1. Introduction
In recent years, photocatalysts have attracted much attention due to their wide range of applications, from generating hydrogen by splitting water to decomposing organic contaminants in wastewater [
1]. In the decomposition of organic contaminants such as dyes in wastewater, the photocatalyst uses visible light or ultraviolet (UV) light as an irradiating source to mineralize the contaminants in order to produce the end products such as carbon dioxide and water. TiO
2 has been the subject of early study in photocatalytic materials. However, due to its wide band gap, it can only work under ultraviolet irradiation for photocatalytic activation, which leads to very low energy efficiency in utilizing solar light. This is because UV light is only utilizing 5% solar energy, a small fraction compared to visible light, which is 45% [
2]. Therefore, research has been focused on finding a method or materials that can work efficiently under visible light irradiation.
Among the widely studied materials are bismuth-based photocatalysts, which have been proven to be suitable in the degradation of organic contaminants under visible light irradiation [
3]. This is because bismuth compound such as Bi
2O
3, BiVO
4, NaBiO
3 has a band gap range less than 3.0 eV and is environmentally friendly. Various approaches in synthesizing BiVO
4 have been reported. A monoclinic BiVO
4 has successfully been prepared by solid state reaction (SSR) and by melting at a high temperature [
4], whereas, a tetragonal BiVO4 has been synthesized by a precipitation method at a room temperature [
5]. In addition, a simple aqueous process in preparing both monoclinic and tetragonal phase was also reported [
6]. In addition, the use of hydrothermal process in creating various structural, morphologies and phase were also being reported. Since BiVO
4 can be prepared by using a simple method, it is one of the suitable candidates in the study of a low-cost synthesis method [
7].
Besides that, nano-size photocatalytic materials have been proven to have a high degradation rate in degrading organic contaminants, due to their having a greater surface area, which provides more fine contact among catalyst particles and organic substrate, good dispersion, and abundant active sites. However, the recovery process becomes a drawback for this nano-size photocatalyst, since the powder-like nano-photocatalyst often suffers from deactivation, agglomeration, and difficulty settling for recycling after the initial run. This makes it hard to recycle the photocatalyst, and nonrecyclable photocatalytic materials will increase operating cost, which significantly limits their application in wastewater treatment. In most wastewater treatment, photocatalysts are suspended in aqueous solution. Separation of photocatalysts must be done by settling with the aid of gravity or mechanical centrifugation. Settling of ultrafine photocatalyst particles with the aid of gravity has been commonly observed to proceed very slowly.
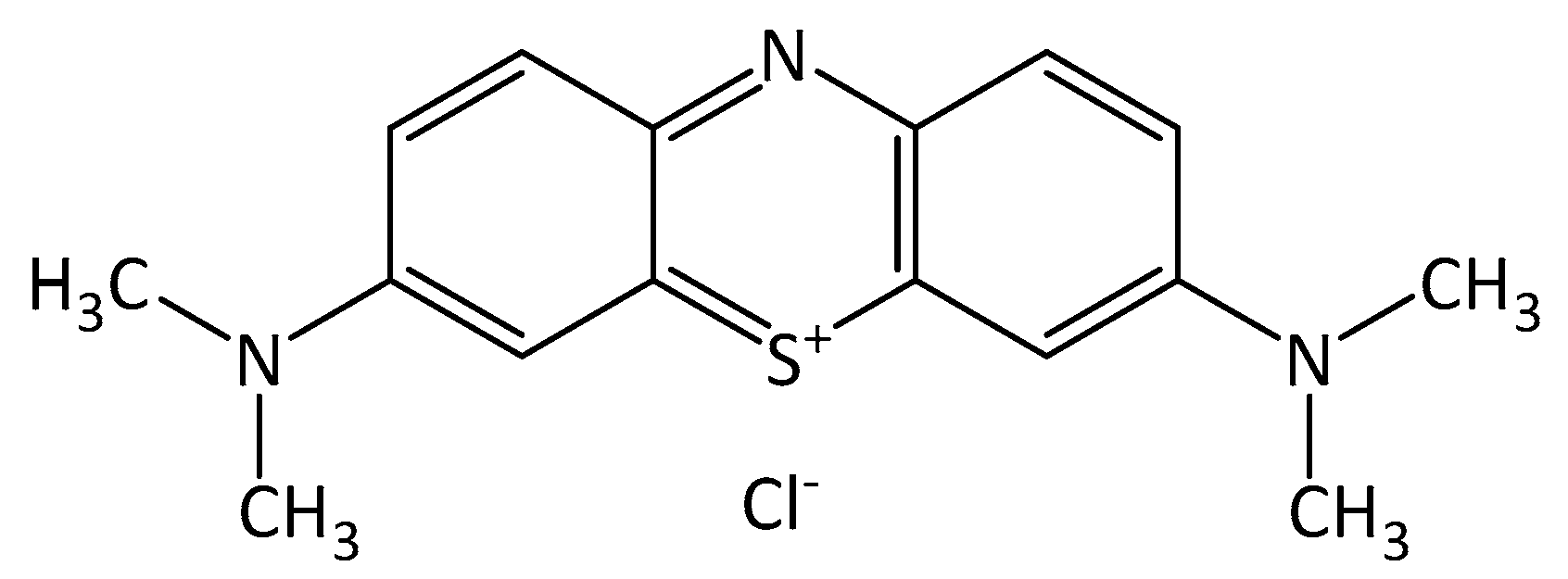
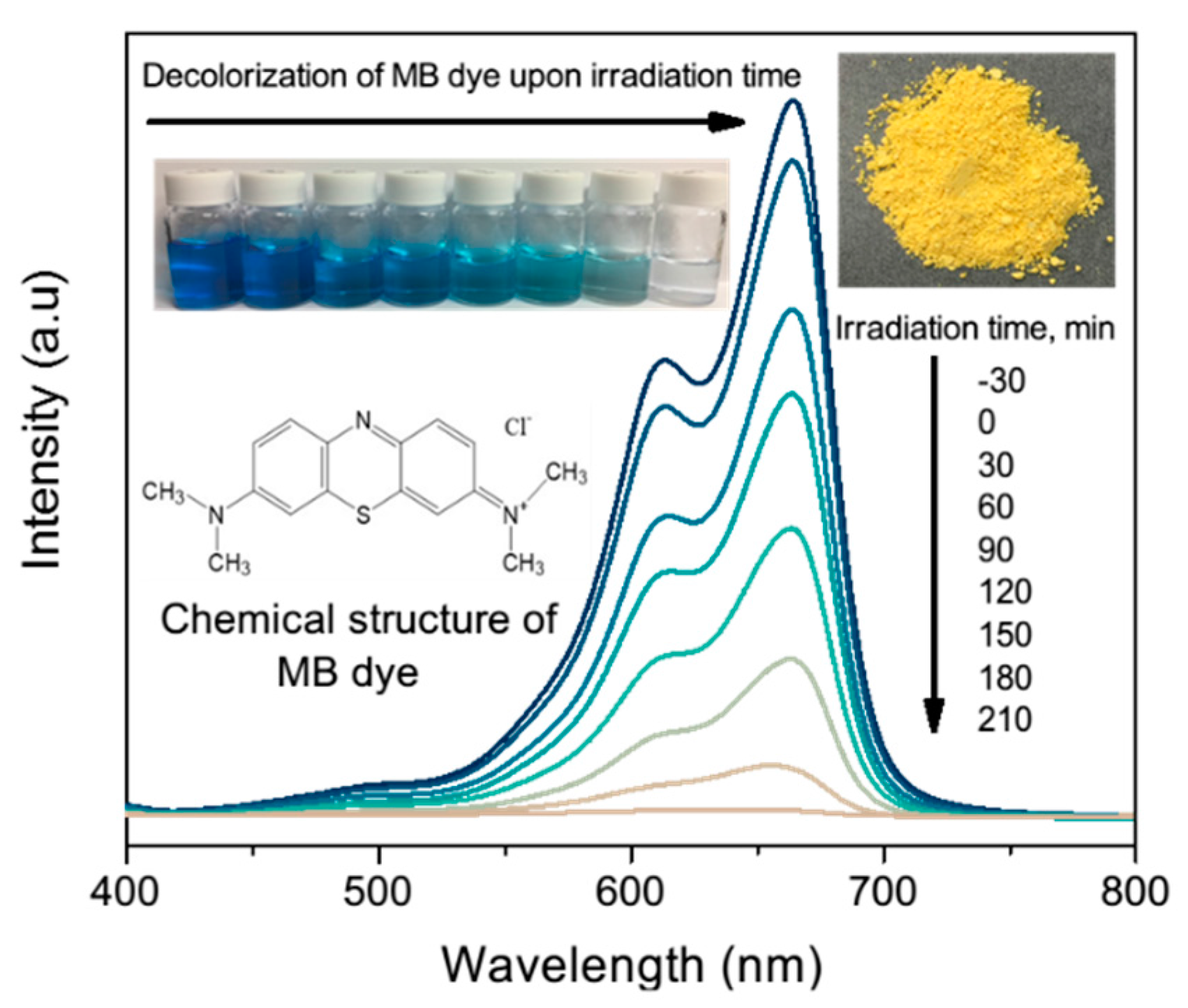
Therefore, in this study, we developed a microstructure photocatalyst composed of nanosheets to enhance the process by using a simple, low-cost method that results in the formation of a flower-like bismuth vanadate microstructure that has a greater surface area, more stability, and higher efficiency in organic dye degradation under visible light irradiation. As for the dye contaminant, we chose methylene blue (MB) dye because it is a frequently used industrial dye. MB dye has the chemical structure of C
16H
18N
3SCl and is shown in
Figure 1.
