Design Considerations for Murine Retinal Imaging Using Scattering Angle Resolved Optical Coherence Tomography
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Murine Eye Model
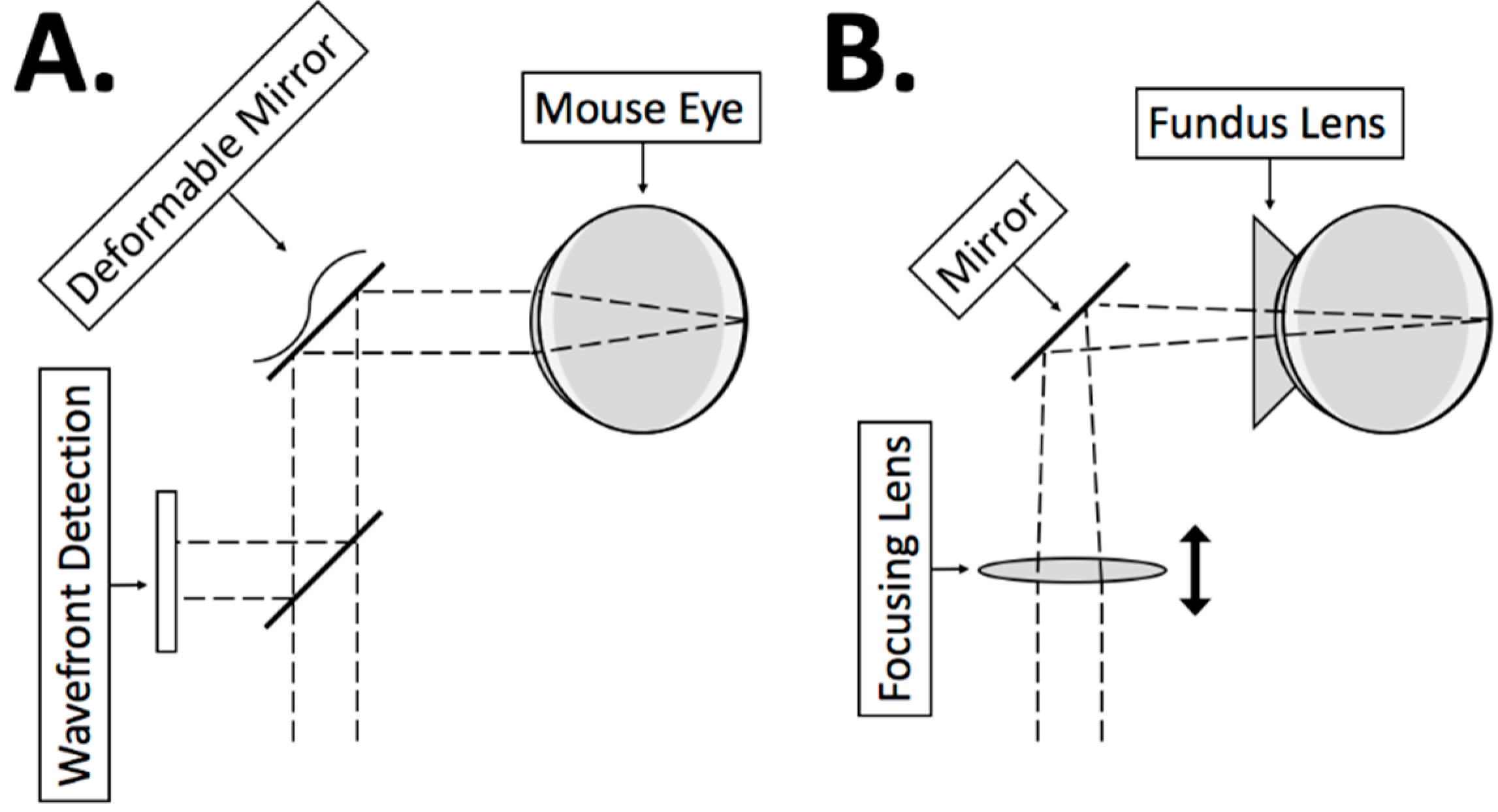
2.2. System Design
2.2.1. 2D MEMS Mirror
2.2.2. Corneal Contact
2.2.3. Focusing Lens
2.2.4. Dispersion Compensation
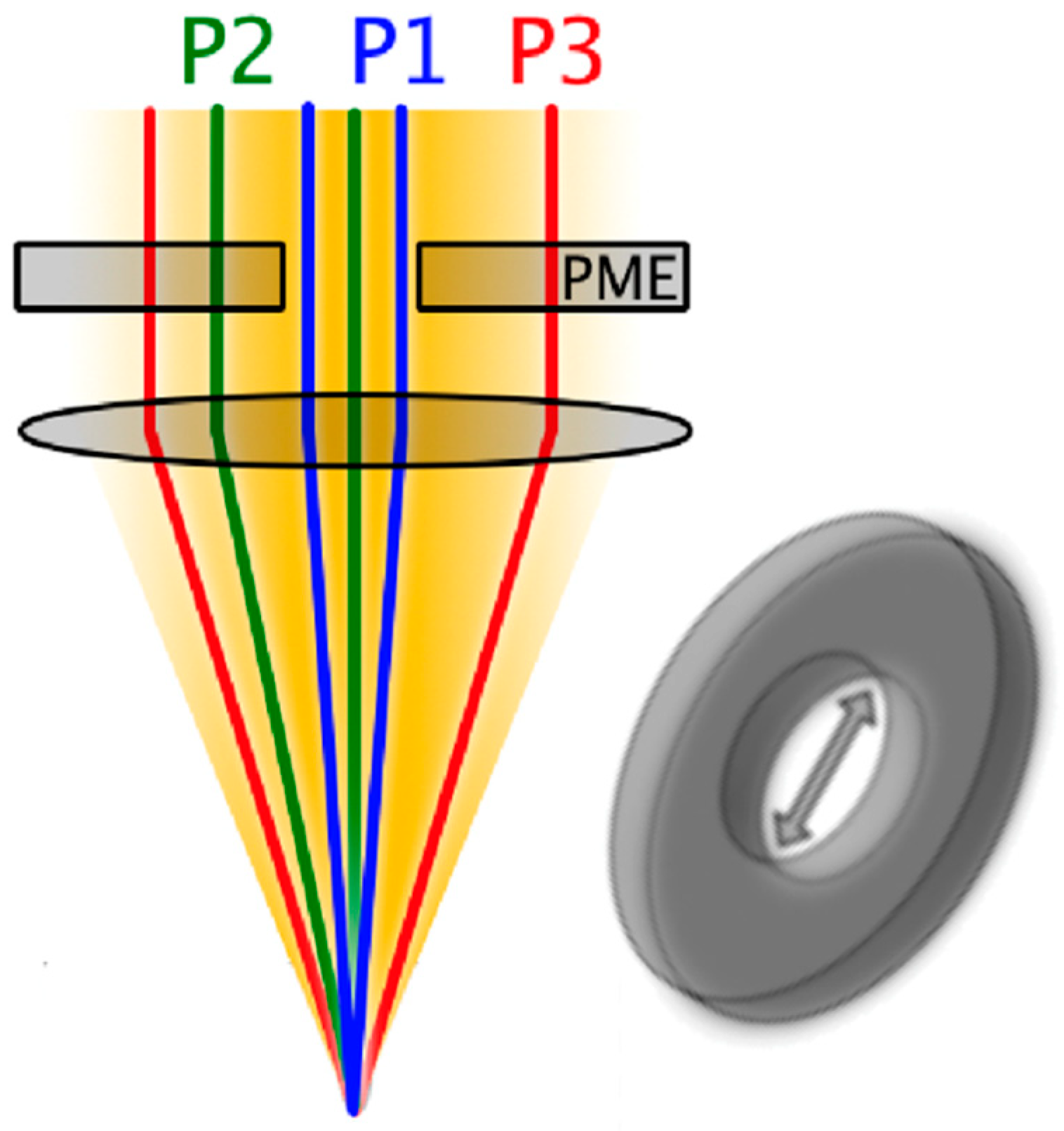
2.2.5. Pathlength Multiplexing Element
2.2.6. System Construction
2.3. System Characterization
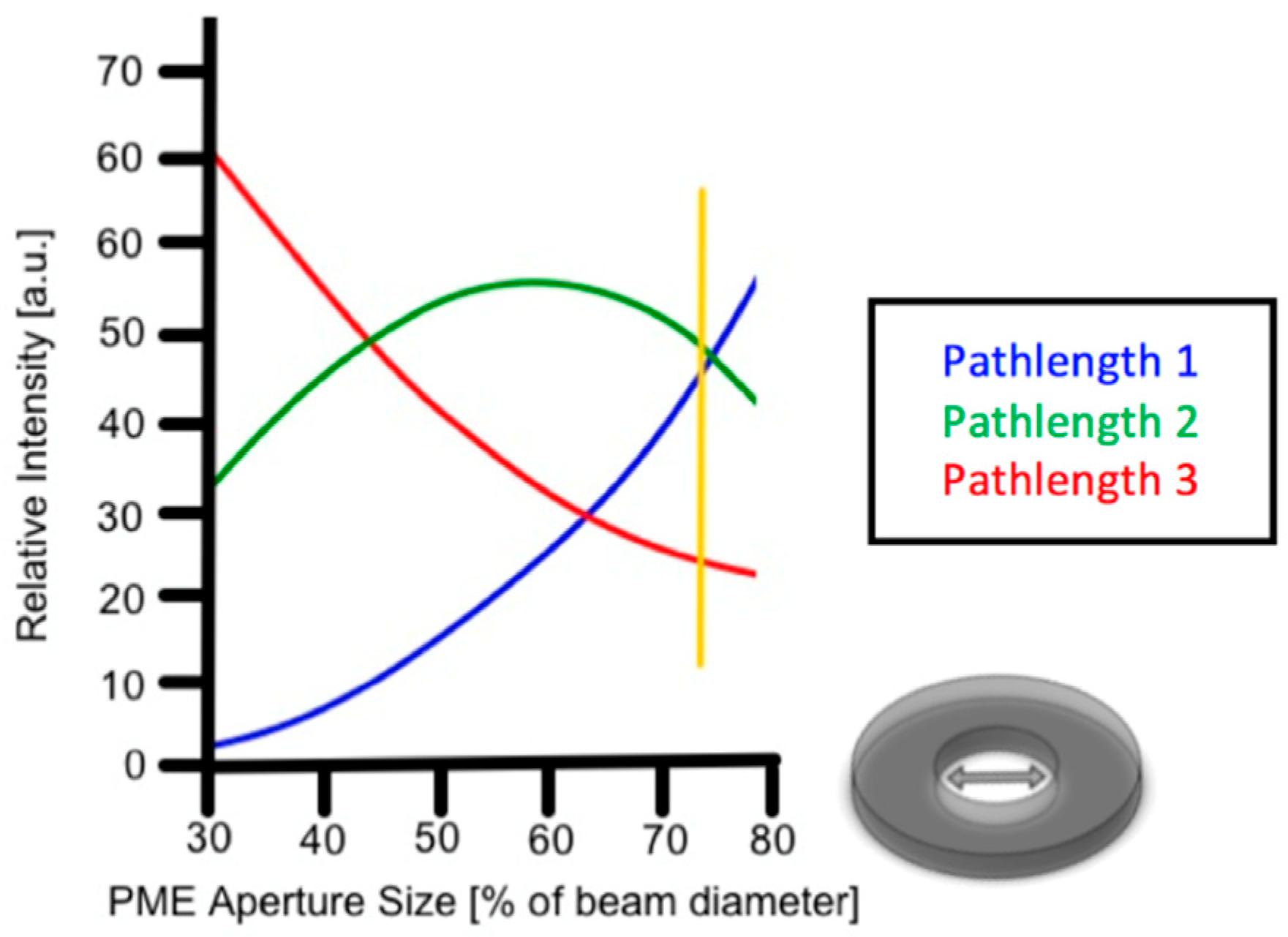
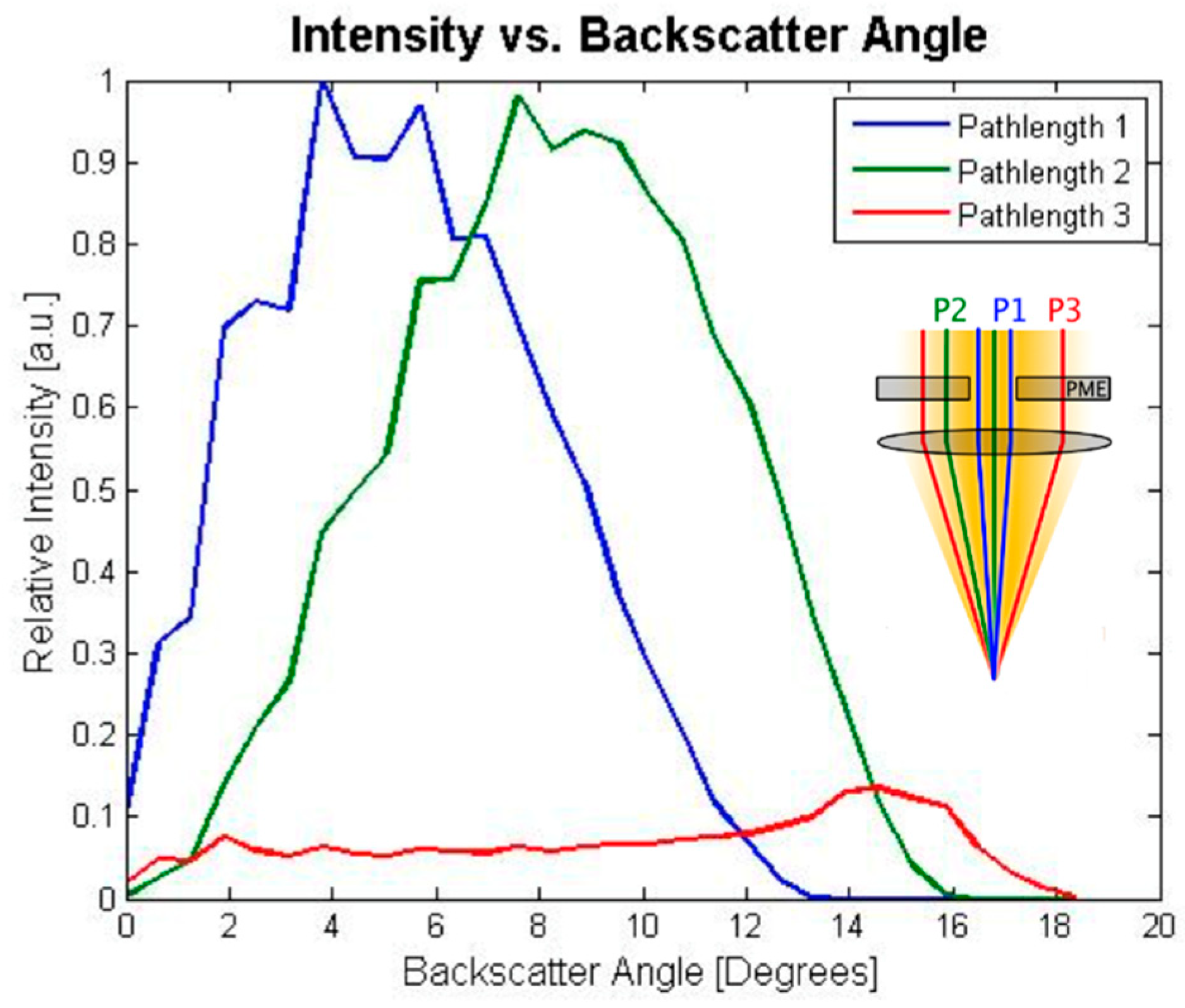
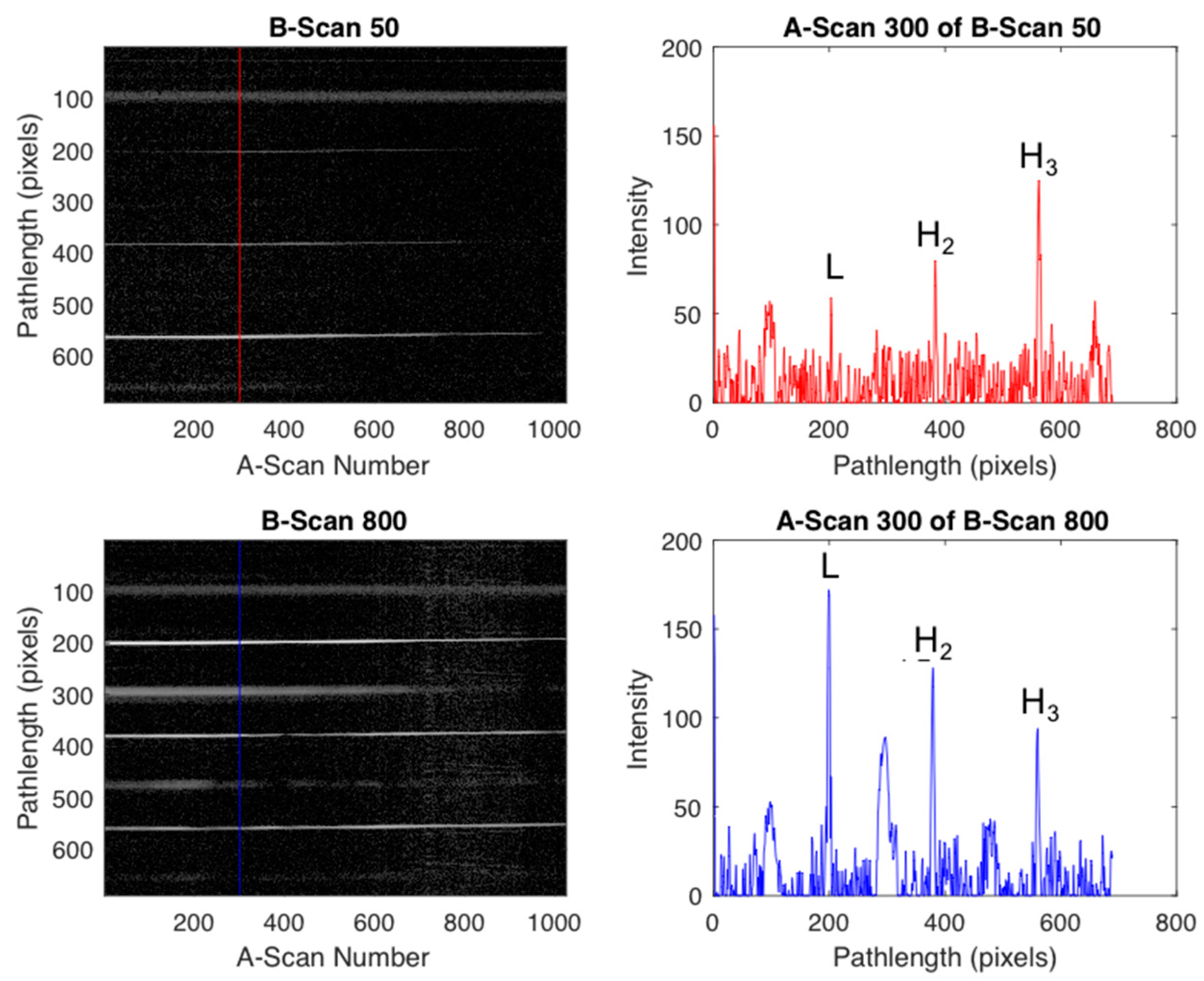
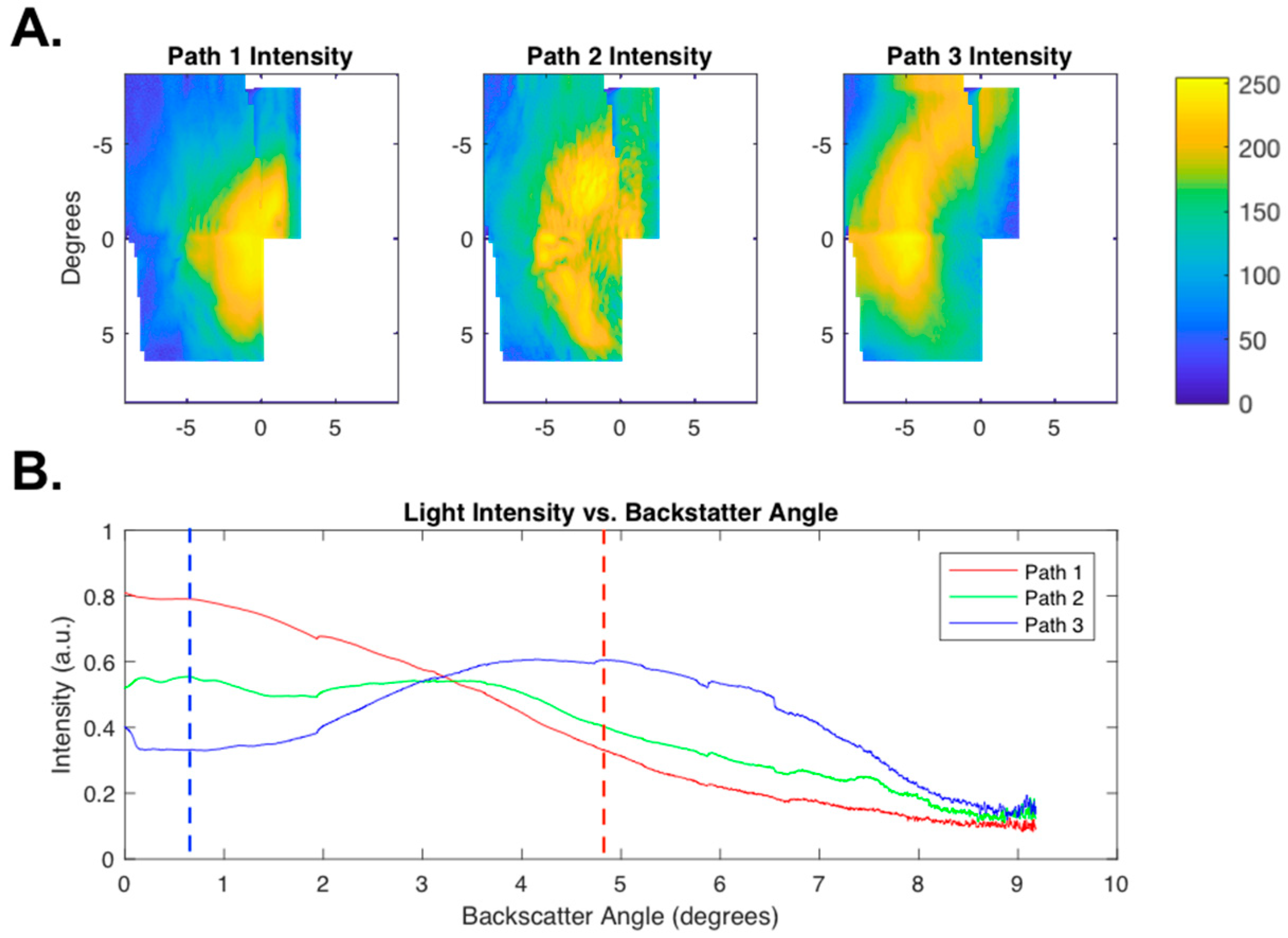
2.3.1. Scattering Angle Characterization
2.3.2. Imaging Protocol
2.4. Image Processing
2.4.1. Initial Processing
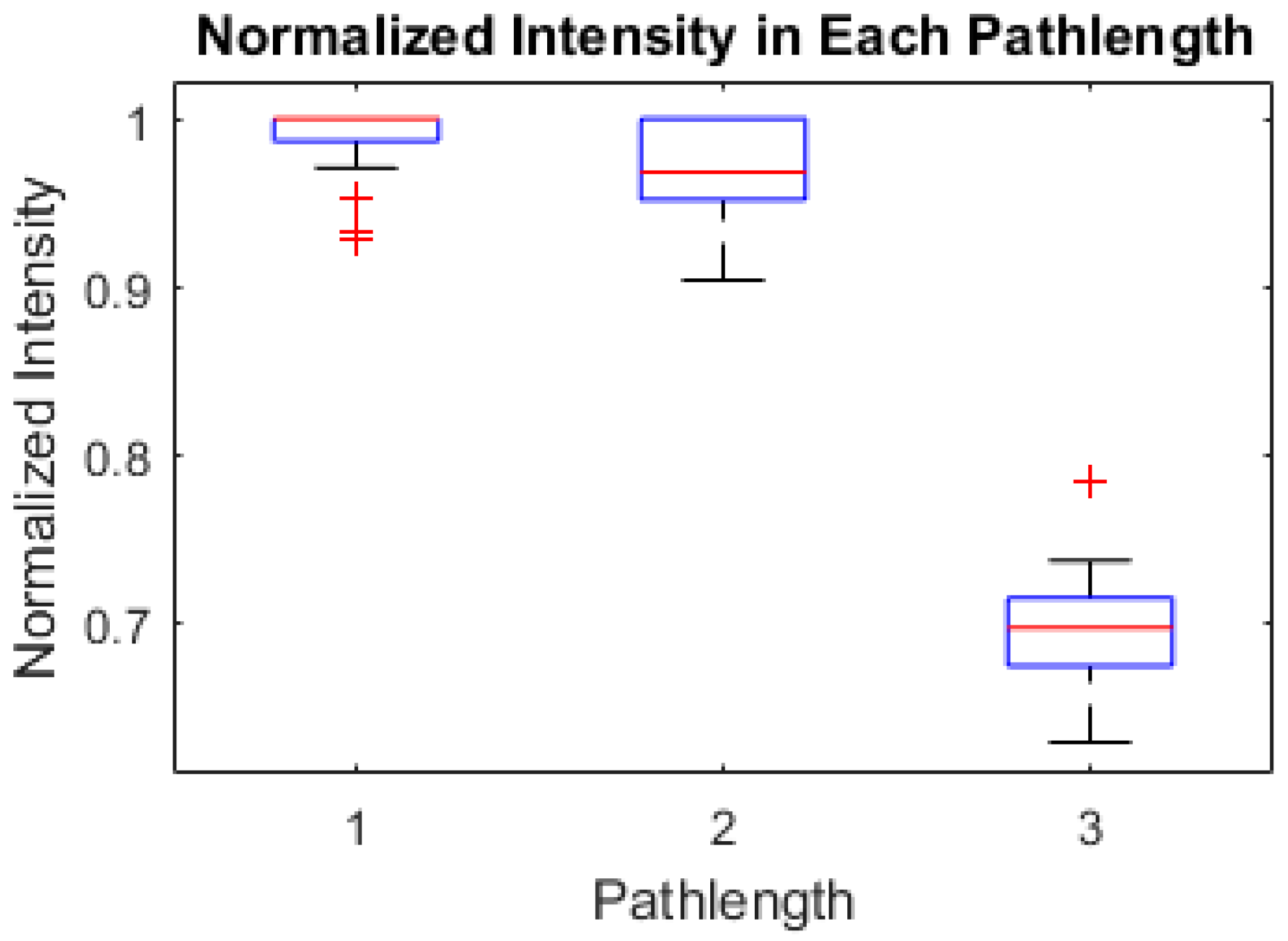
2.4.2. Intensity Processing
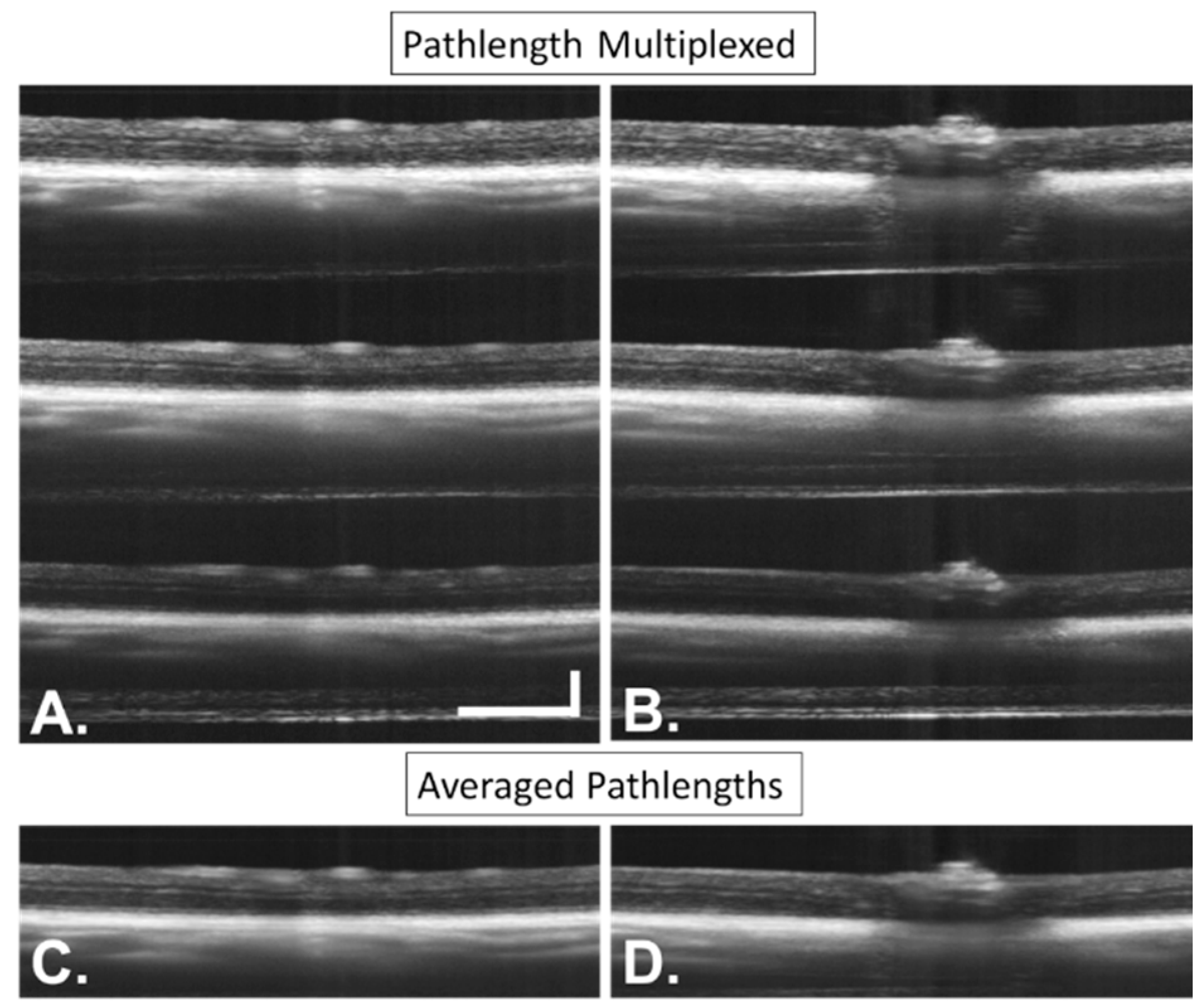
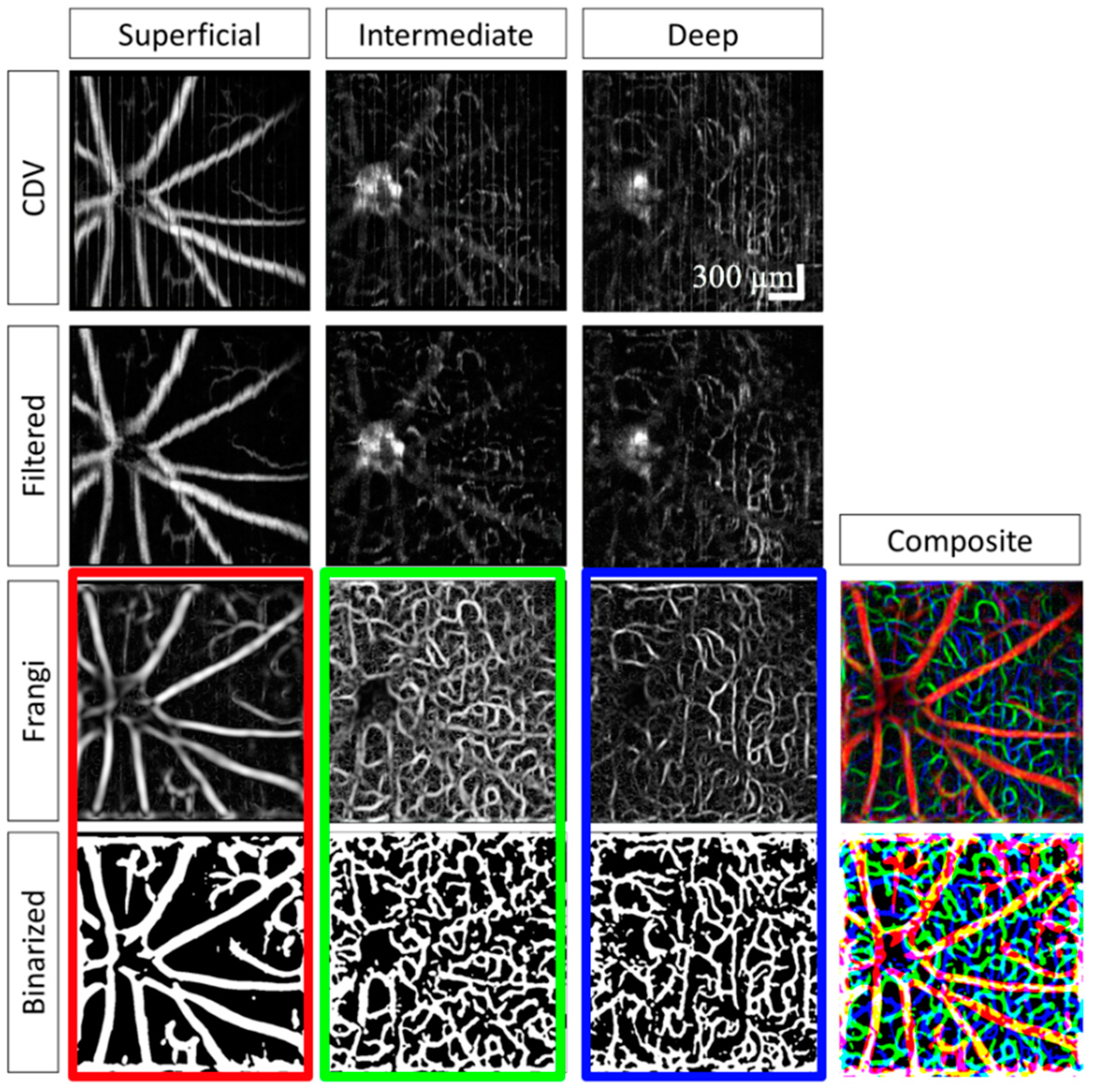
2.4.3. Speckle Processing
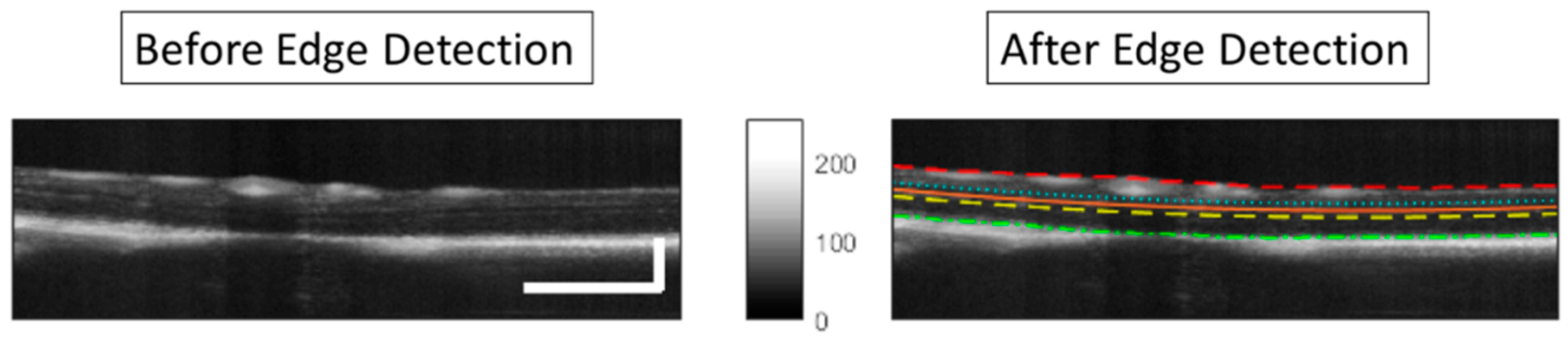
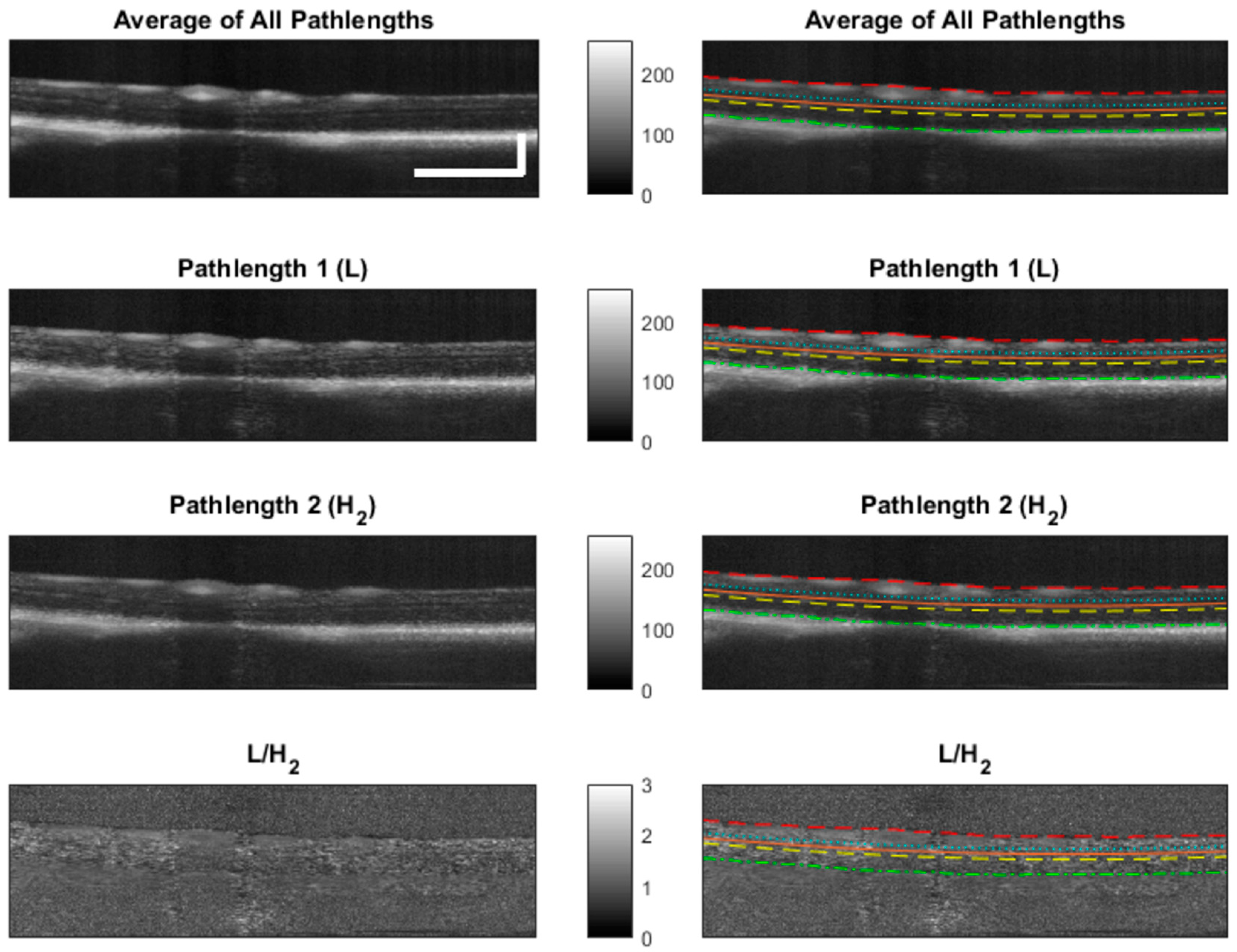
2.4.4. Segmentation
2.4.5. Scattering Angle Feature
3. Results and Discussion
3.1. Mouse Eye Optics
3.2. Scattering Angle Characterization
3.3. Imaging
3.4. Image Processing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, D.; Swanson, E.A.E.A.; Lin, C.P.C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, J.G. Optical Coherence Tomography for Retinal Imaging. Biomed. Photonics Handb. 2003, 4, 402–403. [Google Scholar] [CrossRef]
