Karabutov, A.A.; Podymova, N.B.; Simonova, V.A. Use of acoustoelasticity effect with application of laser sources of the ultrasound for control of the stress state of railbars of the continuous welded rails. Int. J. Appl. Eng. Res. 2015, 10, 41121–41128. [Google Scholar]
- Kremkau, F.W. Diagnostic Utrasound. Principles and Instruments, 6th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 2002. [Google Scholar]
- Chen, S.-L. Review of laser-generated ultrasound transmitters and their applications to all-optical ultrasound transducers and imaging. Appl. Sci. 2017, 7, 25. [Google Scholar] [CrossRef]
- Simonova, V.A.; Karabutov, A.A.; Khokhlova, T.D. Wideband focused transducer array for optoacoustic tomography. Akust. J. 2009, 55, 822–827. [Google Scholar] [CrossRef]
- Gusev, V.E.; Karabutov, A.A. Laser Opto-Acoustics; AIP Press: New York, NY, USA, 1993. [Google Scholar]
- Oraevsky, A.A.; Karabutov, A.A.; Solomatin, S.V.; Savateeva, E.V.; Andreev, V.A.; Gatalica, Z.; Singh, H.; Fleming, R.D. Laser optoacoustic imaging of breast cancer in vivo. Proc. SPIE 2001, 4256, 6–15. [Google Scholar]
- Buehler, A.; Rosenthal, A.; Razansky, D.; Ntziachristos, V. Model-based optoacoustic inversions with incomplete projection data. Med. Phys. 2011, 38, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Köstli, K.P.; Beard, P.C. Two-dimensional photoacoustic imaging by use of Fourier-transform image reconstruction and a detector with an anisotropic response. Appl. Opt. 2003, 42, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Universal back-projection algorithm for photoacoustic computed tomography. Phys. Rev. E 2005, 71, 016706. [Google Scholar] [CrossRef] [PubMed]
- Dean-Ben, X.L.; Buehler, A.; Ntziachristos, V.; Razansky, D. Accurate model-based reconstruction algorithm for three-dimensional optoacoustic tomography. IEEE Trans. Med. Imaging 2012, 31, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Razansky, D.; Ntziachristos, V. Fast semi-analytical model-based acoustic inversion for quantitative optoacoustic tomography. IEEE Trans. Med. Imaging 2010, 29, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Norton, S.J.; Linzer, M. Ultrasonic reflectivity imaging in three dimensions: Exact inverse scattering solutions for plane, cylindrical, and spherical apertures. IEEE Trans. Ultrason. 1981, 2, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Drinkwater, B.W.; Wilcox, P.D. Comparison of ultrasonic array imaging algorithms for nondestructive evaluation. IEEE Trans. Ultrason. 2013, 60, 1732–1745. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, S.; Jensen, J.A. Comparison between different encoding schemes for synthetic aperture imaging. Proc. SPIE 2002, 4687, 1–12. [Google Scholar] [CrossRef]
- Langenberg, K.J.; Berger, M.; Kreutter, T.; Mayer, K.; Schmitz, V. Synthetic aperture focusing technique signal processing. NDT Int. 1986, 19, 177–189. [Google Scholar] [CrossRef]
- Wurzinger, G.; Nuster, R.; Schmitner, N.; Gratt, S.; Meyer, D.; Paltauf, G. Simultaneous three-dimensional photoacoustic and laser-ultrasound tomography. Biomed. Opt. Express 2013, 4, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.S.; Cherepetskaya, E.B.; Karabutov, A.A.; Makarov, V.A. Toroidal sensor arrays for real-time photoacoustic imaging. J. Biomed. Opt. 2017, 22, 076003. [Google Scholar] [CrossRef] [PubMed]
- Bychkov, A.S.; Cherepetskaya, E.B.; Karabutov, A.A.; Makarov, V.A. Improvement of image spatial resolution in optoacoustic tomography with the use of a confocal array. Acoust. Phys. 2018, 64, 77–82. [Google Scholar] [CrossRef]
- Dima, A.; Burton, N.C.; Ntziachristos, V. Multispectral optoacoustic tomography at 64, 128, and 256 channels. J. Biomed. Opt. 2014, 19, 036021. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Ntziachristos, V.; Razansky, D. Acoustic inversion in optoacoustic tomography: A review. Curr. Med. Imaging Rev. 2013, 9, 318–336. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.V.; Ambartsoumian, G.; Kuchment, P. Reconstructions in limited-view thermoacoustic tomography. Med. Phys. 2004, 31, 724–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean-Ben, X.L.; Ding, L.; Razansky, D. Dynamic particle enhancement in limited-view optoacoustic tomography. Opt. Lett. 2017, 42, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Pichler, B.J.; Judenhofer, M.S.; Wehrl, H.F. PET/MRI hybrid imaging: Devices and initial results. Eur. Radiol. 