2. Materials and Methods
Bismuth nitrate pentahydrate (Bi(NO3)3∙5H2O) crystal and nitric acid (HNO3) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Sodium hydroxide (NaOH) granule was purchased from Kanto Chemical Co., Inc., (Tokyo, Japan) vanadium (III) acetylacetonate (C10H14O5V) crystal from Strem Chemicals Inc., (Newburyport, MA, USA) and methylene blue dye powder from Waldeck GmbH & Co. KG (Münster, Germany). Ultrapure water was used in all experiments. All chemicals were analytical grade and used as received, without further purification.
Flowerlike BiVO4 microspheres were synthesized by a simple template-free precipitation method at 60 °C for 24 h. Then, 4.2 g of Bi(NO3)3∙5H2O and 0.27 g of C10H14O5V were dissolved in 100 mL of HNO3 at a concentration of 4 mol L–1. Ultrapure water was added until it reached a final volume of 500 mL. The solution was stirred until a clear light blue solution was formed. Then, the pH of the solution was adjusted to 12 by adding NaOH. The solution changes color from clear light blue to bluish precipitate and brownish precipitate, which indicates the change from acidic to neutral to alkaline state. The suspension was inserted into an oil bath at 60 °C for 24 h without stirring. The resulting yellow precipitate was retrieved by centrifugation, and washed several times with distilled water and absolute ethanol. The yellow precipitate was then dried at 60 °C for 24 h and was calcined at different temperatures (300 °C, 400 °C, 500 °C and 600 °C) for further study.
The samples were analyzed with an X-ray diffractometer (XRD; RINT 2000, Rigaku Corp., Tokyo, Japan) operated at 30 kV and 40 mA with Cu Kα radiation (λ = 1.54178 Å). Data were collected in the angular range of 2θ = 15–80°. Field emission scanning electron microscopy images were obtained from a Hitachi FE-SEM SU8000 (Tokyo, Japan). UV-visible diffusion reflectance spectra were measured at room temperature with a JASCO V-7200 UV-Vis-NIR Spectrophotometer (Jasco Products Company, Oklahoma City, OK, USA). The reflectance was then converted to absorbance by using the Kubelka–Munk method. The size of the particles was recorded by zeta potential and an Otsuka Electronics ELSZ-2000 particle analyzer (Otsuka Electronics Co., Ltd., Osaka. Japan) at room temperature, using ultrapure water as a solution. Further analysis was conducted with a Thermo Scientific Nicolet 4700 Fourier-transform infrared spectroscopy analyzer (Thermo Fisher Scientific, Waltham, MA, USA), a Horiba-Jovin Yvon T64000 micro Raman spectrometer (HORIBA Jobin Yvon IBH Ltd., Paris, France), and a JASCO FP8500 fluorescence spectrometer.
Photocatalysis evaluation test was conducted as follows: 4 mL of methylene blue solution at a concentration of 0.3 mg/100 mL was placed in a 100 mL Pyrex vessel. Then, 96 mL of ultrapure water was added to the aqueous solution. After that, 0.3 g of BiVO4 powder was added for each experiment. The experiments were conducted under the presence of visible light (λ > 400 nm). The solution was magnetically stirred to ensure uniformity. The samples were then filtered by using a syringe membrane filter (Millex; Millipore Corp., Burlington, MA, USA) to remove the photocatalyst particles. Samples were taken from the solution every 30 min and placed inside a UV-Vis spectrophotometer cell in order to measure the maximum absorption of wavelength for the dye and the rate of degradation. The percentage of degradation was calculated by the formula [1 − (C/C0)] × 100%, where C0 is the initial concentration of the dye solution and C is the concentration of the dye at each 30-min interval.
