Novel Applications of Microbial Fuel Cells in Sensors and Biosensors
Abstract
:1. Introduction
2. MFC Power Supply for Sensors and Biosensors
2.1. Effect of Temperature
2.2. Effect of pH
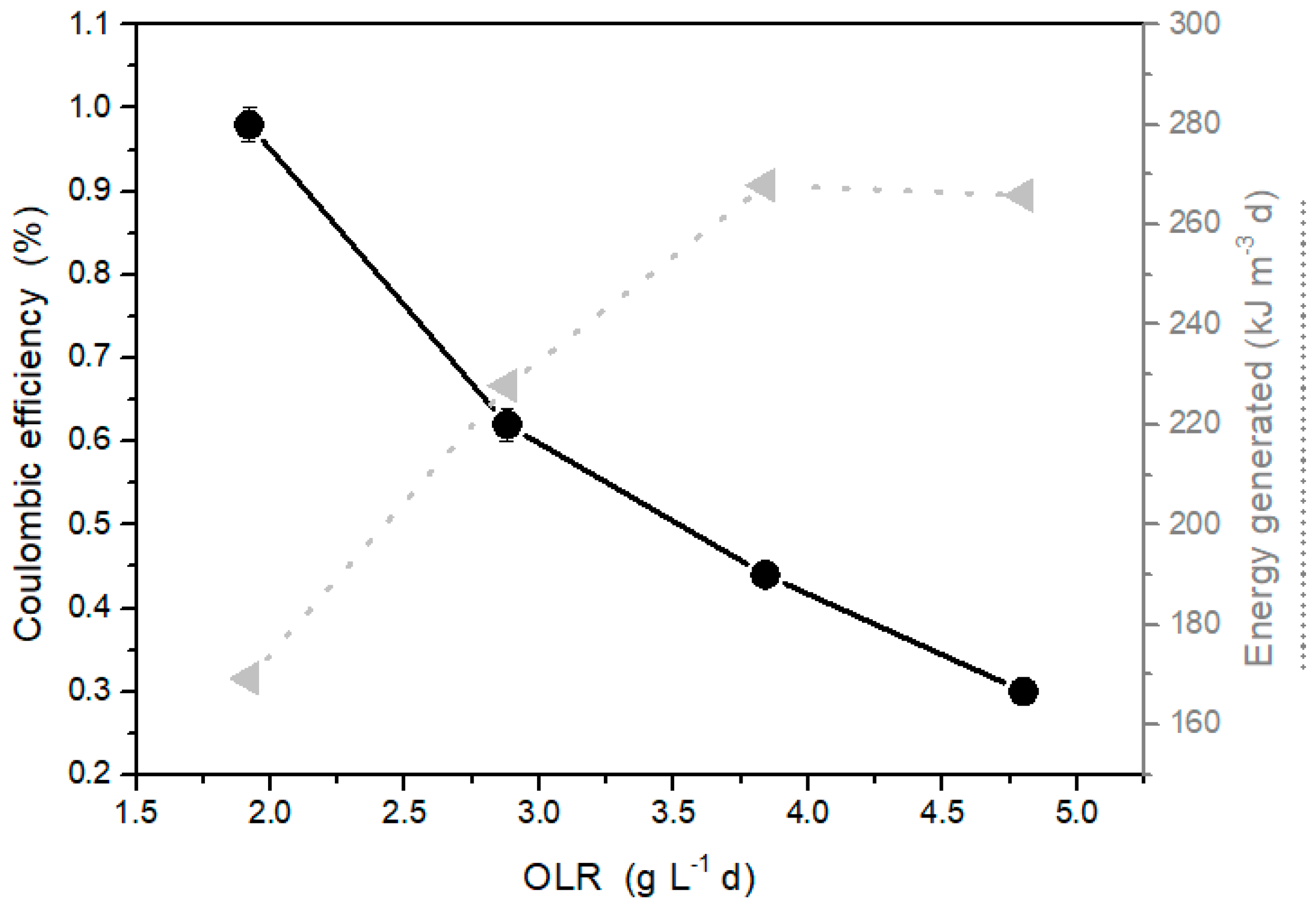
2.3. Effect of Organic Loading Rate
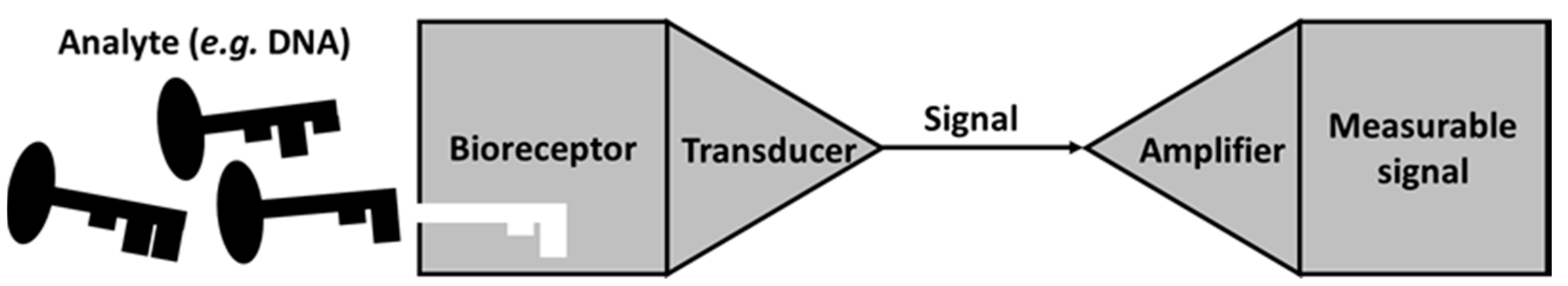
3. MFC as a Self-Powered Biosensor versus a Traditional Whole-Cell Biosensor
4. MFC-Based Biosensors for BOD Detection
5. MFC-Based Biosensors for Water Toxicity Detection
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mashkour, M.; Rahimnejad, M.; Mashkour, M. Bacterial cellulose-polyaniline nano-biocomposite: A porous media hydrogel bioanode enhancing the performance of microbial fuel cell. J. Power Sources 2016, 325, 322–328. [Google Scholar] [CrossRef]
- Santoro, C.; Arbizzani, C.; Erable, B.; Ieropoulos, I. Microbial fuel cells: From fundamentals to applications. A review. J. Power Sources 2017, 356, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, Y.Q.; Doherty, L.; Hu, Y.S.; Hao, X.D. The integrated processes for wastewater treatment based on the principle of microbial fuel cells: A review. Crit. Rev. Environ. Sci. Technol. 2016, 46, 60–91. [Google Scholar] [CrossRef]
- Nevin, K.P.; Woodard, T.L.; Franks, A.E.; Summers, Z.M.; Lovley, D.R. Microbial Electrosynthesis: Feeding Microbes Electricity to Convert Carbon Dioxide and Water to Multicarbon Extracellular Organic Compounds. mBio 2010, 1, e00103-10. [Google Scholar] [CrossRef] [PubMed]
- Villano, M.; Monaco, G.; Aulenta, F.; Majone, M. Electrochemically assisted methane production in a biofilm reactor. J. Power Sources 2011, 196, 9467–9472. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.A.; Schrorder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, S.B.; Srikanth, S.; Mohan, S.V.; Pant, D. Development of exoelectrogenic bioanode and study on feasibility of hydrogen production using abiotic VITO-CoRETM and VITO-CASE(TM) electrodes in a single chamber microbial electrolysis cell (MEC) at low current densities. Bioresour. Technol. 2015, 195, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Minteer, S.D.; Liaw, B.Y.; Cooney, M.J. Enzyme-based biofuel cells. Curr. Opin. Biotechnol. 2007, 18, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Luckarift, H.R.; Atanassov, P.B.; Johnson, G.R. (Eds.) Enzymatic Fuel Cells: From Fundamentals to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 1–468. [Google Scholar]
- Down, S. Microbial fuel cells. Chem. World 2005, 2, 12. [Google Scholar]
- Rahimnejad, M.; Adhami, A.; Darvari, S.; Zirepour, A.; Oh, S.E. Microbial fuel cell as new technology for bioelectricity generation: A review. Alex. Eng. J. 2015, 54, 745–756. [Google Scholar] [CrossRef]