- Wilson, J.D.; Bigelow, C.E.; Calkins, D.J.; Foster, T.H. Light Scattering from Intact Cells Reports Oxidative-Stress-Induced Mitochondrial Swelling. Biophys. J. 2005, 88, 2929–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boustany, N.N.; Drezek, R.; Thakor, N.V. Calcium-Induced Alterations in Mitochondrial Morphology Quantified in Situ with Optical Scatter Imaging. Biophys. J. 2002, 83, 1691–1700. [Google Scholar] [CrossRef] [Green Version]
- Pasternack, R.M.; Zheng, J.-Y.; Boustany, N.N. Optical scatter changes at the onset of apoptosis are spatially associated with mitochondria. J. Biomed. Opt. 2010, 15, 040504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boustany, N.N.; Tsai, Y.-C.; Pfister, B.; Joiner, W.M.; Oyler, G.A.; Thakor, N.V. BCL-xL-dependent light scattering by apoptotic cells. Biophys. J. 2004, 87, 4163–4171. [Google Scholar] [CrossRef] [PubMed]
- Beauvoit, B.; Evans, S.M.; Jenkins, T.W.; Miller, E.E.; Chance, B. Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors. Anal. Biochem. 1995, 226, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Heflin, S.; Kresty, L.A.; Halling, M.; Perez, L.N.; Ho, D.; Crose, M.; Brown, W.; Farsiu, S.; Arshavsky, V.; et al. Analyzing spatial correlations in tissue using angle-resolved low coherence interferometry measurements guided by co-located optical coherence tomography. Biomed. Opt. Express 2016, 7, 1400–1414. [Google Scholar] [CrossRef] [PubMed]