3. Result and Discussion
3.1. Material Characterization
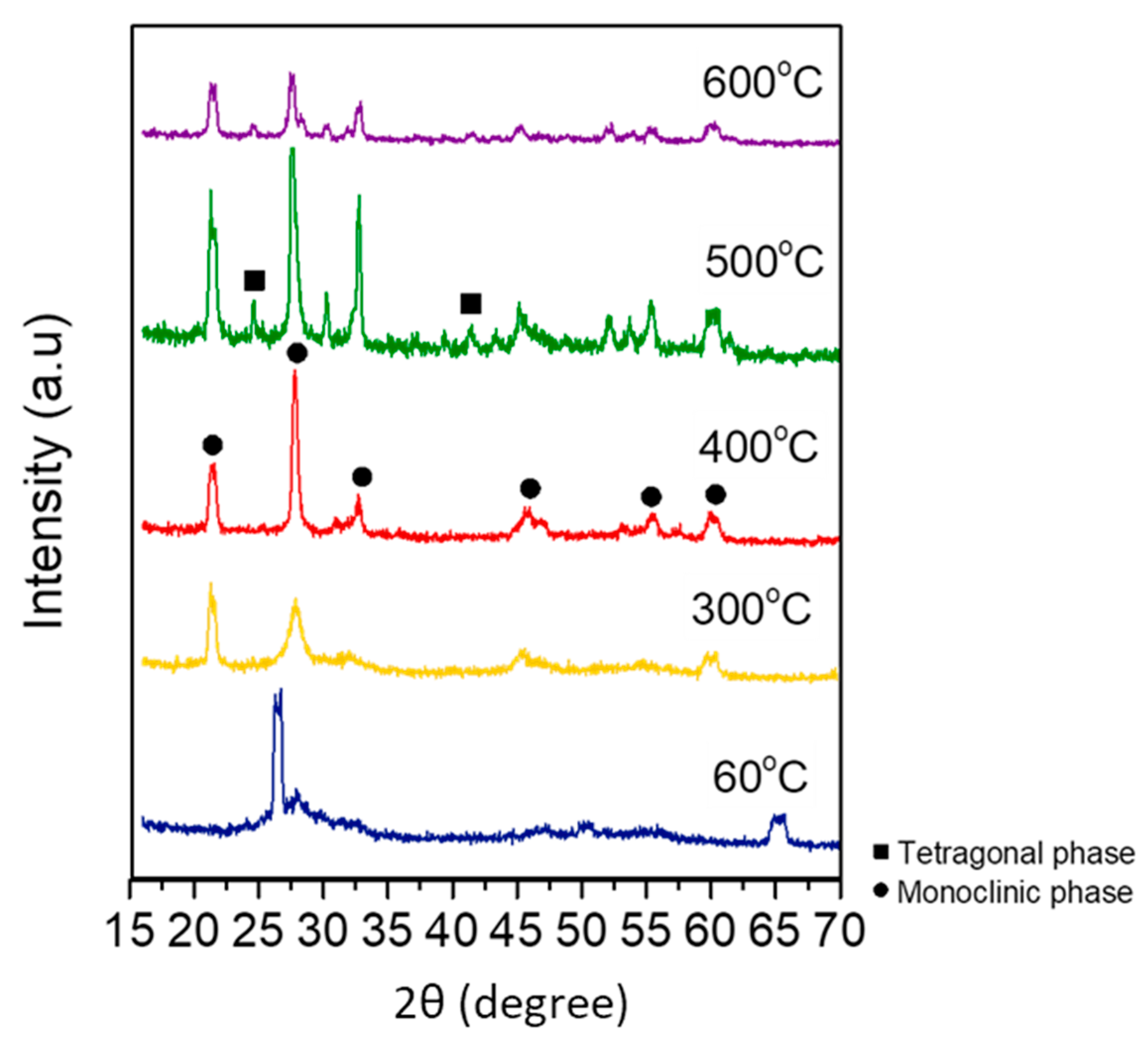
XRD was used to characterize the phase structure of the obtained samples.
Figure 2 shows the XRD pattern of the BiVO
4 microspheres prepared for each calcination temperature. The XRD pattern at 400 °C indicates that the sample was mainly in the monoclinic phase, which exhibits high photocatalytic activity compared to other phases. In addition, a well-defined sharp peak can be seen after calcination at 300 °C. This indicates that the sample achieved a well-defined organizational structure, which leads to better photocatalytic activity.
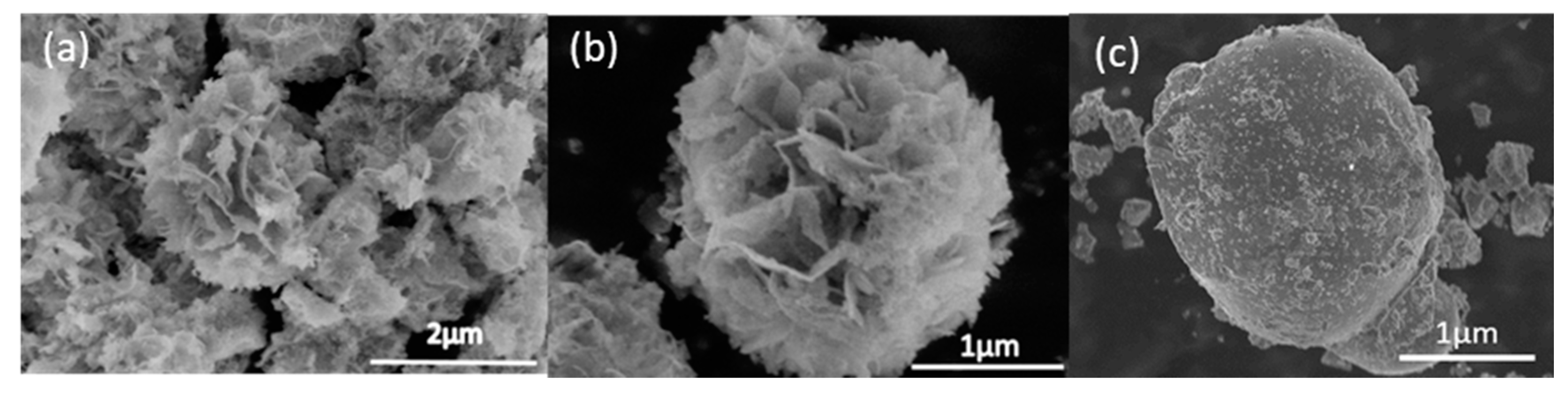
As shown in the scanning electron microscopy (SEM) observation images in
Figure 3a–c, after calcination at 400 °C, the products formed were uniform. The SEM images of commercial BiVO
4 can be seen as bulky, and not much surface area is available.
At 500 °C (Figure 6), the mixture of flower-like structure and agglomerates started to form, while, at 600 °C, the particles started to form only agglomerates, and no flower-like structures were found.
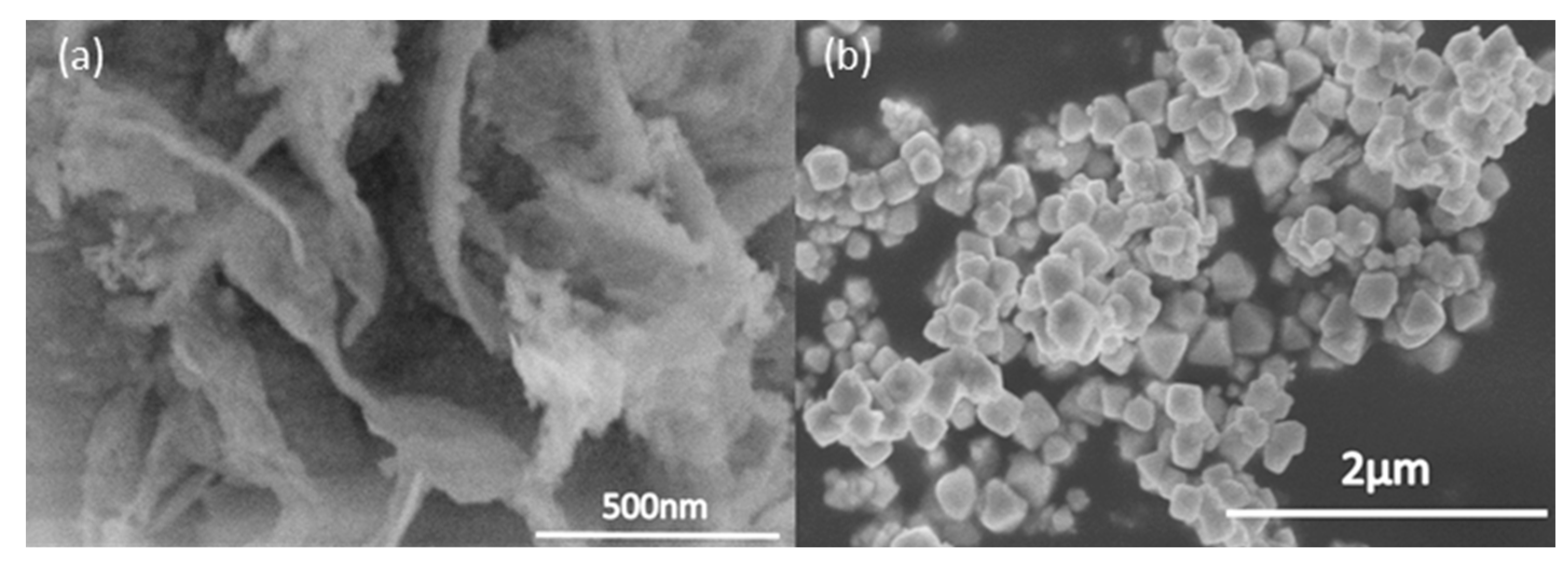
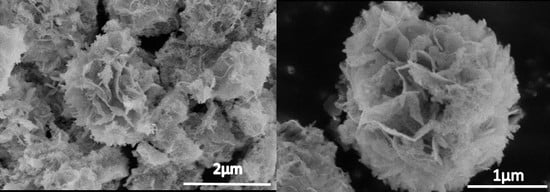
To further study the morphologic structure, we observed the samples under high magnification SEM (
Figure 4a,b), and the flower-like structures of the sample were composed of numerous nanosheets with a thickness of about 100 nm. The nanosheets intercrossed to form a flower-like microsphere via self-assembly and the Ostwald ripening process. These flower-like structures resulted in an increased surface area, which leads to better photocatalytic performance compared to commercial BiVO
4, which has a bulky structure and low surface area. Besides that, the large surface area provided by the flower-like structure can allow more rapid diffusion of the reactants and products during the reaction process. If we increased the pH of the synthesis condition to more than 12, the flower-like structure could not form, and only microspheres/microcubes would be observed.
