Brevinin-2 Drug Family—New Applied Peptide Candidates Against Methicillin-Resistant Staphylococcus aureus and Their Effects on Lys-7 Expression of Innate Immune Pathway DAF-2/DAF-16 in Caenorhabditis elegans
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and C. elegans
2.2. Preparation of Antibacterial Peptides in the Brevinin-2 Family
2.3. Survival Assay of C. elegans
2.4. MIC and MBC Assays Used to Determine Brevinin-2 Family Antibacterial Peptides
2.5. Anti-Infective Screening of the Brevinin-2 Family
2.6. Infective Tharepy of Brevinin-2 Family Peptides
2.7. Isolation of RNA in C. elegans
2.8. Quantitative PCR Analysis of lys-7 in C. elegans
2.9. Western Blot Analysis of lys-7 in C. elegans
2.10. Statistical Analysis
3. Results
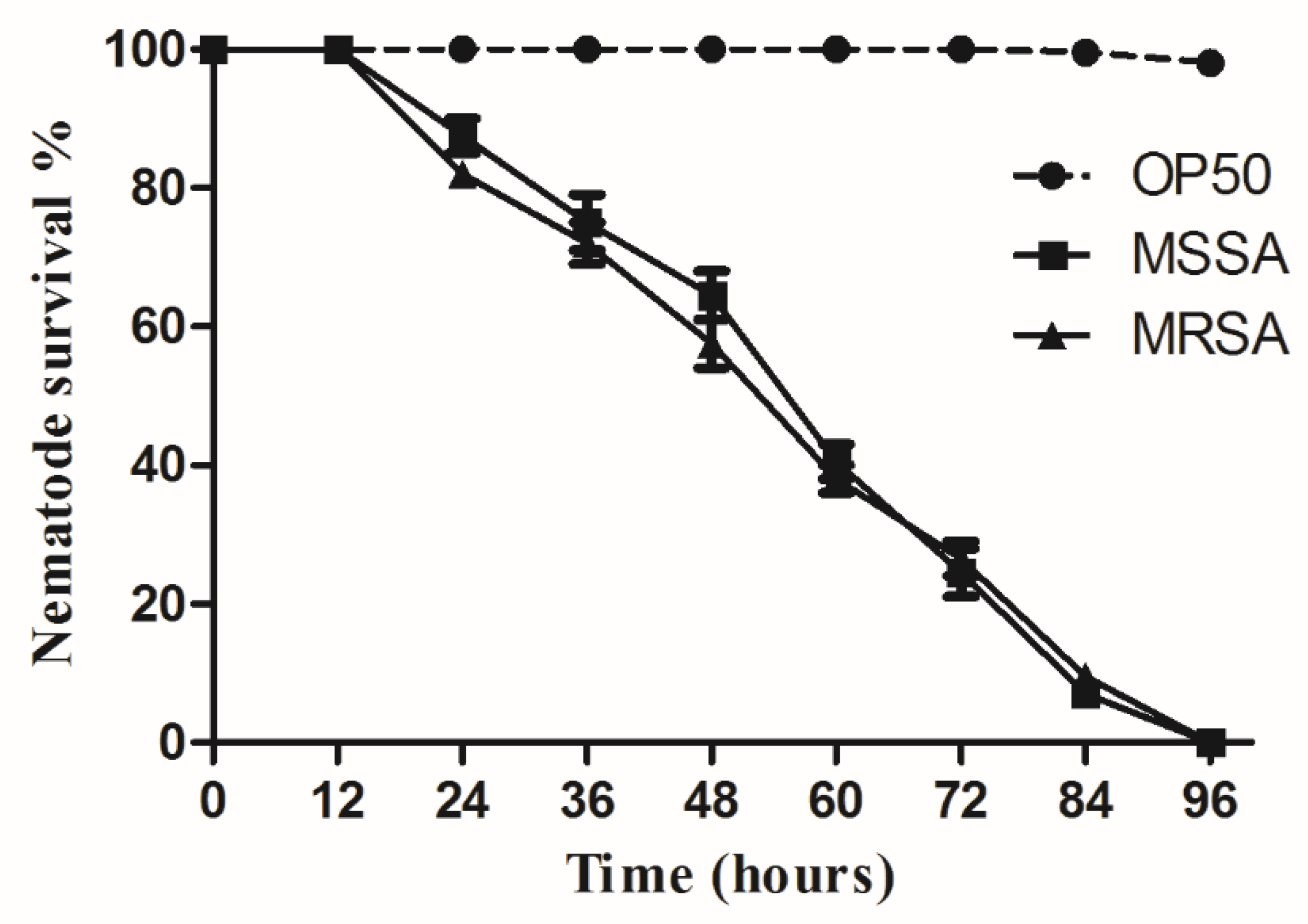
3.1. Assessment of C. elegans Survival Using Liquid-Based Screening
3.2. The MIC and MBC of 13 Peptides Exhibited Different Anti-MRSA Activity
3.3. Anti-Infective Screening of 13 Brevinin-2 Family Peptides against MRSA
3.4. Brevinin-2 Family Peptides Used for Anti-Infective Therapy
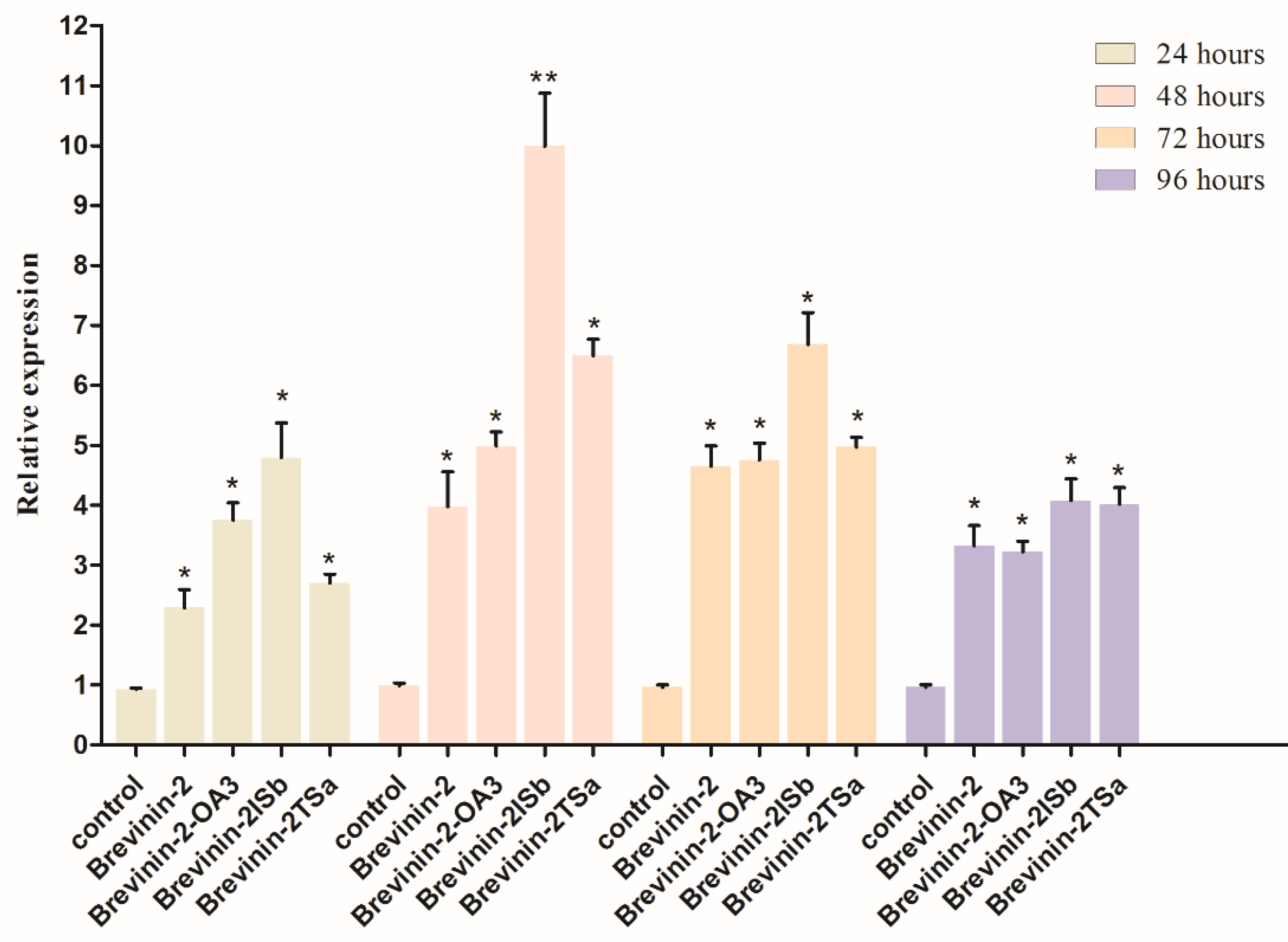
3.5. Confirmation of lys-7 Expression by Real-Time RT-PCR
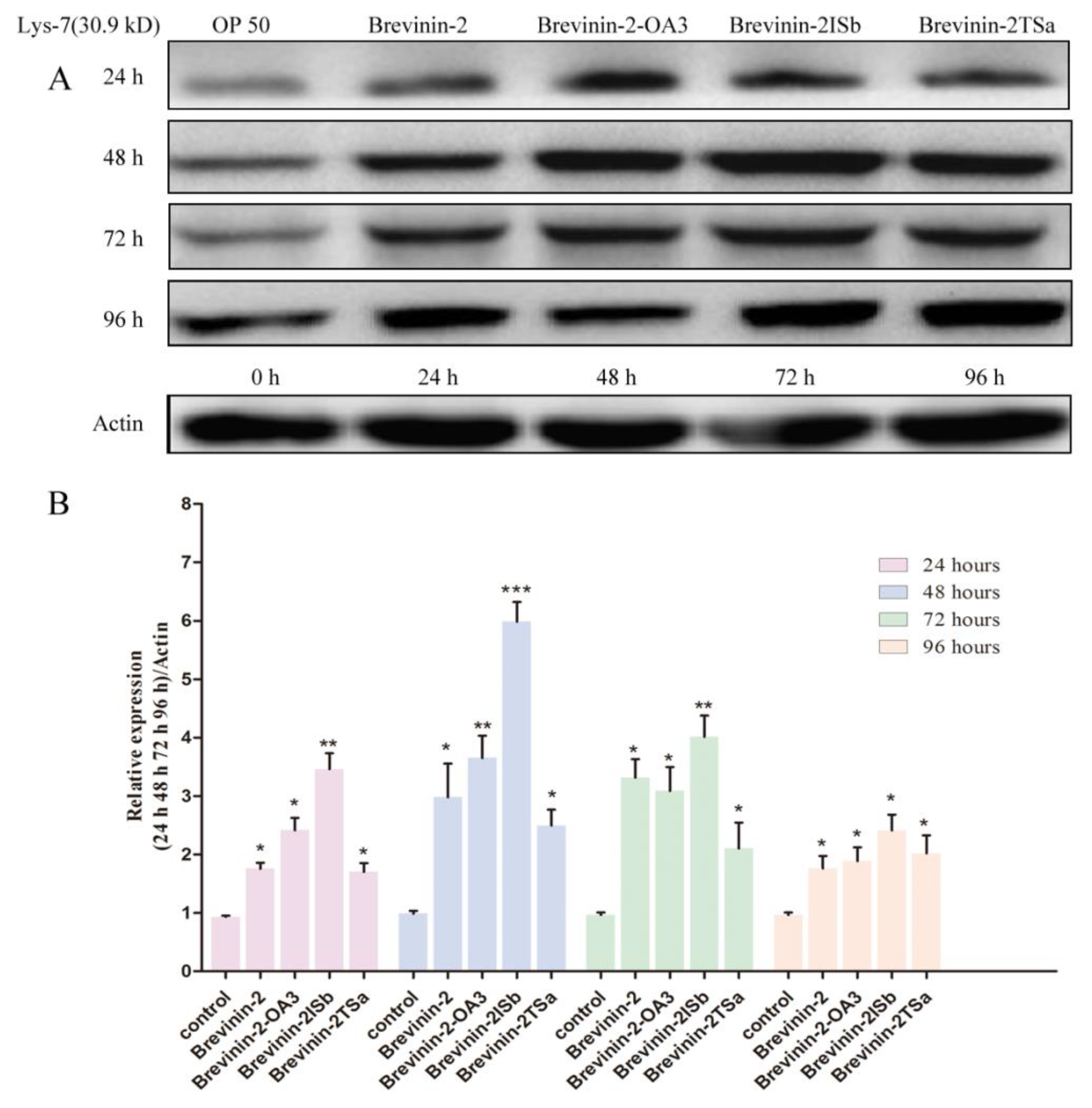
3.6. Confirmation of lys-7 Expression by Western Blot
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, Q.; Xie, H.; Song, H.J.; Nie, F.Y.; Wang, J.H.; Chen, D.; Wang, F. In Vivo Wound Healing Activity of Abrus cantoniensis Extract. Evid. Based Complement. Altern. Med. 2016, 2016, 6568528. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.R.; Wang, J.; Wang, K.Y.; Chen, D.F.; Dong, X.W.; Liu, T.; Zeng, Y.K.; Wang, X.L.; Wu, D.M. Expression, Purification and Antibacterial Activity of NK-Lysin Mature Peptides from the Channel Catfish (Ictalurus punctatus). Appl. Sci. 2016, 6, 240. [Google Scholar] [CrossRef]