- Wax, A.; Yang, C.; Backman, V.; Badizadegan, K.; Boone, C.W.; Dasari, R.R.; Feld, M.S. Cellular Organization and Substructure Measured Using Angle-Resolved Low-Coherence Interferometry. Biophys. J. 2002, 82, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Dwelle, J.; Liu, S.; Wang, B.; McElroy, A.; Ho, D.; Markey, M.K.; Milner, T.; Rylander, H.G., 3rd; Grady Rylander, H. Thickness, phase retardation, birefringence, and reflectance of the retinal nerve fiber layer in normal and glaucomatous non-human primates. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4380–4395. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, B.; Yin, B.; Milner, T.E.; Markey, M.K.; McKinnon, S.J.; Rylander, H.G., III. Retinal nerve fiber layer reflectance for early glaucoma diagnosis. J. Glaucoma 2014, 23, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yin, B.; Dwelle, J.; Rylander, H.G.; Markey, M.K.; Milner, T.E. Path-length-multiplexed scattering-angle-diverse optical coherence tomography for retinal imaging. Opt. Lett. 2013, 38, 4374–4377. [Google Scholar] [CrossRef] [PubMed]
- Wartak, A.; Augustin, M.; Haindl, R.; Beer, F.; Salas, M.; Laslandes, M.; Baumann, B.; Pircher, M.; Hitzenberger, C.K. Multi-directional optical coherence tomography for retinal imaging. Biomed. Opt. Express 2017, 8, 5560–5578. [Google Scholar] [CrossRef] [PubMed]