We also proposed the formation mechanism of the flower-like BiVO
4 microspheres, as shown in
Figure 5. A precipitation process occurred by the nucleation and growth of the second phase from a supersaturated solution. With a two-phase mixture composed of a dispersed second phase in a matrix, the mixture does not satisfy thermodynamic equilibrium. This is due to small particles with a higher surface ratio and a higher energy state leading to excess surface energy, which does not satisfy the requirement of minimum energy configuration. The total energy of this system can be decreased via an increase in the scale of the second phase, thus decreasing the total interfacial area. This will result in a continuous nucleation and growth process until a single precipitate particle forms, which introduces a new particle of a given size class, which, in this case, was the flower-like microsphere structure [
8,
9].
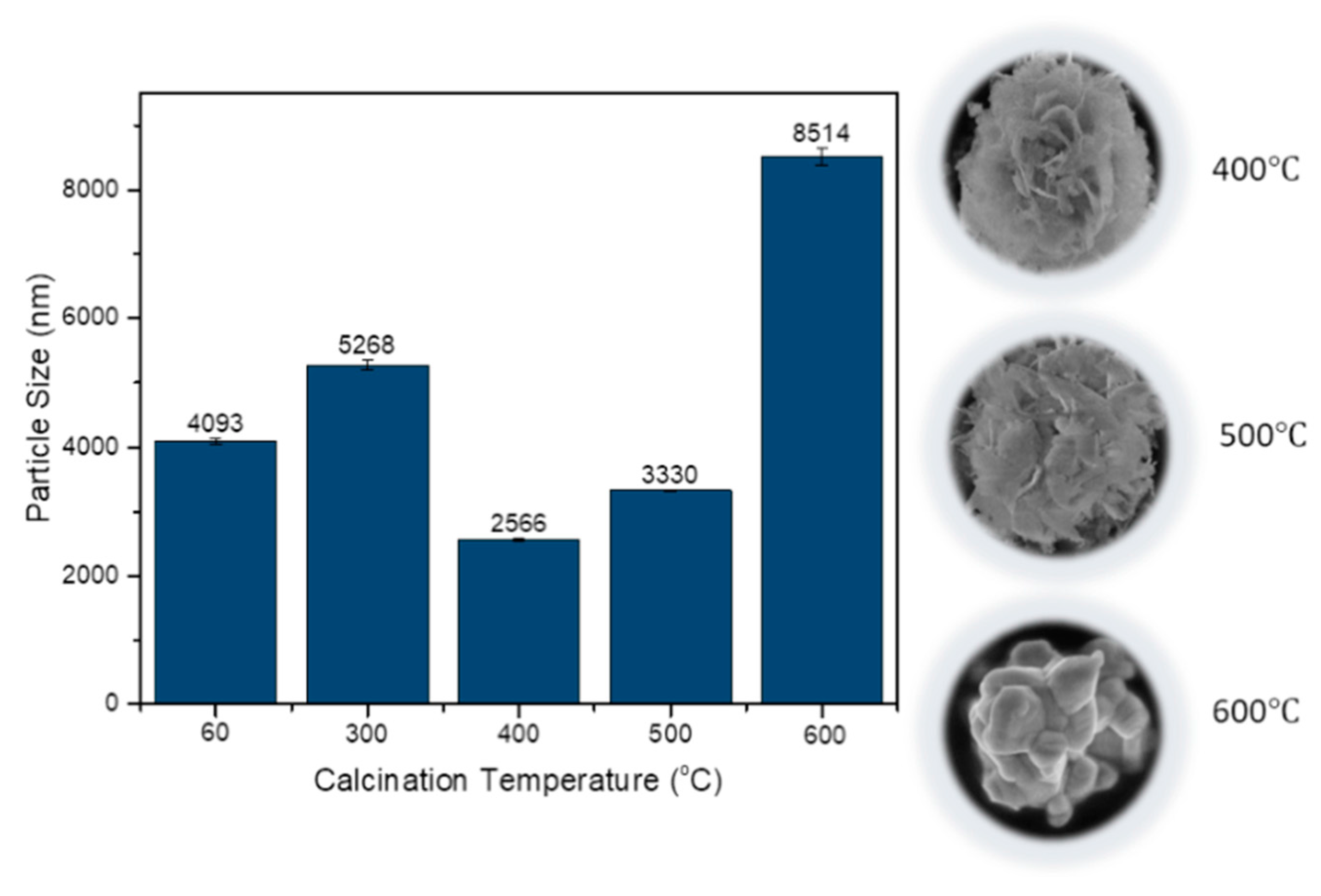
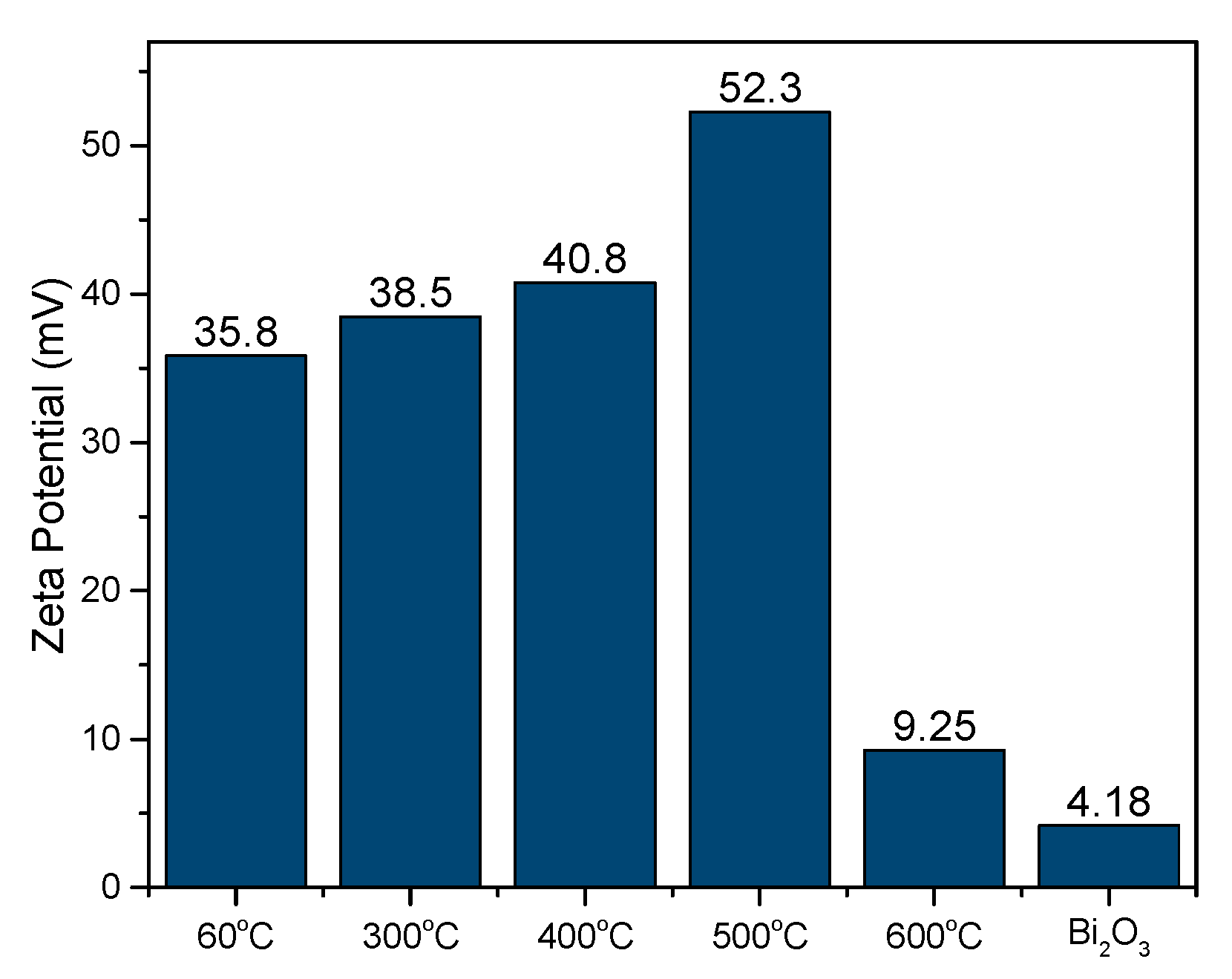
The mean particle size for the obtained samples was also studied using the zeta potential and particle analyzer. As shown in
Figure 6, the smallest size was at 400 °C, which was 2566 nm with a slight error value. At 60 °C and 300 °C, the sample consisted of impurities, which led to a larger mean particle size. However, at 500 °C, the mean particle size was larger than at 400 °C, because a mixture of 3D microsphere structure and agglomerates started to form. The mean particle size became the largest, with a substantial error value at 60 °C due to most of the sample being made up of large agglomerates, contributing to poor photocatalytic performance.