- Plowman, R.; Graves, N.; Griffin, M.A.S.; Roberts, J.A.; Swan, A.V.; Cookson, B.; Taylor, L. The rate and cost of hospital-acquired infections occurring in patients admitted to selected specialties of a district general hospital in England and the national burden imposed. J. Hosp. Infect. 2001, 47, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Mellbye, B.; Schuster, M. The Sociomicrobiology of Antivirulence Drug Resistance: A Proof of Concept. mBio 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Yehye, W.A.; Abd Rahman, N.; Tan, M.W.; Nathan, S. Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement. Altern. Med. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.S.; Ali, M.A. Novel Pyrazolo[3,4-b]pyridine Derivatives: Synthesis, Characterization, Antimicrobial and Antiproliferative Profile. Biol. Pharm. Bull. 2016, 39, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niwa, T.; Watanabe, T.; Goto, T.; Ohta, H.; Nakayama, A.; Suzuki, K.; Shinoda, Y.; Tsuchiya, M.; Yasuda, K.; Murakami, N.; et al. Daily Review of Antimicrobial Use Facilitates the Early Optimization of Antimicrobial Therapy and Improves Clinical Outcomes of Patients with Bloodstream Infections. Biol. Pharm. Bull. 2016, 39, 721–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickelgren, I. As the worm ages: Epilepsy drugs lengthen nematode life span. Science 2005, 307, 193. [Google Scholar] [CrossRef] [PubMed]
- Garsin, D.A.; Villanueva, J.M.; Begun, J.; Kim, D.H.; Sifri, C.D.; Calderwood, S.B.; Ruvkun, G.; Ausubel, F.M. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science 2003, 300, 1921. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.S.; Narasimhan, S.D.; Yen, K.; Tissenbaum, H.A. A new DAF-16 isoform regulates longevity. Nature 2010, 466, 498–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla, P.A.; Garcia, A.M.; Ladage, M.L.; Toni, L.S. Caenorhabditis elegans: An Old Genetic Model Can Learn New Epigenetic Tricks. Integr. Comp. Biol. 2014, 54, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Pukkila-Worley, R.; Feinbaum, R.; Kirienko, N.V.; Larkins-Ford, J.; Conery, A.L.; Ausubel, F.M. Stimulation of Host Immune Defenses by a Small Molecule Protects C. elegans from Bacterial Infection. PLoS Genet. 2012, 8, e1002733. [Google Scholar] [CrossRef] [PubMed]