2008, 18, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Wang, T.; Kumavor, P.D.; Aguirre, A.; Shung, K.K.; Zhou, Q.; Sanders, M.; Brewer, M.; Zhu, Q. Integrated optical coherence tomography, ultrasound and photoacoustic imaging for ovarian tissue characterization. Biomed. Opt. Express 2011, 2, 2551–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merčep, E.; Dean-Ben, X.L.; Razansky, D. Combined pulse-echo ultrasound and multispectral optoacoustic tomography with a multi-segment detector array. IEEE Trans. Med. Imaging 2017, 36, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Kramer, C.M. Multimodality Imaging in Cardiovascular Medicine; Demos Med. Publishing: New York, NY, USA, 2010. [Google Scholar]
- Curiel, L.; Chopra, R.; Hynynen, K. Progress in multimodality imaging: Truly simultaneous ultrasound and magnetic resonance imaging. IEEE Trans. Med. Imaging 2007, 26, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Choyke, P.L. Abstract IA23: Diagnosing prostate cancer with image fusion (MRI, PET, CT, US). Cancer Res. 2012, 72, IA23. [Google Scholar] [CrossRef]
- Culver, J.; Akers, W.; Achilefu, S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J. Nucl. Med. 2008, 49, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.; Li, L.; Maslov, K.; Wang, L. Hybrid-scanning optical resolution photoacoustic microscopy for in vivo vasculature imaging. Opt. Lett. 2010, 35, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Iftimia, N.; Ferguson, R.D.; Mujat, M.; Patel, A.H.; Zhang, E.Z.; Fox, W.; Rajadhyaksha, M. Combined reflectance confocal microscopy/optical coherence tomography imaging for skin burn assessment. Biomed. Opt. Express 2013, 4, 680–695. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-C.A.; Chekkoury, A.; Omar, M.; Schmitt-Manderbach, T.; Koberstein-Schwarz, B.; Mappes, T.; López-Schier, H.; Razansky, D.; Ntziachristos, V. Selective plane illumination optical and optoacoustic microscopy for postembryonic imaging. Laster Photonics Rev. 2015, 9, L29–L34. [Google Scholar] [CrossRef]
- Dean-Ben, X.L.; Merčep, E.; Razansky, D. Hybrid-array-based optoacoustic and ultrasound (OPUS) imaging of biological tissues. Appl. Phys. Lett. 2017, 110, 203703. [Google Scholar] [CrossRef]
- Jose, J.; Willemink, R.G.H.; Steenbergen, W.; Slump, C.H.; van Leeuwen, T.G.; Manohar, S. Speed-of-sound compensated photoacoustic tomography for accurate imaging. Med. Phys. 2012, 39, 7262–7271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jose, J.; Willemink, R.G.H.; Resink, S.; Piras, D.; van Hespen, J.G.; Slump, C.H.; Steenbergen, W.; van Leeuwen, T.; Manohar, S. Passive element enriched photoacoustic computed tomography (PER PACT) for simultaneous imaging of acoustic propagation properties and light absorption. Opt. Express 2011, 19, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Resink, S.; Jose, J.; Willemink, R.G.H.; Slump, C.H.; Steenbergen, W.; van Leeuwen, T.G.; Manohar, S. Multiple passive element enriched photoacoustic computed tomography. Opt. Lett. 2011, 36, 2809–2811. [Google Scholar] [CrossRef] [PubMed]
- Wurzinger, G.; Nuster, R.; Gratt, S.; Paltauf, G. Combined photoacoustic and speed-of-sound imaging using integrating optical detection. Proc. SPIE 2014, 8943. [Google Scholar] [CrossRef]
- Nuster, R.; Schmitner, N.; Wurzinger, G.; Gratt, S.; Salvenmoser, W.; Meyer, D.; Paltauf, G.G. Hybrid photoacoustic and ultrasound section imaging with optical ultrasound detection. J. Biophotonics 2013, 6, 549–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ermilov, S.A.; Su, R.; Conjusteau, A.; Anis, F.; Nadvoretskiy, V.; Anastasio, M.A.; Oraevsky, A.A. Three-Dimensional Optoacoustic and Laser-Induced Ultrasound Tomography System for Preclinical Research in Mice: Design and Phantom Validation. Ultrason. Imaging 2016, 38, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, R.G.M.; Brands, P.J.; Steenbergen, W.; van Leeuwen, T.G.C. Real-time in vivo photoacoustic and ultrasound imaging. J. Biomed. Opt. 2008, 13, 050510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montilla, L.G.; Olafsson, R.; Bauer, D.R.; Witte, R.S. Real-time photoacoustic and ultrasound imaging: A simple solution for clinical ultrasound systems with linear arrays. Phys. Med. Biol. 2013, 58, N1–N12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Xu, G.; Yu, Y.; Zhou, Y.; Carson, P.L.; Wang, X.; Liu, X. Real-time photoacoustic and ultrasound dual-modality imaging system facilitated with graphics processing unit and code parallel optimization. J. Biomed. Opt. 2013, 18, 086001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqasemi, U.; Li, H.; Aguirre, A.; Zhu, Q. FPGA-Based Reconfigurable Processor for Ultrafast Interlaced Ultrasound and Photoacoustic Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, M.W.; Hunt, H.K. Hand-held optoacoustic imaging: A review. Photoacoustics 2018, 11, 14–27. [Google Scholar] [CrossRef] [PubMed]