3.2. Optical Properties
The UV-Visible optical absorption spectrum of BiVO
4 microspheres was measured and compared with commercial BiVO
4 and synthesized BiVO
4 microspheres, as shown in
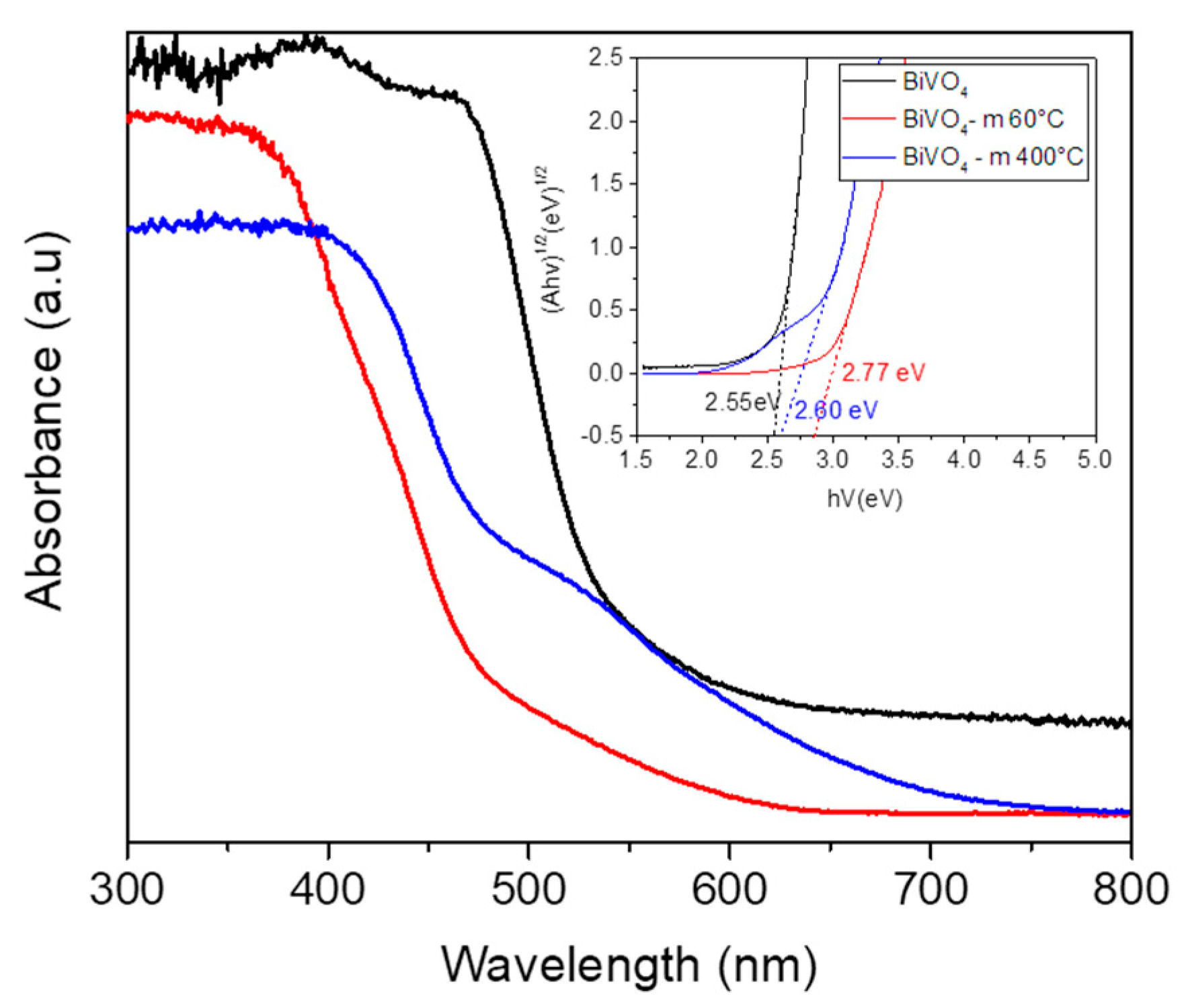
Figure 7. A broad absorption spectrum could be seen from 300 to 400 nm. At around 420 nm, an abrupt cutoff absorption edge was observed.
The absorption band contains a tail extending rightward until about 800 nm. This may be a result of crystal defects formation during the growth of the BiVO
4 microspheres. The band gap energy was determined by Kubelka–Munk derivation (
Figure 7). The band gap of this photocatalyst is 2.60 eV, and it has yellowish colored powder. This shows that this photocatalyst can work efficiently under visible light irradiation. In addition, the presence of the monoclinic phase which has superior photocatalytic activity contributed to the enhancement of the photocatalytic character of this photocatalyst.
Photoluminescence spectroscopy was studied at the excitation wavelength λ
ex = 325 nm. As shown in
Figure 8, a strong peak can be seen at around 560 nm for both synthesized microspheres and commercial BiVO
4. The strong emission at 560 nm corresponds to recombination of the hole formed from the hybrid orbitals of Bi 6s and O 2p and the electron generated from the V 3d orbitals. In addition, the emission bands of BiVO
4–m have a blue shift compared to commercial BiVO
4, which shows that it has a lower recombination rate.