- Shivers, R.P.; Youngman, M.J.; Kim, D.H. Transcriptional responses to pathogens in Caenorhabditis elegans. Curr. Opin. Microbiol. 2008, 11, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Dosanjh, L.; Lao, L.X.; Tan, M.; Shim, B.S.; Luo, Y. Cinnamomum cassia Bark in Two Herbal Formulas Increases Life Span in Caenorhabditis elegans via Insulin Signaling and Stress Response Pathways. PLoS ONE 2010, 5, e9339. [Google Scholar] [CrossRef] [PubMed]
- Orosz, L.; Papanicolaou, E.G.; Seprenyi, G.; Megyeri, K. IL-17A and IL-17F induce autophagy in RAW 264.7 macrophages. Biomed. Pharmacother. 2016, 77, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Tan, M.W.; Nathan, S. Orthosiphon stamineus protects Caenorhabditis elegans against Staphylococcus aureus infection through immunomodulation. Biol. Open 2014, 3, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Marudhupandiyan, S.; Balamurugan, K. Intrinsic JNK-MAPK pathway involvement requires daf-16-mediated immune response during Shigella flexneri infection in C. elegans. Immunol. Res. 2017, 65, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Hung, T.M.; Huang, H.C.; Lee, I.J.; Chang, C.C.; Cheng, J.J.; Lin, L.C.; Huang, C. Bai-Hu-Jia-Ren-Shen-Tang Decoction Reduces Fatty Liver by Activating AMP-Activated Protein Kinase In Vitro and In Vivo. Evid. Based Complement. Altern. Med. 2015, 2015, 651734. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ma, C.; Zhou, M.; Wang, L.; Li, R.; Chen, T.; Shaw, C.; Li, W. Identification and bioactivity evaluation of a novel bradykinin inhibitory peptide from the skin secretion of Chinese large odorous frog, Odorrana livida. J. Pept. Sci. 2016, 22, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Q.; Li, Z.; Zhang, Y.; Zhao, J.; Wang, L. Molecular Cloning, Expression, Purification, and Functional Characterization of Palustrin-2CE, an Antimicrobial Peptide of Rana chensinensis. Biosci. Biotechnol. Biochem. 2012, 76, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morikawa, N.; Hagiwara, K.; Nakajima, T. Brevinin-1 and -2, unique antimicrobial peptides from the skin of the frog, Rana brevipoda porsa. Biochem. Biophys. Res. Commun. 1992, 189, 184–190. [Google Scholar] [CrossRef]