- Wartak, A.; Haindl, R.; Trasischker, W.; Baumann, B.; Pircher, M.; Hitzenberger, C.K. Active-passive path-length encoded (APPLE) Doppler OCT. Biomed. Opt. Express 2016, 7, 5233–5251. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Schery, L.A.; Sharma, R.; Dubra, A.; Ahmad, K.; Libby, R.T.; Williams, D.R. Optical properties of the mouse eye. Biomed. Opt. Express 2011, 2, 717–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remtulla, S.; Hallett, P.E. A schematic eye for the mouse, and comparisons with the rat. Vis. Res. 1985, 25, 21–31. [Google Scholar] [CrossRef]
- Schmucker, C.; Schaeffel, F. A paraxial schematic eye model for the growing C57BL/6 mouse. Vis. Res. 2004, 44, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Conrady, A.E. Applied Optics and Optical Design, Part Two, 1st ed.; Kingslake, R., Ed.; Dover Publications, Inc.: New York, NY, USA, 1960. [Google Scholar]
- Gardner, M.R.; Katta, N.; McElroy, A.; Baruah, V.; Rylander, H.G.; Milner, T.E. Scattering angle resolved optical coherence tomography for in vivo murine retinal imaging. In SPIE BiOS; International Society for Optics and Photonics: San Franscisco, CA, USA, 2017; p. 100531O. [Google Scholar]
- Swanson, E.A.; Izatt, J.A.; Lin, C.P.; Fujimoto, J.G.; Schuman, J.S.; Hee, M.R.; Huang, D.; Puliafito, C.A. In vivo retinal imaging by optical coherence tomography. Opt. Lett. 1993, 18, 1864–1866. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Zevallos, O.; Nankivil, D.; Keller, B.; Viehland, C.; Lujan, B.J.; Izatt, J.A. Pupil tracking optical coherence tomography for precise control of pupil entry position. Biomed. Opt. Express 2015, 6, 3405–3419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Condit, J.C.; McElroy, A.; Kemp, N.J.; Hoshino, K.; Milner, T.E.; Zhang, X. Fast 3D in vivo swept-source optical coherence tomography using a two-axis MEMS scanning micromirror. J. Opt. A Pure Appl. Opt. 2008, 10, 044013. [Google Scholar] [CrossRef]
- Aguirre, A.D.; Herz, P.R.; Chen, Y.; Fujimoto, J.G.; Piyawattanametha, W.; Fan, L.; Wu, M.C. Two-axis MEMS scanning catheter for ultrahigh resolution three-dimensional and en face imaging. Opt. Express 2007, 15, 2445–2453. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.D.; Kraus, M.F.; Potsaid, B.; Liu, J.J.; Choi, W.; Jayaraman, V.; Cable, A.E.; Hornegger, J.; Duker, J.S.; Fujimoto, J.G. Handheld ultrahigh speed swept source optical coherence tomography instrument using a MEMS scanning mirror. Biomed. Opt. Express 2014, 5, 293–311. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, F.; Nankivil, D.; DuBose, T.; Toth, C.A.; Farsiu, S.; Izatt, J.A. In vivo cellular-resolution retinal imaging in infants and children using an ultracompact handheld probe. Nat. Photonics 2016, 10, 580–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Cera, E.G.; Rodríguez, G.; Llorente, L.; Schaeffel, F.; Marcos, S. Optical aberrations in the mouse eye. Vis. Res. 2006, 46, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, T.; Meadway, A.; Wang, X.; Zhang, Y. High-speed