- VanderLaan, D.; Karpiouk, A.; Yeager, D.; Emelianov, S. Real-Time Intravascular Ultrasound and Photoacoustic Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, K.; van den Berg, P.; Rabot, O.; Kohl, A.; Tisserand, S.; Brands, P.; Steenbergen, W. Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express 2014, 22, 26365–26374. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, R.; Sahin, O.; Emelianov, S. Ultrasound-guided photoacoustic imaging: Current state and future development. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 450–466. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable Real-time Clinical Photoacoustic and Ultrasound Imaging System. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.-W.; Nguyen, T.-M.; Xia, J.; Arnal, B.; Wong, E.Y.; Pelivanov, I.M.; O’Donnell, M. Real-Time Integrated Photoacoustic and Ultrasound (PAUS) Imaging System to Guide Interventional Procedures: Ex Vivo Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Su, J.L.; Karpiouk, A.B.; Wang, B.; Emelianov, S.Y. Photoacoustic imaging of clinical metal needles in tissue. J. Biomed. Opt. 2010, 15, 021309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, W.; Mosse, C.A.; Colchester, R.J.; Mari, J.M.; Nikitichev, D.I.; West, S.J.; Ourselin, S.; Beard, P.C.; Desjardins, A.E. Fiber optic photoacoustic probe with ultrasonic tracking for guiding minimally invasive procedures. Proc. SPIE 2015, 9539. [Google Scholar] [CrossRef]
- Colchester, R.; Mosse, C.A.; Nikitichev, D.I.; Zhang, E.Z.; West, S.; Beard, P.C.; Papakonstantinou, I.; Desjardins, A.E. Real-time needle guidance with photoacoustic and laser-generated ultrasound probes. Proc. SPIE 2015, 9323. [Google Scholar] [CrossRef]
- Finlay, M.C.; Mosse, C.A.; Colchester, R.J.; Noimark, S.; Zhang, E.Z.; Ourselin, S.; Beard, P.C.; Schilling, R.J.; Parkin, I.P.; Papakonstantinou, I.; et al. Through-needle all-optical ultrasound imaging in vivo: A preclinical swine study. Light-Sci. Appl. 2017, 6, e17103. [Google Scholar] [CrossRef] [PubMed]
- Dean-Ben, X.L.; Lopez-Schier, H.; Razansky, D. Optoacoustic micro-tomography at 100 volumes per second. Sci. Rep. 2017, 7, 6850. [Google Scholar] [CrossRef] [PubMed]
- Levesque, D.; Bescond, C.; Lord, M.; Cao, X.; Wanjara, P.; Monchalin, J.P. Inspection of additive manufactured parts using laser ultrasonics. Proc. Rev. Progr. QNDE AIP 2015, 1706, 130003. [Google Scholar]
- Bolognesi, G.; Cottin-Bizonne, C.; Guene, E.M.; Teisseire, J.; Pirat, C. A novel technique for simultaneous velocity and interface profile measurements on micro-structured surfaces. Soft Matter 2013, 9, 2239–2244. [Google Scholar] [CrossRef]
- Li, C.; Hall, G.; Zhu, D.; Li, H.; Eliceiri, K.W.; Jiang, H. Three-dimensional surface profile measurement of microlenses using the Shack-Hartmann wavefront sensor. J. Microelectromech. Syst. 2012, 21, 530–540. [Google Scholar] [CrossRef]
- Brekhovskih, L. Waves in Layered Media; Academic Press: New York, NY, USA, 1976. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bychkov, A.; Simonova, V.; Zarubin, V.; Cherepetskaya, E.; Karabutov, A. The Progress in Photoacoustic and Laser Ultrasonic Tomographic Imaging for Biomedicine and Industry: A Review. Appl. Sci. 2018, 8, 1931. https://doi.org/10.3390/app8101931
Bychkov A, Simonova V, Zarubin V, Cherepetskaya E, Karabutov A. The Progress in Photoacoustic and Laser Ultrasonic Tomographic Imaging for Biomedicine and Industry: A Review. Applied Sciences. 2018; 8(10):1931. https://doi.org/10.3390/app8101931
Chicago/Turabian StyleBychkov, Anton, Varvara Simonova, Vasily Zarubin, Elena Cherepetskaya, and Alexander Karabutov. 2018. "The Progress in Photoacoustic and Laser Ultrasonic Tomographic Imaging for Biomedicine and Industry: A Review" Applied Sciences 8, no. 10: 1931. https://doi.org/10.3390/app8101931



