Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Nanoparticles
2.2. Characterization of the Nanoparticles
2.3. Biological Studies
3. Results
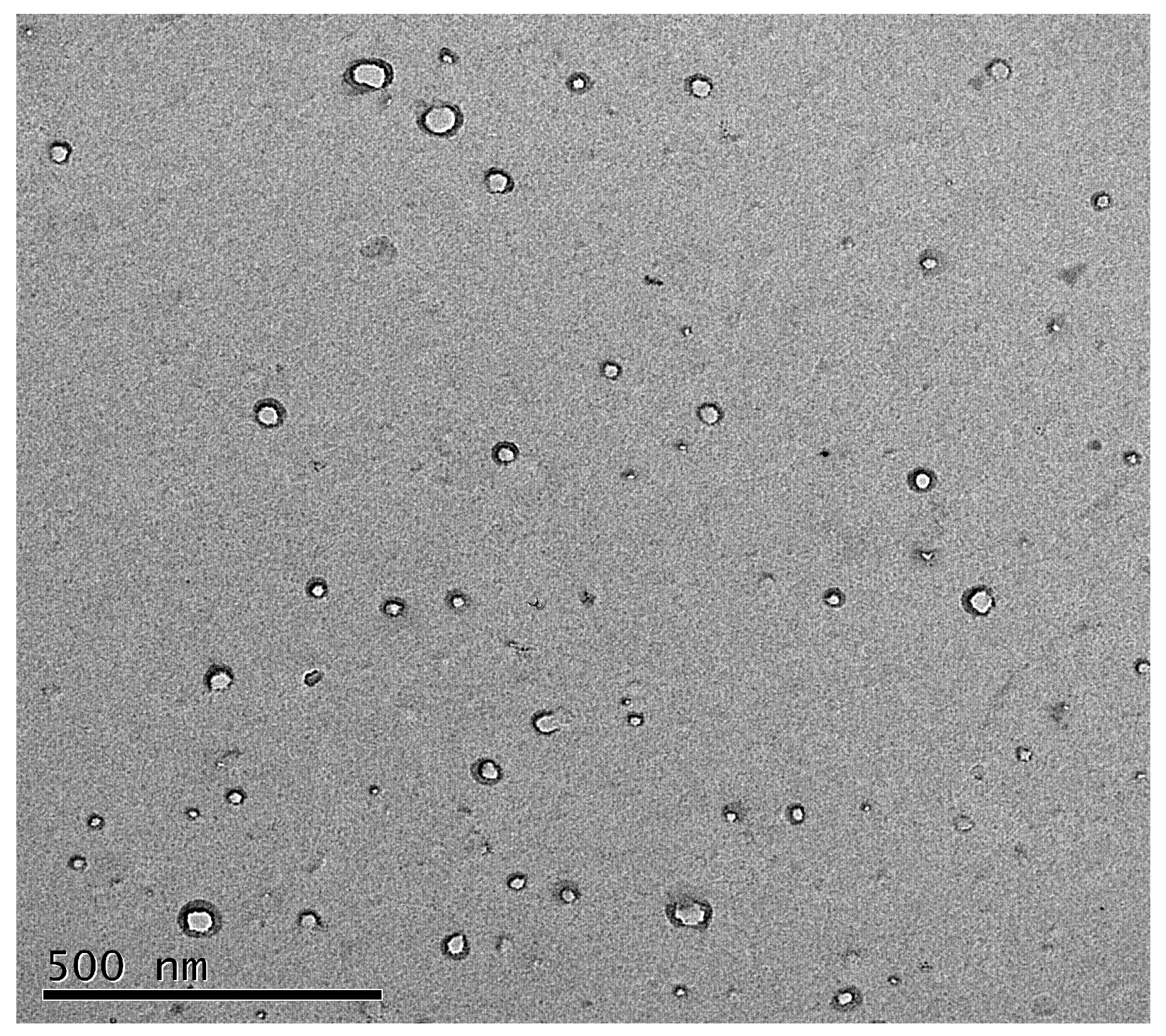
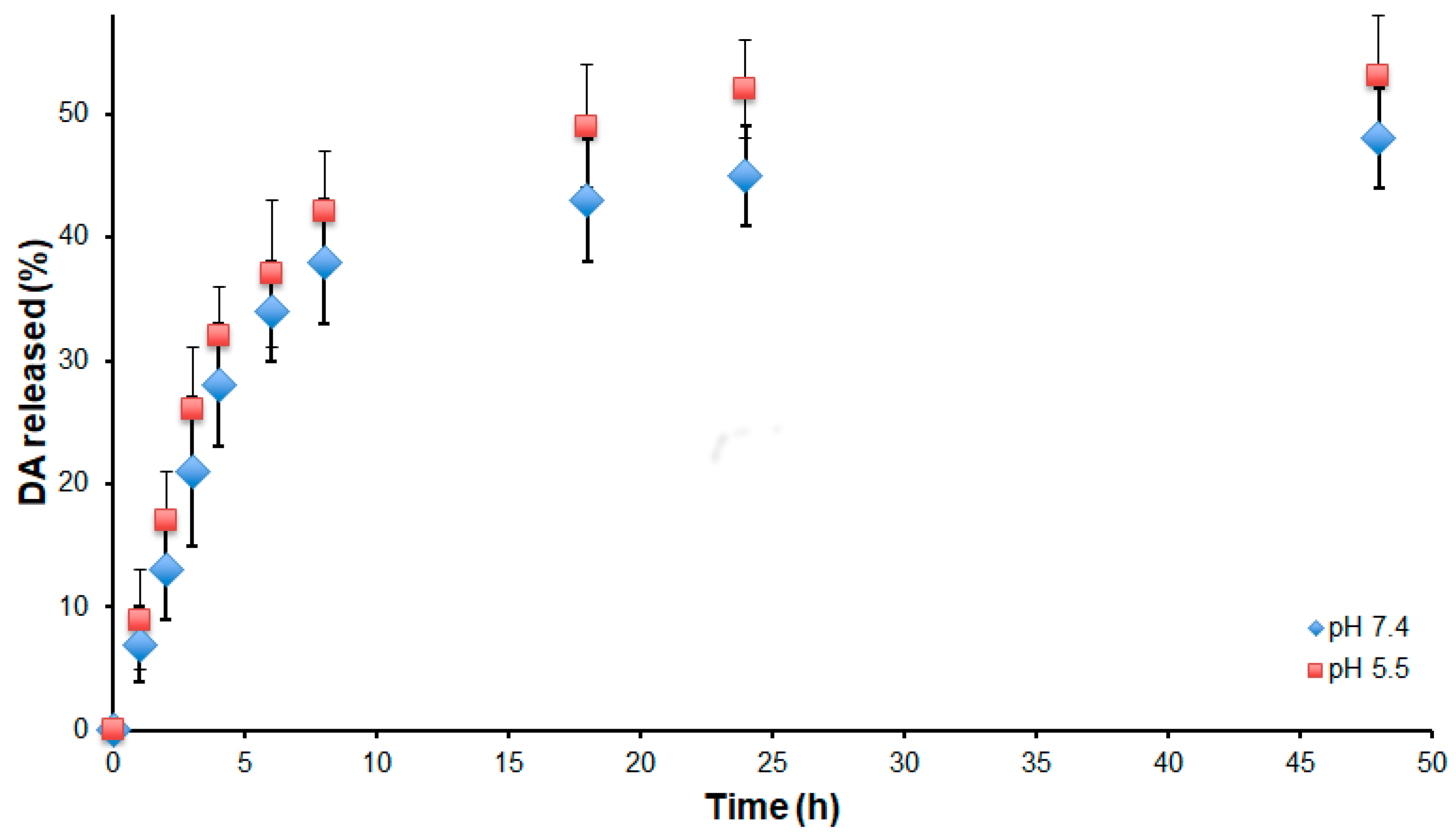
3.1. Preparation and Characterization of the NPs
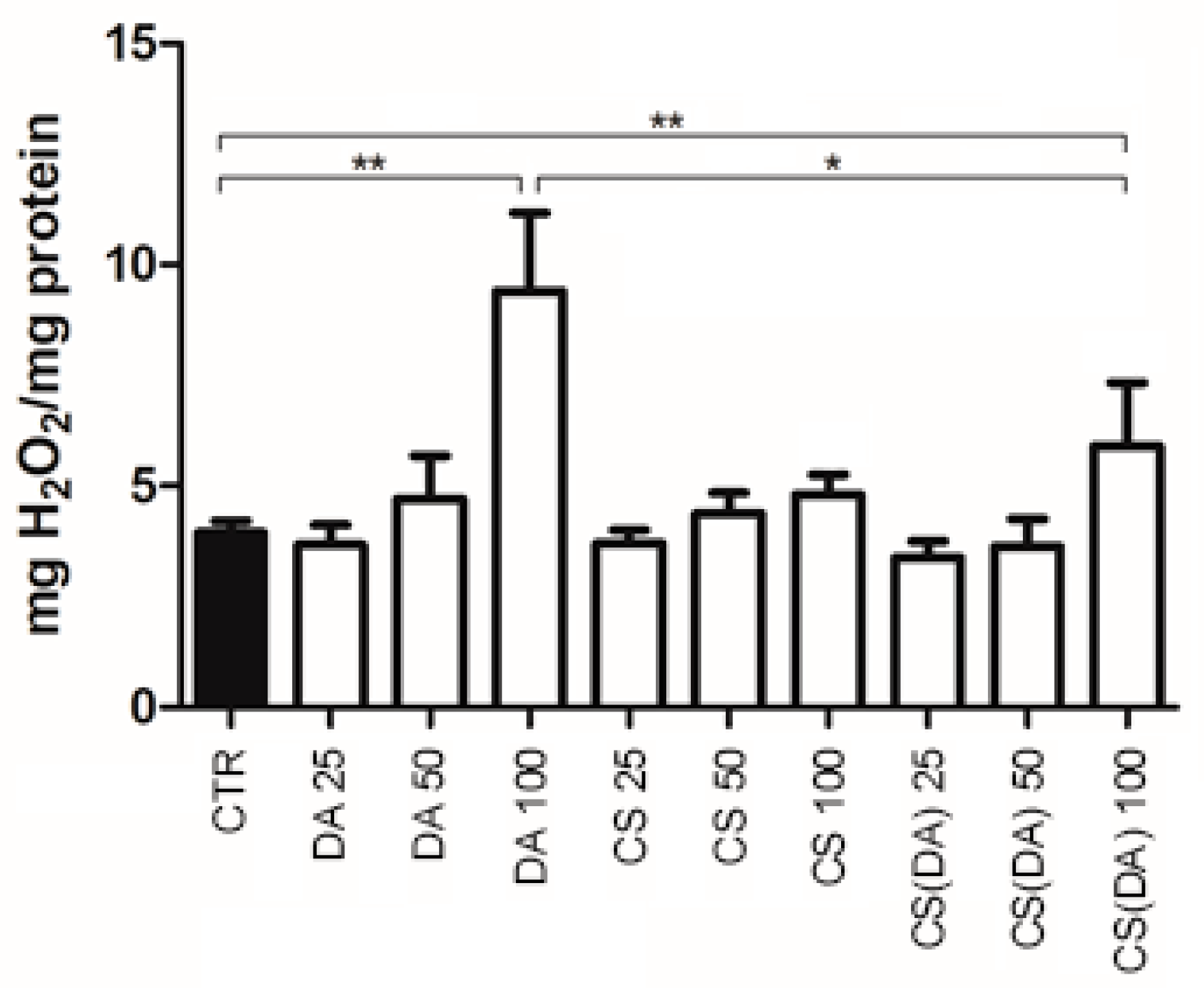
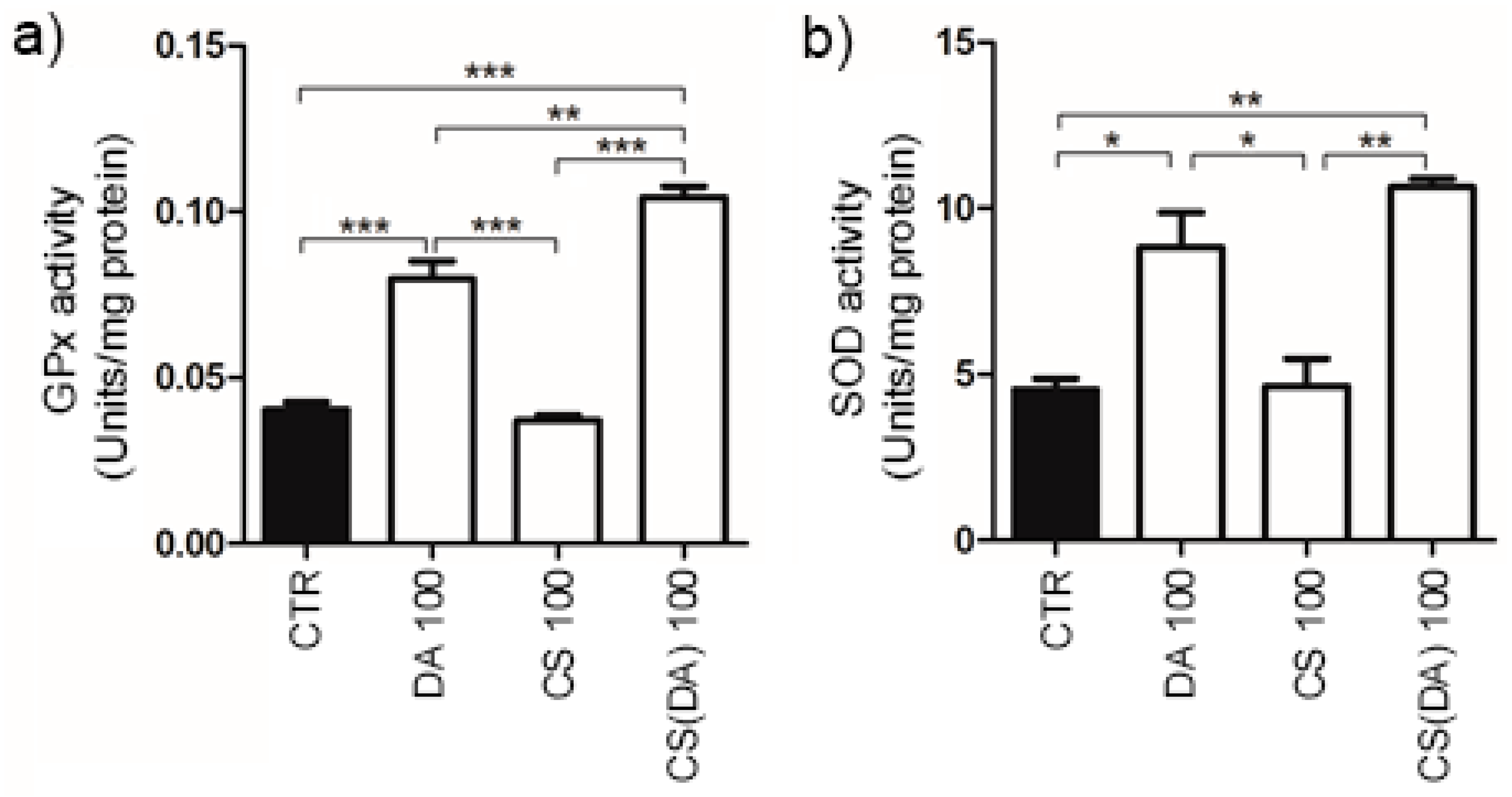
3.2. ROS Neuroprotection by CS(DA) NPs and Enzymatic Activities
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schapira, A.H. Causes of neuronal death in Parkinson’s disease. Adv. Neurol. 2001, 86, 155–162. [Google Scholar] [PubMed]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Garbayo, E.; Ansorena, E.; Blanco-Prieto, M.J. Drug development in Parkinson’s disease: From emerging molecules to innovative drug delivery systems. Maturitas 2013, 76, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Eaton, P.; Ragusa, A.; Clavel, C.; Rojas, C.T.; Graham, P.; Durán, R.V.; Penadés, S. Glyconanoparticle-DNA interactions: An atomic force microscopy study. IEEE Trans. Nanobiosci. 2007, 6, 309–318. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Di Corato, R.; Curcio, A.; Melisi, D.; Rimoli, M.G.; Tortiglione, C.; Tino, A.; George, C.; Brunetti, V.; Cingolani, R.; et al. Multiple Functionalization of Fluorescent Nanoparticles for Specific Biolabeling and Drug Delivery of Dopamine. Nanoscale 2011, 3, 5110–5119. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, F.; Conversano, F.; Soloperto, G.; Casciaro, E.; Ragusa, A.; Sbenaglia, E.A.; Dipaola, L.; Casciaro, S. Epithelial cell biocompatibility of silica nanospheres for contrast-enhanced ultrasound molecular imaging. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Conversano, F.; Soloperto, G.; Greco, A.; Ragusa, A.; Casciaro, E.; Chiriacò, F.; Demitri, C.; Gigli, G.; Maffezzoli, A.; Casciaro, S. Echographic detectability of optoacoustic signals from low-concentration PEG-coated gold nanorods. Int. J. Nanomed. 2012, 7, 4373–4389. [Google Scholar] [CrossRef] [Green Version]
- Ragusa, A.; Garcia, I.; Penades, S.; García, I.; Penadés, S.; Garcia, I.; Penades, S. Nanoparticles as nonviral gene delivery vectors. IEEE Trans. Nanobiosci. 2007, 6, 319–330. [Google Scholar] [CrossRef]
- Malvindi, M.A.; Greco, A.; Conversano, F.; Figuerola, A.; Corti, M.; Bonora, M.; Lascialfari, A.; Doumari, H.A.; Moscardini, M.; Cingolani, R.; et al. Magnetic/silica nanocomposites as dual-mode contrast agents for combined magnetic resonance imaging and ultrasonography. Adv. Funct. Mater. 2011, 21, 2548–2555. [Google Scholar] [CrossRef]