- Nimje, V.R.; Chen, C.C.; Chen, H.R.; Chen, C.Y.; Tseng, M.J.; Cheng, K.C.; Shih, R.C.; Chang, Y.F. A Single-Chamber Microbial Fuel Cell without an Air Cathode. Int. J. Mol. Sci. 2012, 13, 3933–3948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.A.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Mashkour, M.; Rahimnejad, M.; Pourali, S.M.; Ezoji, H.; ElMekawy, A.; Pant, D. Catalytic performance of nano-hybrid graphene and titanium dioxide modified cathodes fabricated with facile and green technique in microbial fuel cell. Prog. Nat. Sci. Mater. Int. 2017, 27, 647–651. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M.; Mashkour, M.; Bakeri, G.; Luque, R.; Oh, S.E. Application of wet nanostructured bacterial cellulose as a novel hydrogel bioanode for microbial fuel cells. ChemElectroChem 2017, 4, 648–654. [Google Scholar] [CrossRef]
- Abrevaya, X.C.; Sacco, N.J.; Bonetto, M.C.; Hilding-Ohlsson, A.; Corton, E. Analytical applications of microbial fuel cells. Part II: Toxicity, microbial activity and quantification, single analyte detection and other uses. Biosens. Bioelectron. 2015, 63, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, S.F.Y. Cathode reactions and applications in microbial fuel cells: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2504–2525. [Google Scholar] [CrossRef]
- Ucar, D.; Zhang, Y.; Angelidaki, I. An overview of electron acceptors in microbial fuel cells. Front. Microbiol. 2017, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, J.; Gong, B. Series and parallel connection of anaerobic fluidized bed microbial fuel cells (MFCs). Int. J. Appl. Microbiol. Biotechnol. Res. 2016, 4, 7–14. [Google Scholar]
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Jafary, T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 2011, 88, 3999–4004. [Google Scholar] [CrossRef]
- Wang, M.; Li, Z.; Fang, X.; Wang, L.; Qu, Y. Biotechnology in China III: Biofuels and Bioenergy; Springer: Berlin, Germany, 2012; ISBN 9783642284786. [Google Scholar]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. Trends Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Lissens, G.; Siciliano, S.D.; Verstraete, W. A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol. Lett. 2003, 25, 1531–1535. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, K.; Boon, N.; Siciliano, S.D.; Verhaege, M.; Verstraete, W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 2004, 70, 5373–5382. [Google Scholar] [CrossRef] [PubMed]