- Wang, G.S.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Power, G.J.; Abdel-Wahab, Y.H.; Flatt, P.R.; Jiansheng, H.; Coquet, L.; Leprince, J.; Jouenne, T.; Vaudry, H. A potent, non-toxic insulin-releasing peptide isolated from an extract of the skin of the Asian frog, Hylarana guntheri (Anura:Ranidae). Regul. Pept. 2008, 151, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Di Grazia, A.; Cappiello, F.; Imanishi, A.; Mastrofrancesco, A.; Picardo, M.; Paus, R.; Mangoni, M.L. The Frog Skin-Derived Antimicrobial Peptide Esculentin-1a(1-21)NH2 Promotes the Migration of Human HaCaT Keratinocytes in an EGF Receptor-Dependent Manner: A Novel Promoter of Human Skin Wound Healing? PLoS ONE 2015, 10, e0128663. [Google Scholar] [CrossRef] [PubMed]
- Van der Meijden, B.; Robinson, J.A. Synthesis of a polymyxin derivative for photolabeling studies in the gram-negative bacterium Escherichia coli. J. Pept. Sci. 2015, 21, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, Y.; Li, Z.; Su, Q. Molecular cloning of novel antimicrobial peptide genes from the skin of the Chinese brown frog, Rana chensinensis. Zool. Sci. 2011, 28, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, A.N.; Liu, Y.Q.; Wang, T.F.; Musgrave, I.F.; Pukala, T.L.; Tabor, R.F.; Martin, L.L.; Carver, J.A.; Bowie, J.H. The Amyloid Fibril-Forming Properties of the Amphibian Antimicrobial Peptide Uperin3.5. ChemBioChem 2016, 17, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.M.; Woodhams, D.; Howard, C.; LaFonte, B.E.; Gregory, J.R.; Johnson, P.T.J. Role of Antimicrobial Peptides in Amphibian Defense Against Trematode Infection. EcoHealth 2016, 13, 383–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, M.; Bauer, M.; Neubauer, D.; Kamysz, W.; Dawgul, M. Influence of Amphibian Antimicrobial Peptides and Short Lipopeptides on Bacterial Biofilms Formed on Contact Lenses. Materials 2016, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Hou, X.; Wang, L.; Gao, Y.; Wu, D.; Xi, X.; Zhou, M.; Kwok, H.F.; Duan, J.; Chen, T.; et al. Two Novel