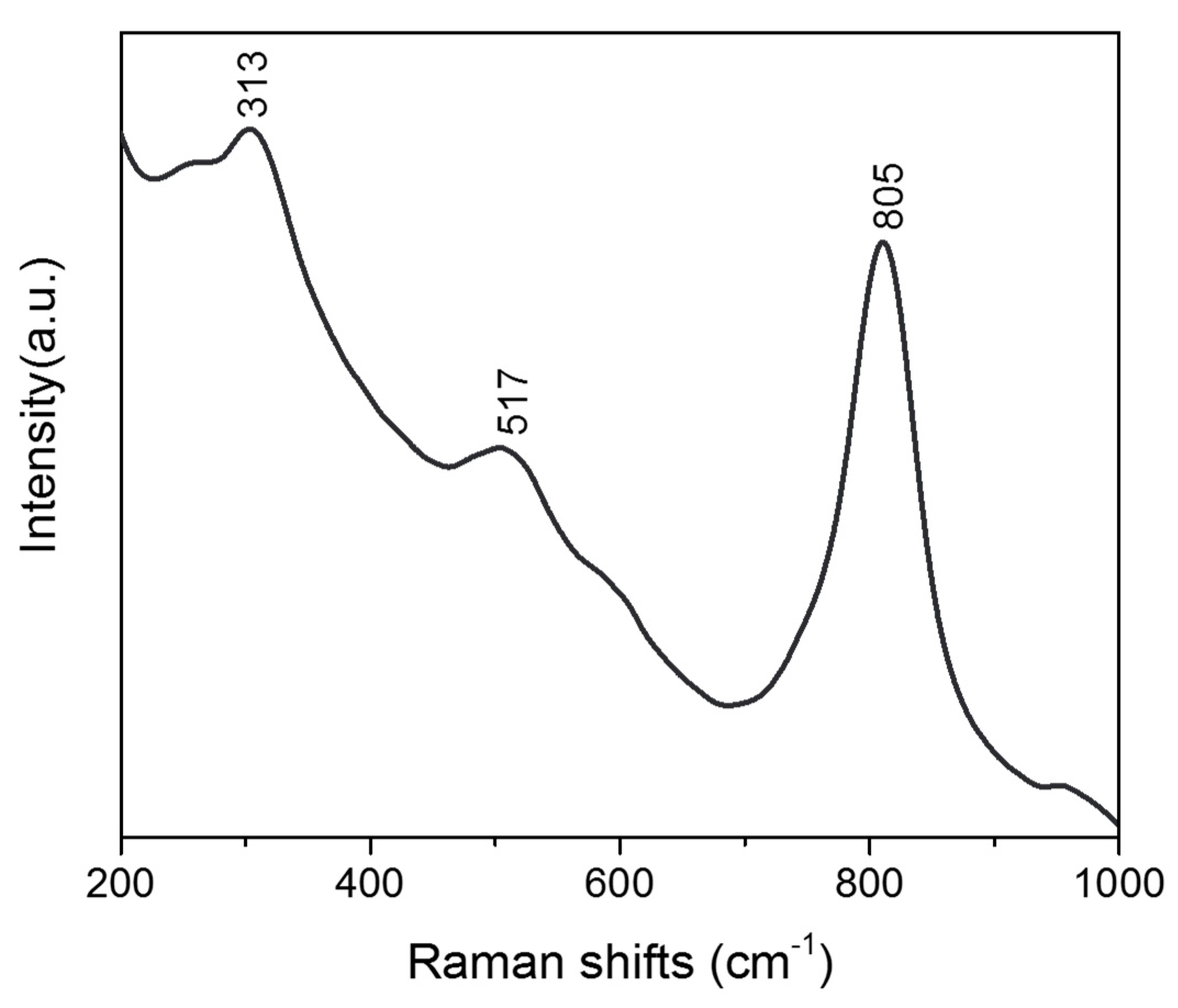
Raman spectroscopy was also investigated, as shown in
Figure 9. The Raman bands at 313 cm
−1 are the typical vibrations of BiVO
4 and can be assigned to the asymmetric and symmetric deformation modes of the VO
43− tetrahedron. It has been reported that there were two Raman bands, at 710 cm
−1 and 805 cm
−1, which were attributed to the stretching modes of two different types of Vanadium–Oxygen bands in the Raman spectrum of BiVO
4. However, only one band around 805 cm
−1 was found in our case. The absence of a 710 cm
−1 band might be attributed to the local structure of porous microspheres, which were composed of nanosheets. Additionally, the Raman band at 517 cm
−1 indicates the characteristics of monoclinic scheelite BiVO
4 that made up these microsphere structures.
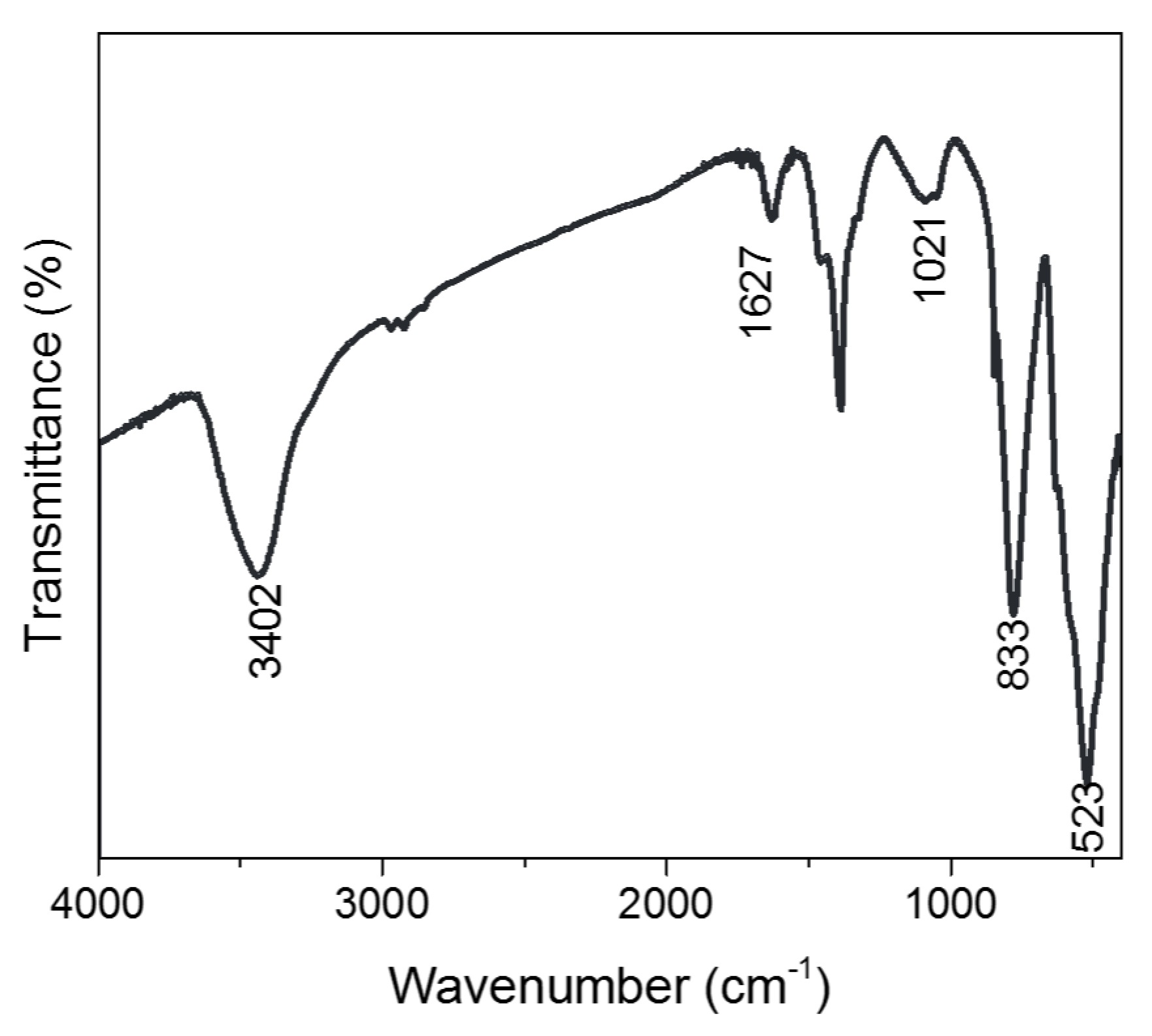
From the Fourier-transform infrared spectroscopy analysis we conducted (
Figure 10), we can understand the bonding group presence in these BiVO
4 microspheres. At 1021 cm
−1, there is an unshared V = O stretching vibration, followed by 833 cm
−1, which shows the presence of an asymmetric stretching vibration of the bound oxygen that is shared by two vanadium atoms (V–O–V). Furthermore, at 523 cm
−1, a symmetric stretching mode of the V–O–V unit is present, and finally, at 3402 and 1627 cm
−1, it shows an O–H stretching and bending vibration of the lattice water molecules [
10].