- Schroder, U.; Niessen, J.; Scholz, F. A generation of microbial fuel cells with current outputs boosted by more than one order of magnitude. Angew. Chem. Int. Ed. 2003, 42, 2880–2883. [Google Scholar] [CrossRef] [PubMed]
- Constructed Wetlands. Available online: https://www.epa.gov/wetlands/constructed-wetlands (accessed on 17 July 2018).
- Corbella, C.; Puigagut, J.; Garfi, M. Life cycle assessment of constructed wetland systems for wastewater treatment coupled with microbial fuel cells. Sci. Total Environ. 2017, 584, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Zhou, M.H.; Liu, M.M.; Yang, W.L.; Gu, T.Y. Microbial fuel cells for biosensor applications. Biotechnol. Lett. 2015, 37, 2357–2364. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y.H. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Shantaram, A.; Beyenal, H.; Raajan, R.; Veluchamy, A.; Lewandowski, Z. Wireless sensors powered by microbial fuel cells. Environ. Sci. Technol. 2005, 39, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, A.; Sergeev, N.; Herold, K.E. Biosensor technologies for microbial and environmental analysis. Min. Biotecnol. 2007, 19, 105–116. [Google Scholar]
- Wilson, G.S.; Hu, Y.B. Enzyme based biosensors for in vivo measurements. Chem. Rev. 2000, 100, 2693–2704. [Google Scholar] [CrossRef] [PubMed]
- Rechnitz, G.A. Biosensors based on cell and tissue material. J. Biotechnol. 1990, 15, 201–217. [Google Scholar] [CrossRef]
- Wang, J.; Rivas, G.; Cai, X.; Palecek, E.; Nielsen, P.; Shiraishi, H.; Dontha, N.; Luo, D.; Parrado, C.; Chicharro, M.; et al. DNA electrochemical biosensors for environmental monitoring: A review. Anal. Chim. Acta 1997, 347, 1–8. [Google Scholar] [CrossRef]
- Tombelli, S.; Minunni, M.; Mascini, M. Piezoelectric biosensors: Strategies for coupling nucleic acids to piezoelectric devices. Methods 2005, 37, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Renschler, C.L.; White, C.A.; Carter, R.M. Piezoelectric Biosensor with a Ladder Polymer Substrate Coating. U.S. Patent US5814525A, 29 September 1998. [Google Scholar]
- Mosbach, K. Thermal biosensors. Biosens. Bioelectron. 1991, 6, 179–182. [Google Scholar] [CrossRef]
- Schaertel, B.J.; Firstenbergeden, R. Biosensors in the food-industry—Present and future. J. Food Prot. 1988, 51, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Bouvrette, P.; Male, K.B. Developments and applications of biosensors in food analysis. Trends Biotechnol. 1997, 15, 369–377. [Google Scholar] [CrossRef]
- Spichiger-Keller, U.E. Chemical Sensors and Biosensors for Medical and Biological Applications; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2007; ISBN 9783527288557. [Google Scholar]