adaptive optics for imaging of the living human eye. Opt. Express 2015, 23, 23035–23052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawadzki, R.J.; Jones, S.M.; Olivier, S.S.; Zhao, M.; Bower, B.A.; Izatt, J.A.; Choi, S.; Laut, S.; Werner, J.S. Adaptive-optics optical coherence tomography for high-resolution and high-speed 3D retinal in vivo imaging. Opt. Express 2005, 13, 8532–8546. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Xu, J.; Gradowski, M.A.; Bonora, S.; Zawadzki, R.J.; Sarunic, M.V. Wavefront sensorless adaptive optics optical coherence tomography for in vivo retinal imaging in mice. Biomed. Opt. Express 2014, 5, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Zawadzki, R.J.; Sarunic, M.V. Adaptive optics optical coherence tomography for in vivo mouse retinal imaging. J. Biomed. Opt. 2013, 18, 056007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wang, C.-H.; Dai, C.; Camesa, A.; Zhang, H.F.; Jiao, S. Effect of Contact Lens on Optical Coherence Tomography Imaging of Rodent Retina. Curr. Eye Res. 2013, 38, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Mocci, J.; Wahl, D.J.; Meleppat, R.K.; Manna, S.K.; Quintavalla, M.; Muradore, R.; Sarunic, M.V.; Bonora, S.; Pugh, E.N.; et al. Effect of a contact lens on mouse retinal in vivo imaging: Effective focal length changes and monochromatic aberrations. Exp. Eye Res. 2018, 172, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Dwelle, J.; Wang, B.; Wang, T.; Feldman, M.D.; Rylander, H.G., 3rd; Milner, T.E. Fourier optics analysis of phase-mask-based path-length-multiplexed optical coherence tomography. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2015, 32, 2169–2177. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.; Roggan, A.; Schweitzer, D.; Muller, G. Optical properties of ocular fundus tissues—An in vitro study using the double-integrating-sphere technique and inverse Monte Carlo simulation. Phys. Med. Biol. 1995, 40, 963. [Google Scholar] [CrossRef] [PubMed]
- Nam, A.S.; Chico-Calero, I.; Vakoc, B.J. Complex differential variance algorithm for optical coherence tomography angiography. Biomed. Opt. Express 2014, 5, 3822–3832. [Google Scholar] [CrossRef] [PubMed]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, Cambridge, MA, USA, 11–13 October 1998; Springer: New York, NY, USA, 1998; pp. 130–137. [Google Scholar]
- Pratt, W.K. Digital Image Processing; John Wiley & Sons, Inc.: New York, NY, USA, 1978; ISBN 0-471-01888-0. [Google Scholar]

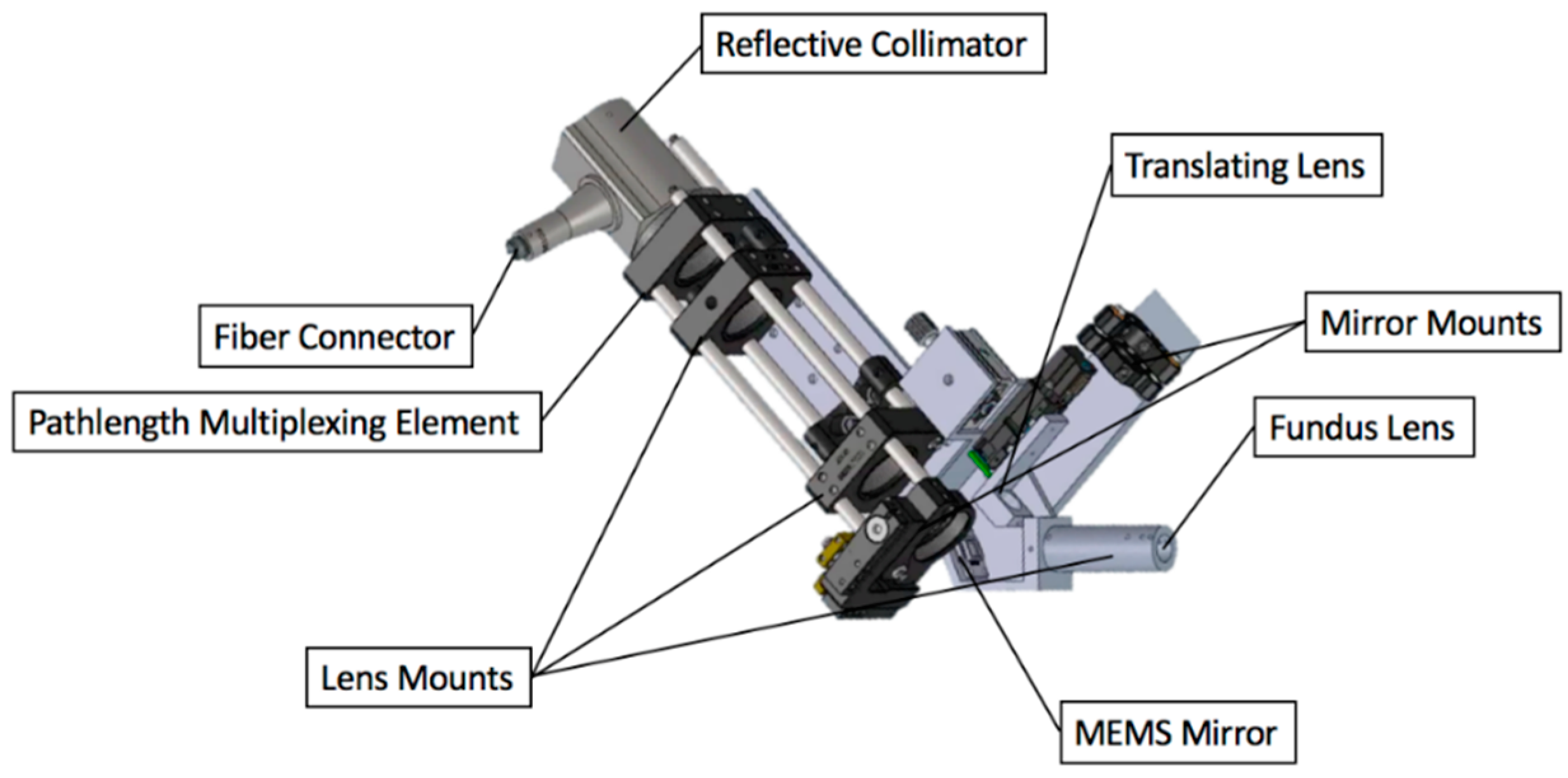
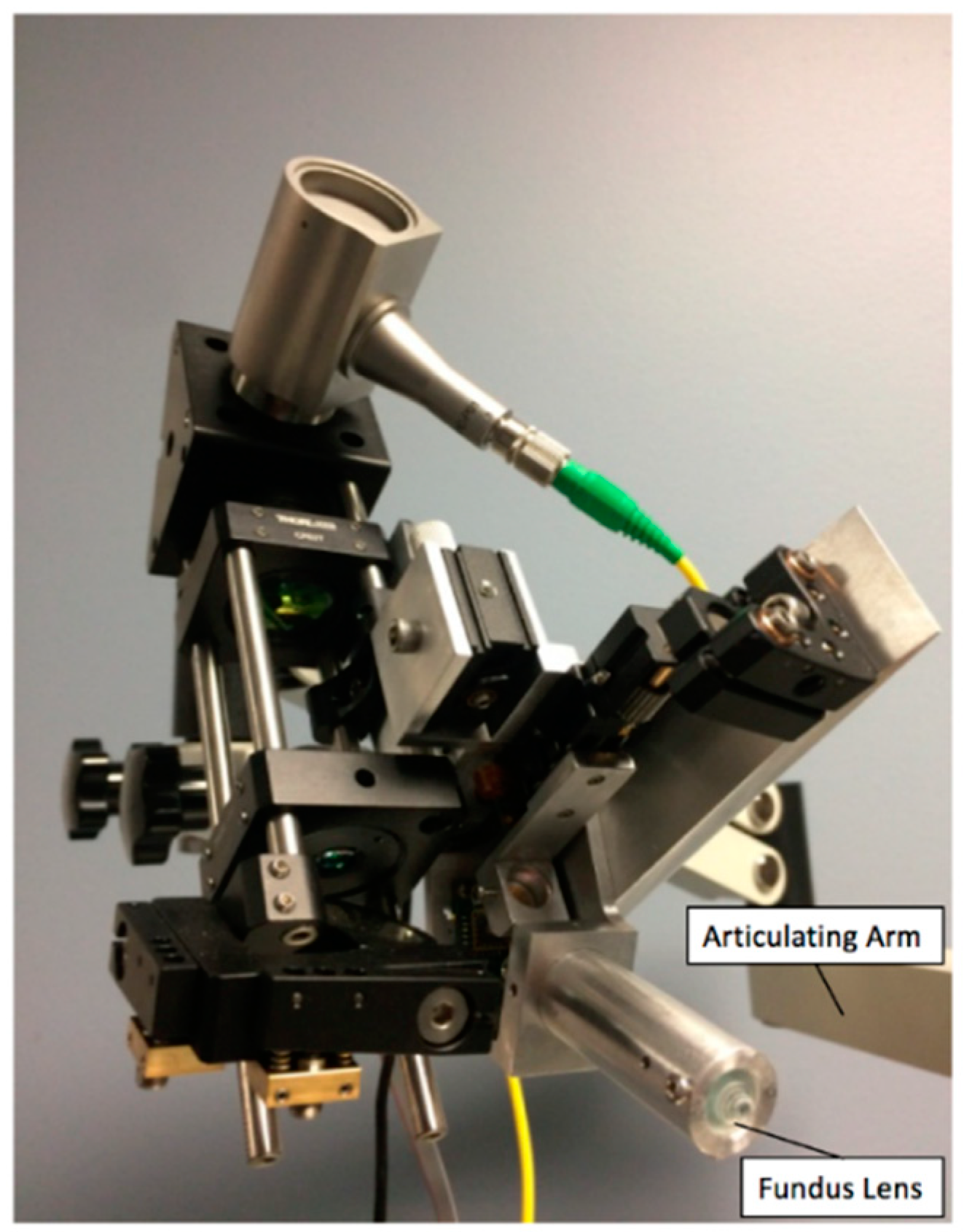
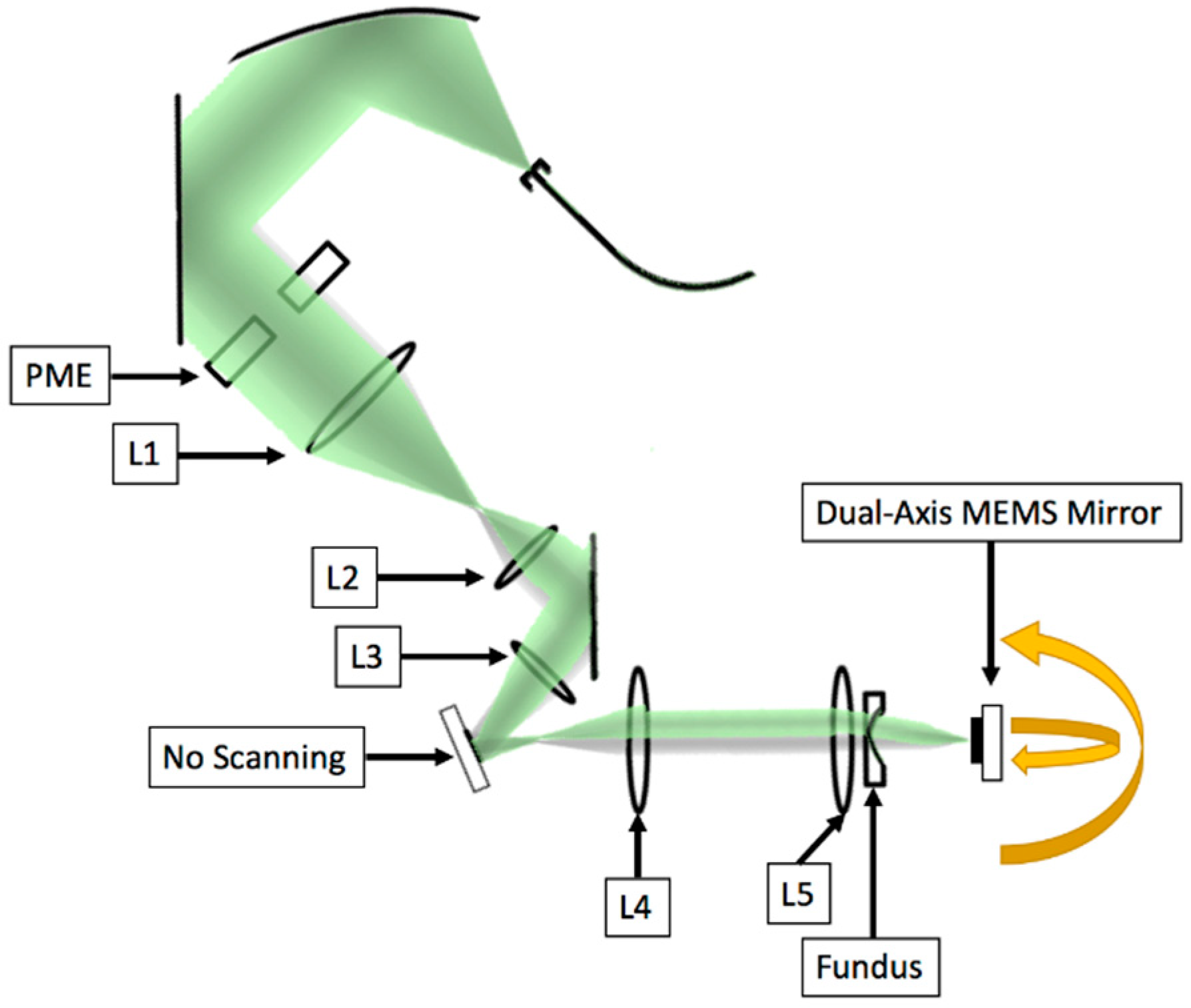
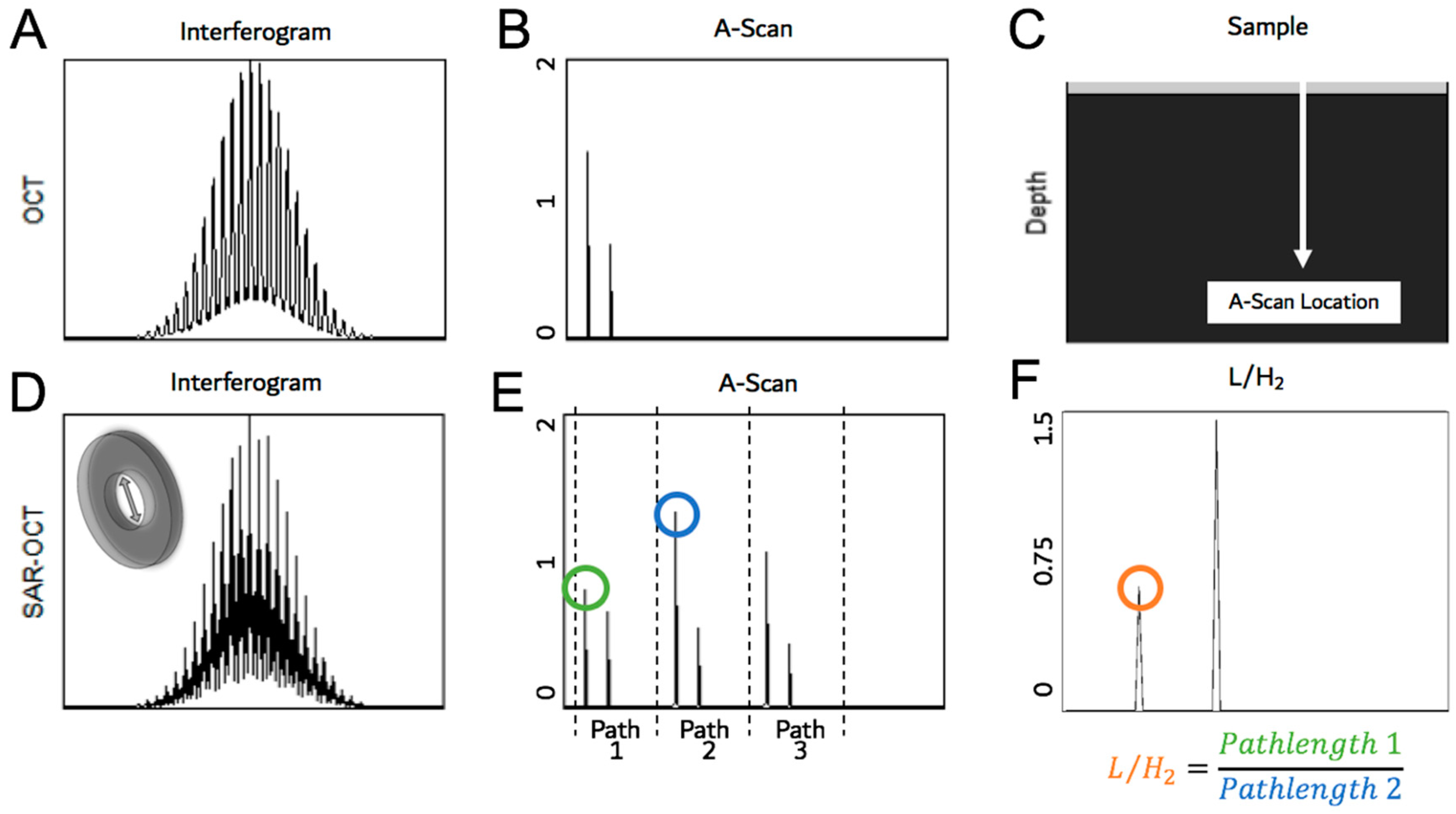

| Measurement Taken after: | Combined 2nd Order Terms after Element (s2) | Individual Contribution (s2) |
|---|---|---|
| Reflective Collimator | 5.40 × 10−31 | 5.40 × 10−31 |
| Lens 1 | 1.77 × 10−28 | 1.76 × 10−28 |