We also conducted a zeta potential analysis to study the stability of this flower-like BiVO
4 in terms of the colloidal dispersion, as shown in
Figure 11. This shows that it has high stability compared to commercial BiVO
4, since it possesses a higher zeta potential value. When the potential is small, attractive forces can exceed this repulsion and the dispersion can break and flocculate. Therefore, colloids with high zeta potential (negative or positive) are electrically stabilized, while colloids with low zeta potential tend to coagulate or flocculate [
11,
12].
3.3. Photocatalytic Properties
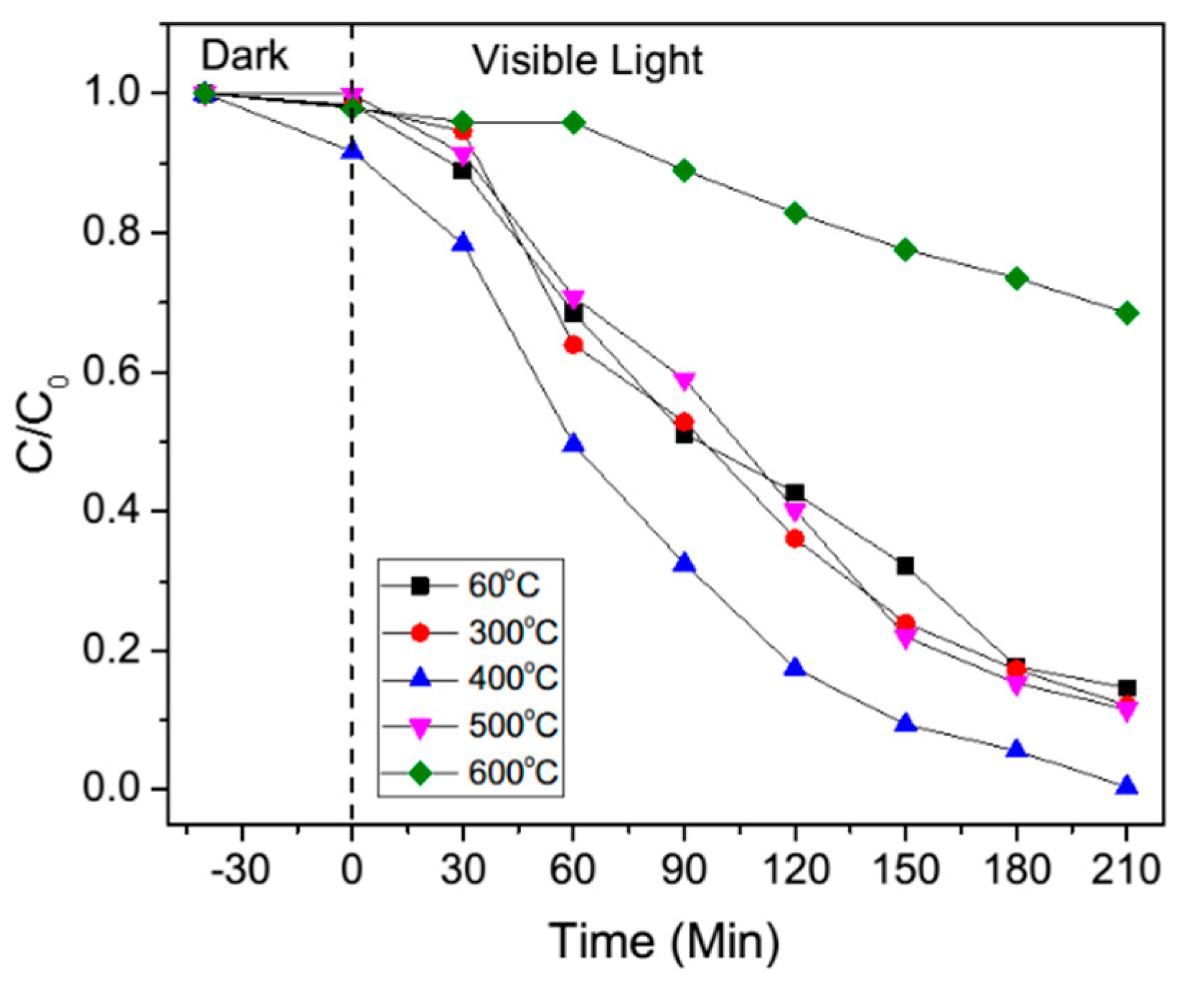
Photocatalysis of the samples was evaluated by decolorization of MB aqueous solution under visible light irradiation, and the results are shown in
Figure 12 and
Figure 13. Absorption of MB aqueous solution at λ = 664 nm was used to monitor the MB dye decolorization rate process. The decolorization rate sequence can be concluded as 400 °C, 500 °C, 60 °C and 300 °C, followed by 600 °C. Improved performance was observed for 60 °C, 300 °C, 400 °C and 500 °C. However, due to the presence of only agglomeration and bulk structures at 600 °C, the reaction rate declined.