Dermaseptin-Like Antimicrobial Peptides with Anticancer Activities from the Skin Secretion of Pachymedusa dacnicolor. Toxins 2016, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Lee, W.H.; Yang, X.W.; Zhang, Y. Novel Peptides from Skins of Amphibians Showed Broad-Spectrum Antimicrobial Activities. Chem. Biol. Drug Des. 2016, 87, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Irazoqui, J.E.; Urbach, J.M.; Ausubel, F.M. Evolution of host innate defence: Insights from Caenorhabditis elegans and primitive invertebrates. Nat. Rev. Immunol. 2010, 10, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.S.; Maki, T.; Shah, M.M.; Ichinose, Y. Synthesis and Antimicrobial Activity of Nitrobenzyl-oxy-phenol Derivatives. Biol. Pharm. Bull. 2016, 39, 1888–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Riveros, M.; De-la-Pinta, I.; Marcos-Arias, C.; Ezpeleta, G.; Quindos, G.; Eraso, E. Usefulness of the Non-conventional Caenorhabditis elegans Model to Assess Candida Virulence. Mycopathologia 2017, 182, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Tabara, H.; Hill, R.J.; Mello, C.C.; Priess, J.R.; Kohara, Y. Pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans. Development 1999, 126, 1–11. [Google Scholar] [PubMed]
- Giacomotto, J.; Segalat, L.; Carre-Pierrat, M.; Gieseler, K. Caenorhabditis elegans as a chemical screening tool for the study of neuromuscular disorders. Manual and semi-automated methods. Methods 2012, 56, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, S.; Yi, M.; Wu, X.; Ding, Y. MIC as an appropriate method to construct the brain functional network. BioMed Res. Int. 2015, 2015, 825136. [Google Scholar] [CrossRef] [PubMed]
- Gowrishankar, S.; Sivaranjani, M.; Kamaladevi, A.; Ravi, A.V.; Balamurugan, K.; Karutha Pandian, S. Cyclic dipeptide cyclo(l-leucyl-l-prolyl) from marine Bacillus amyloliquefaciens mitigates biofilm formation and virulence in Listeria monocytogenes. Pathog. Dis. 2016, 74, ftw017. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.S.; Freitas, T.S.; Cruz, R.P.D.; Costa, M.D.S.; Pereira, R.L.S.; Quintans-Junior, L.J.; Andrade, T.A.; Menezes, P.D.P.; Sousa, B.M.H.; Nunes, P.S.; et al. Evaluation of the antibacterial and modulatory potential of α-bisabolol, β-cyclodextrin and α-bisabolol/β-cyclodextrin complex. Biomed. Pharmacother. 2017, 92, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.A.; Cheng, H.; Willeford, K.O.; Wu, S. Interleukin-6 expression in response to innate immune regulatory factor stimulation. Biomed. Pharmacother. 2011, 65, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Peng, D.; Cheng, C.; Zhou, W.; Ju, S.; Wan, D.; Yu, Z.; Shi, J.; Deng, Y.; Wang, F.; et al. Bacillus thuringiensis Crystal Protein Cry6Aa Triggers Caenorhabditis elegans Necrosis Pathway Mediated by Aspartic Protease (ASP-1). PLoS Pathog. 2016, 12, e1005389. [Google Scholar] [CrossRef] [PubMed]
- Moy, T.I.; Conery, A.L.; Larkins-Ford, J.; Wu, G.; Mazitschek, R.; Casadei, G.; Lewis, K.; Carpenter, A.E.; Ausubel, F.M. High-Throughput Screen for Novel Antimicrobials using a Whole Animal Infection Model. ACS Chem. Biol. 2009, 4, 527–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cespedes, G.F.; Lorenzon, E.N.; Vicente, E.F.; Mendes-Giannini, M.J.; Fontes, W.; Castro, M.S.; Cilli, E.M. Mechanism of action and relationship between structure and biological activity of Ctx-Ha: A new ceratotoxin-like peptide from Hypsiboas albopunctatus. Protein Pept. Lett. 2012, 19, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Bhunia, A. Editorial: Antimicrobial Peptides in Medicinal Chemistry: Advances and Applications. Curr. Top. Med. Chem. 2016, 16, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Biragyn, A.; Kwak, L.W.; Oppenheim, J.J. Mammalian defensins in immunity: More than just microbicidal. Trends Immunol. 2002, 23, 291–296. [Google Scholar] [CrossRef]
- Davidson, D.J.; Currie, A.J.; Reid, G.S.; Bowdish, D.M.; MacDonald, K.L.; Ma, R.C.; Hancock, R.E.; Speert, D.P. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J. Immunol. 2004, 172, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Shagaghi, N.; Palombo, E.A.; Clayton, A.H.; Bhave, M. Archetypal tryptophan-rich antimicrobial peptides: Properties and applications. World J. Microbiol. Biotechnol. 