| Lens 2 | 2.61 × 10−28 | 2.61 × 10−28 |
| Lens 3 | 3.24 × 10−28 | 6.33 × 10−29 |
| Lens 4 | 4.21 × 10−28 | 9.65 × 10−29 |
| Lens 5 | 5.04 × 10−28 | 8.34 × 10−29 |
| Fundus Lens | 5.01 × 10−28 | −2.87 × 10−30 |
| Eye Segment | n0 | B | C | n (at 1.31 µm) | RMS Error |
|---|---|---|---|---|---|
| Cornea | 1.399 | −2.177 × 10−3 | 1.260 × 10−3 | 1.398 | 1.674 × 10-4 |
| Aqueous | 1.289 | 3.277 × 10−2 | −1.382 × 10−3 | 1.313 | 1.834 × 10-4 |
| Lens | 1.635 | 2.831 × 10−3 | 4.411 × 10−3 | 1.639 | 2.567 × 10-4 |
| Vitreous | 1.319 | 8.008 × 10−3 | 2.551 × 10−4 | 1.326 | 1.228 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardner, M.R.; Katta, N.; Rahman, A.S.; Rylander, H.G., III; Milner, T.E. Design Considerations for Murine Retinal Imaging Using Scattering Angle Resolved Optical Coherence Tomography. Appl. Sci. 2018, 8, 2159. https://doi.org/10.3390/app8112159
Gardner MR, Katta N, Rahman AS, Rylander HG III, Milner TE. Design Considerations for Murine Retinal Imaging Using Scattering Angle Resolved Optical Coherence Tomography. Applied Sciences. 2018; 8(11):2159. https://doi.org/10.3390/app8112159
Chicago/Turabian StyleGardner, Michael R., Nitesh Katta, Ayesha S. Rahman, Henry G. Rylander, III, and Thomas E. Milner. 2018. "Design Considerations for Murine Retinal Imaging Using Scattering Angle Resolved Optical Coherence Tomography" Applied Sciences 8, no. 11: 2159. https://doi.org/10.3390/app8112159