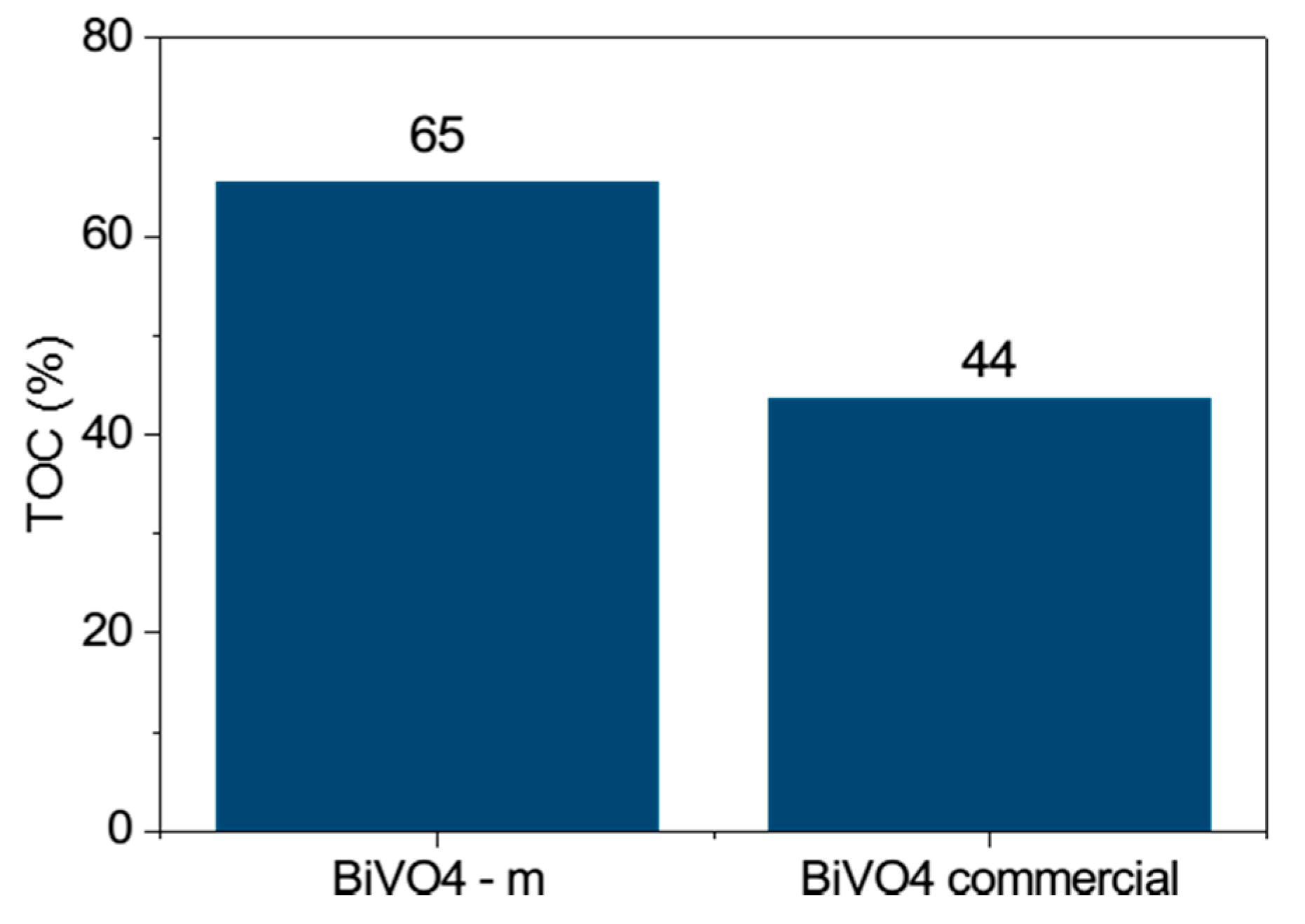
In addition, we conducted a Total Organic Carbon (TOC) study (
Figure 14) on this photocatalyst, showing that it can degrade MB dye better than commercial BiVO
4. Thus, it has better efficiency in decolorizing and mineralizing the dye.
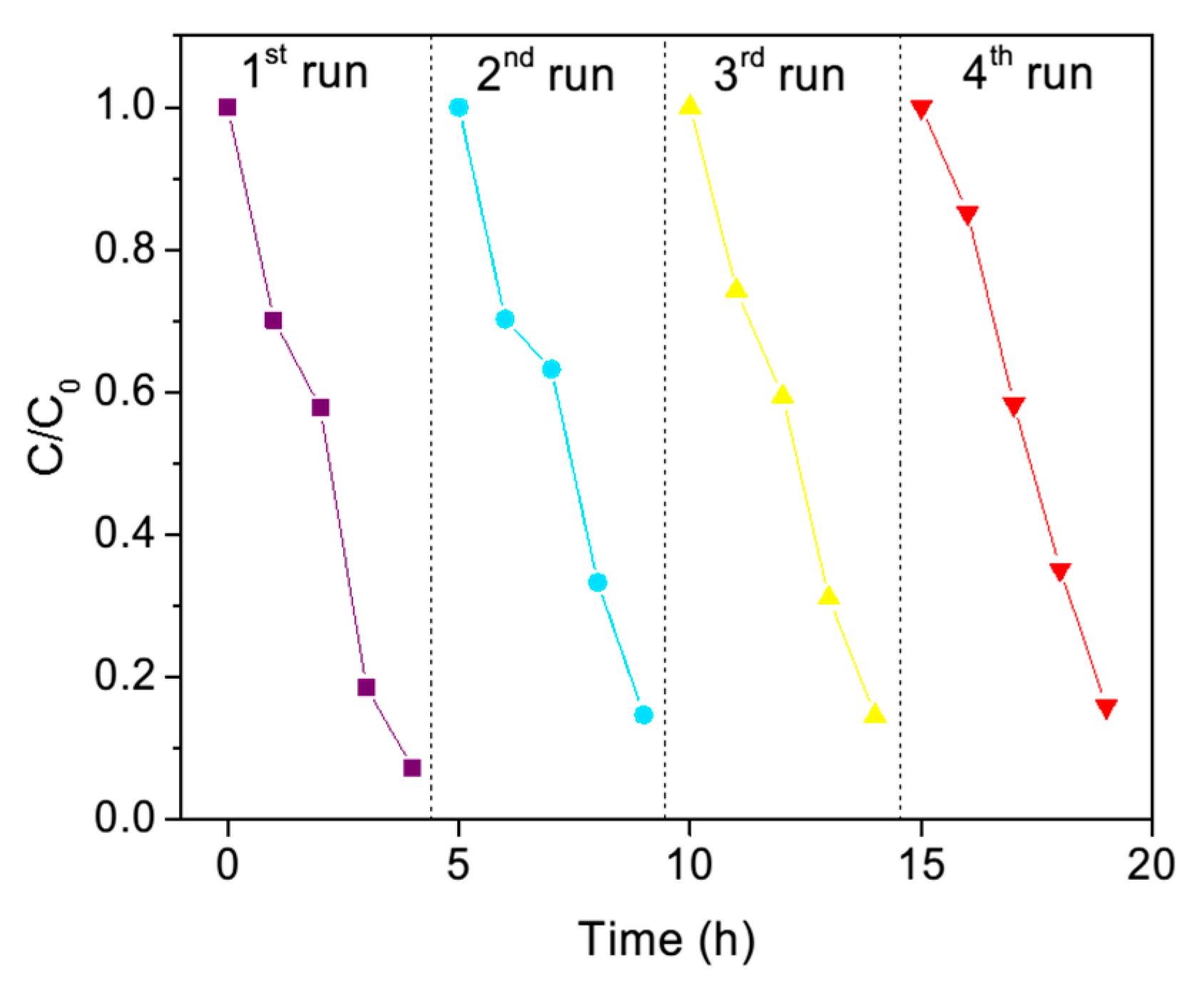
The recyclability of this photocatalyst was also studied (
Figure 15). We found that this material has good stability, since it only declined from 94% to 88% after four cycles of the activity, which indicates that it has good stability in this recyclability study.
4. Conclusions
In conclusion, by adding vanadium (III) acetylacetonate to the system, the flower-like structure can be achieved at a low temperature of 60 °C for 24 h. Although photocatalysis in decolorization just slightly improved compared to commercial BiVO4, it can mineralize the dye better, as can be seen in results of the TOC analysis. We believe that, with further study, the reaction time and photocatalytic performance can be improved. Thus, this work may open the way to possible new and varied low-cost self-assembly synthesizing methods.