- Re, F.; Gregori, M.; Masserini, M. Nanotechnology for neurodegenerative disorders. Maturitas 2012, 73, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Ragusa, A.; Deka, S.; Toitiglione, C.; Tino, A.; Cingolani, R.; Pellegrino, T. Bioconjugation of rod-shaped fluorescent nanocrystals for efficient targeted cell labeling. Langmuir 2009. [Google Scholar] [CrossRef] [PubMed]
- Di Corato, R.; Quarta, A.; Piacenza, P.; Ragusa, A.; Figuerola, A.; Buonsanti, R.; Cingolani, R.; Manna, L.; Pellegrino, T. Water solubilization of hydrophobic nanocrystals by means of poly(maleic anhydride-alt-1-octadecene). J. Mater. Chem. 2008, 18, 1991. [Google Scholar] [CrossRef]
- Di Corato, R.; Bigall, N.C.; Ragusa, A.; Dorfs, D.; Genovese, A.; Marotta, R.; Manna, L.; Pellegrino, T. Multifunctional Nanobeads Based on Quantum Dots and Magnetic Nanoparticles – Synthesis and Cancer Cell Targeting and Sorting. ACS Nano 2011, 5, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Coradini, K.; Lima, F.O.O.; Oliveira, C.M.M.; Chaves, P.S.S.; Athayde, M.L.L.; Carvalho, L.M.M.; Beck, R.C.R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; De Giglio, E.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetani, S.; Cuomo, V.; Trapani, G. Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Trapani, A.; Cafagna, D.; Sabbatini, L.; Cometa, S. Dopamine-loaded chitosan nanoparticles: Formulation and analytical characterization. Anal. Bioanal. Chem. 2011, 400, 1997–2002. [Google Scholar] [CrossRef] [PubMed]
- Di Gioia, S.; Trapani, A.; Mandracchia, D.; De Giglio, E.; Cometa, S.; Mangini, V.; Arnesano, F.; Belgiovine, G.; Castellani, S.; Pace, L.; et al. Intranasal delivery of dopamine to the striatum using glycol chitosan/sulfobutylether-??-cyclodextrin based nanoparticles. Eur. J. Pharm. Biopharm. 2015, 94, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wang, W.; Wang, S.; Zhang, L.; Guo, Y. An overview of the protective effects of chitosan and acetylated chitosan oligosaccharides against neuronal disorders. Mar. Drugs 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Pahuja, R.; Seth, K.; Shukla, A.; Shukla, R.K.; Bhatnagar, P.; Chauhan, L.K.S.; Saxena, P.N.; Arun, J.; Chaudhari, B.P.; Patel, D.K.; et al. Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano 2015, 9, 4850–4871. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, G.H.; Bernuci, M.P.; Bortolanza, M.; Tumas, V.; Issy, A.C.; Del-Bel, E. Nanomedicine to Overcome Current Parkinson’s Treatment Liabilities: A Systematic Review. Neurotox. Res. 2016, 30, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Huenchuguala, S.; Paris, I.; Segura-Aguilar, J. Dopamine oxidation and autophagy. Parkinsons. Dis. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Selvaratnam, T.; Chao, Y.X.; Lim, T.M.; Tan, E.K. Dopamine (DA) Dependent Toxicity Relevant to DA Neuron Degeneration in Parkinson’s Disease (PD). Austin J. Drug Abus. Addict. 2016, 3, 1–9. [Google Scholar]

| Sample | DA Conc. (mg/mL) | Size (nm) | PDI | ζ-Potential (mV) | DA Loading Efficiency (%) |
|---|---|---|---|---|---|
| CS NPs | 0 | 100 ± 18 | 0.3 | +27 ± 0.4 | - |
| CS(DA) NPs | 5 | 109 ± 16 | 0.4 | +34 ± 0.9 | 64% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragusa, A.; Priore, P.; Giudetti, A.M.; Ciccarella, G.; Gaballo, A. Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery. Appl. Sci. 2018, 8, 474. https://doi.org/10.3390/app8040474
Ragusa A, Priore P, Giudetti AM, Ciccarella G, Gaballo A. Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery. Applied Sciences. 2018; 8(4):474. https://doi.org/10.3390/app8040474
Chicago/Turabian StyleRagusa, Andrea, Paola Priore, Anna Maria Giudetti, Giuseppe Ciccarella, and Antonio Gaballo. 2018. "Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery" Applied Sciences 8, no. 4: 474. https://doi.org/10.3390/app8040474