2016, 32, 31. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Powers, J.P.; Straus, S.K.; Hancock, R.E. Lipid-specific binding of the calcium-dependent antibiotic daptomycin leads to changes in lipid polymorphism of model membranes. Chem. Phys. Lipids 2008, 154, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Zhuang, Z.J.; Lin, C.Y.; Chen, W.J. Novel antimicrobial peptides with promising activity against multidrug resistant Salmonella enterica serovar Choleraesuis and its stress response mechanism. J. Appl. Microbiol. 2016, 121, 952–965. [Google Scholar] [CrossRef] [PubMed]
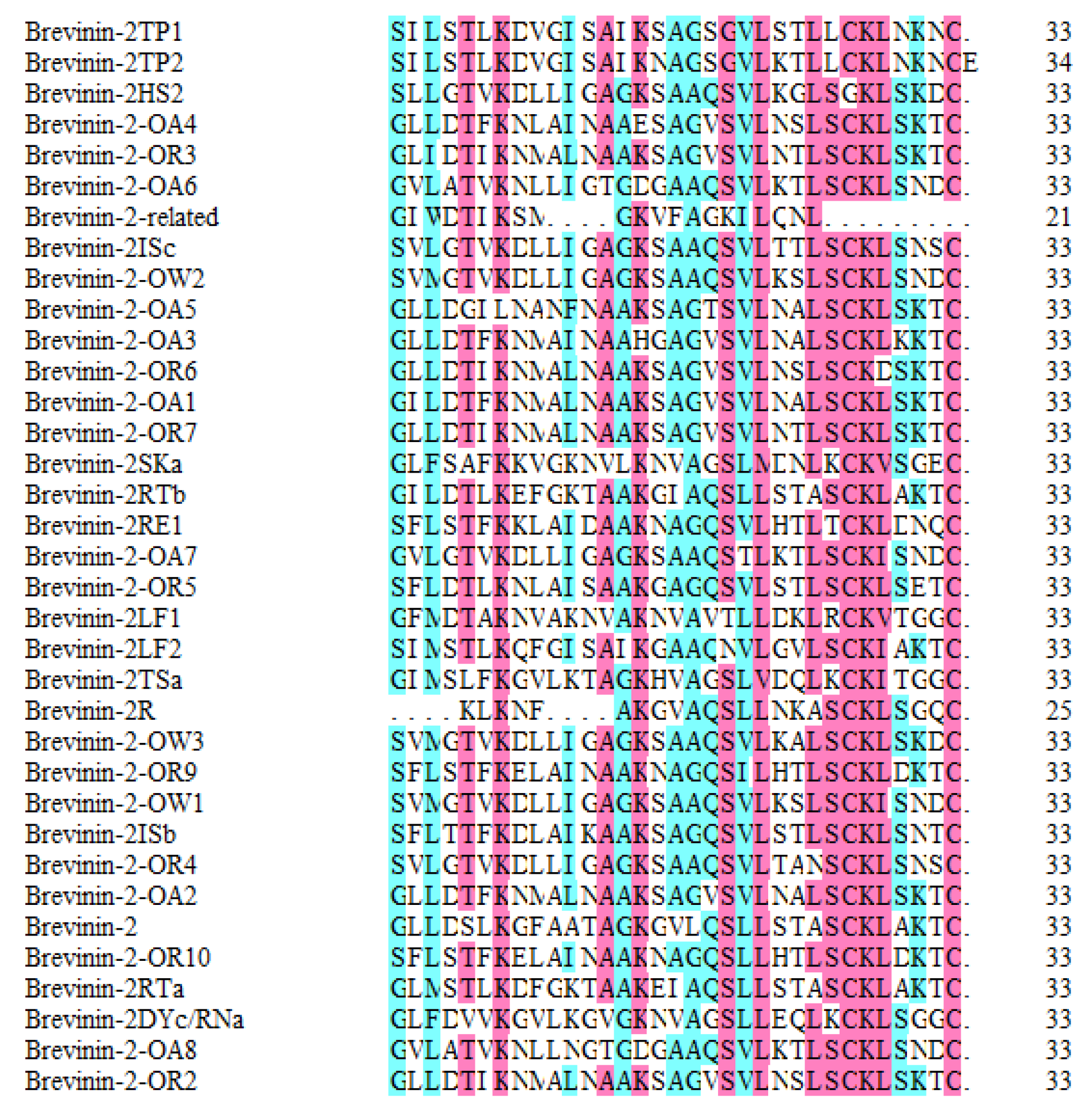


| No. | Name | ADP No. | Peptide Sequences | MIC on APD |
|---|---|---|---|---|
| 1. | Brevinin-2 | AP00075 | GLLDSLKGFAATAGKGVLQSLLSTASCKLAKTC | S. aureus (8 μM) |
| 2. | Brevinin-2DYc/RNa | AP00566 | GLFDVVKGVLKGVGKNVAGSLLEQLKCKLSGGC | S. aureus (7.5 μM) |
| 3. | Brevinin-2HS2 | AP00881 | SLLGTVKDLLIGAGKSAAQSVLKGLSGKLSKDC | S. aureus (19 μM) |
| 4. | Brevinin-2ISb | AP01708 | SFLTTFKDLAIKAAKSAGQSVLSTLSCKLSNTC | S. aureus (6.3–25 μM) |
| 5. | Brevinin-2LF2 | AP02476 | SIMSTLKQFGISAIKGAAQNVLGVLSCKIAKTC | S. aureus (25 μM) |
| 6. | Brevinin-2-OA3 | AP01836 | GLLDTFKNMAINAAHGAGVSVLNALSCKLKKTC | S. aureus (3–13 μM) |
| 7. | Brevinin-2-OR5 | AP01846 | SFLDTLKNLAISAAKGAGQSVLSTLSCKLSETC | S. aureus (6.6–13.2 μM) |
| 8. | Brevinin-2-OW2 | AP01853 | SVMGTVKDLLIGAGKSAAQSVLKSLSCKLSNDC | S. aureus (3.3–6.6 μM) |
| 9. | Brevinin-2RE1 | AP02612 | SFLSTFKKLAIDAAKNAGQSVLHTLTCKLDNQC | S. aureus (20–50 μM) |
| 10. | Brevinin-2-related peptide | AP00599 | GIWDTIKSMGKVFAGKILQNL | S. aureus (25 μM) |
| 11. | Brevinin-2SKa | AP01928 | GLFSAFKKVGKNVLKNVAGSLMDNLKCKVSGEC | S. aureus (50 μM) |
| 12. | Brevinin-2TP1 | AP02465 | SILSTLKDVGISAIKSAGSGVLSTLLCKLNKNC | S. aureus (100 μM) |
| 13. | Brevinin-2TSa | AP00587 | GIMSLFKGVLKTAGKHVAGSLVDQLKCKITGGC | MRSA (≤25 μM) |
| Name | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| lys-7 | TTGTTGACTCATCCCTTCC | TGTCCTGCTGGGTTGTAT |
| actin | AAGACCACGTCATCAAGG | TTCTCCATATCATCCCAGTT |
| No | Name | MIC on APD (S. aureus) | MIC (MRSA) | MBC (MRSA) |
|---|---|---|---|---|
| 1. | Brevinin-2 | 8 μM | 9.6 ± 1.3 μM | 23.4 ± 4.2 μM |
| 2. | Brevinin-2DYc/RNa | 37.5 μM | 32.3 ± 2.7 μM | 82.5 ± 12.4 μM |
| 3. | Brevinin-2HS2 | 19 μM | 21.5 ± 1.1 μM | 39.7 ± 5.7 μM |
| 4. | Brevinin-2ISb | 6.3–25 μM | 8.7 ± 0.9 μM | 33.8 ± 5.4 μM |
| 5. | Brevinin-2LF2 | 25 μM | 23.4 ± 2.1 μM | 61.5 ± 12.6 μM |
| 6. | Brevinin-2-OA3 | 3–13 μM | 6.7 ± 0.7 μM | 31.5 ± 6.2 μM |
| 7. | Brevinin-2-OR5 | 6.6–13.2 μM | 8.6 ± 1.6 μM | 43.3 ± 8.6 μM |
| 8. | Brevinin-2-OW2 | 3.3–6.6 μM | 4.9 ± 1.2 μM | 28.7 ± 3.9 μM |
| 9. | Brevinin-2RE1 | 20–50 μM | 35.9 ± 4.4 μM | 103.8 ± 14.6 μM |
| 10. | Brevinin-2-related peptide | 25 μM | 27.8 ± 2.6 μM | 58.6 ± 13.4 μM |
| 11. | Brevinin-2SKa | 50 μM | 62.3 ± 6.5 μM | 196.3 ± 26.6 μM |
| 12. | Brevinin-2TP1 | 100 μM | 89.7 ± 11.7 μM | 345.7 ± 64.3 μM |
| 13. | Brevinin-2TSa | ≥25 μM (MRSA) | 31.2 ± 6.1 μM | 78.4 ± 12.5 μM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Zhan, Y.; Chen, X.; Zeng, Q.; Chen, D.; Liang, J. Brevinin-2 Drug Family—New Applied Peptide Candidates Against Methicillin-Resistant Staphylococcus aureus and Their Effects on Lys-7 Expression of Innate Immune Pathway DAF-2/DAF-16 in Caenorhabditis elegans. Appl. Sci. 2018, 8, 2627. https://doi.org/10.3390/app8122627
Xie H, Zhan Y, Chen X, Zeng Q, Chen D, Liang J. Brevinin-2 Drug Family—New Applied Peptide Candidates Against Methicillin-Resistant Staphylococcus aureus and Their Effects on Lys-7 Expression of Innate Immune Pathway DAF-2/DAF-16 in Caenorhabditis elegans. Applied Sciences. 2018; 8(12):2627. https://doi.org/10.3390/app8122627
Chicago/Turabian StyleXie, Hui, Yonghua Zhan, Xueli Chen, Qi Zeng, Dan Chen, and Jimin Liang. 2018. "Brevinin-2 Drug Family—New Applied Peptide Candidates Against Methicillin-Resistant Staphylococcus aureus and Their Effects on Lys-7 Expression of Innate Immune Pathway DAF-2/DAF-16 in Caenorhabditis elegans" Applied Sciences 8, no. 12: 2627. https://doi.org/10.3390/app8122627