- Mills, G.; Fones, G. A review of in situ methods and sensors for monitoring the marine environment. Sens. Rev. 2012, 32, 17–28. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhou, M.; Dong, S.J. A Self-Powered Acetaldehyde Sensor Based on Biofuel Cell. Anal. Chem. 2012, 84, 10345–10349. [Google Scholar] [CrossRef] [PubMed]
- Asghary, M.; Raoof, J.B.; Rahimnejad, M.; Ojani, R. A novel self-powered and sensitive label-free DNA biosensor in microbial fuel cell. Biosens. Bioelectron. 2016, 82, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Dumas, C.; Mollica, A.; Feron, D.; Basseguy, R.; Etcheverry, L.; Bergel, A. Marine microbial fuel cell: Use of stainless steel electrodes as anode and cathode materials. Electrochim. Acta 2007, 53, 468–473. [Google Scholar] [CrossRef] [Green Version]
- Chang, I.S.; Jang, J.K.; Gil, G.C.; Kim, M.; Kim, H.J.; Cho, B.W.; Kim, B.H. Continuous determination of biochemical oxygen demand using microbial fuel cell type biosensor. Biosens. Bioelectron. 2004, 19, 607–613. [Google Scholar] [CrossRef]
- Marzorati, S.; Schievano, A.; Colombo, A.; Lucchini, G.; Cristiani, P. Ligno-cellulosic materials as air-water separators in low-tech microbial fuel cells for nutrients recovery. J. Clean. Prod. 2018, 170, 1167–1176. [Google Scholar] [CrossRef]
- Agubra, V.; Fergus, J. Lithium Ion Battery Anode Aging Mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donovan, C.; Dewan, A.; Peng, H.A.; Heo, D.; Beyenal, H. Power management system for a 2.5W remote sensor powered by a sediment microbial fuel cell. J. Power Sources 2011, 196, 1171–1177. [Google Scholar] [CrossRef]
- Zhang, F.; Tian, L.; He, Z. Powering a wireless temperature sensor using sediment microbial fuel cells with vertical arrangement of electrodes. J. Power Sources 2011, 196, 9568–9573. [Google Scholar] [CrossRef]
- Yang, Y.G.; Sun, G.P.; Xu, M.Y. Microbial fuel cells come of age. J. Chem. Technol. Biotechnol. 2011, 86, 625–632. [Google Scholar] [CrossRef]
- Dewan, A.; Ay, S.U.; Karim, M.N.; Beyenal, H. Alternative power sources for remote sensors: A review. J. Power Sources 2014, 245, 129–143. [Google Scholar] [CrossRef]
- Liliana Alzate-Gaviria. Microbial Fuel Cells for Wastewater Treatment, Waste Water—Treatment and Reutilization. Prof., Einschlag, Fernando Sebastián García, Ed.; InTech, 2011. Available online: http://www.intechopen.com/books/waste-water-treatment-and-reutilization/microbial-fuel-cells-for-wastewater-treatment (accessed on 17 July 2018).
- Jiang, Y.; Liang, P.; Liu, P.P.; Yan, X.X.; Bian, Y.H.; Huang, X. A cathode-shared microbial fuel cell sensor array for water alert system. Int. J. Hydrogen Energy 2017, 42, 4342–4348. [Google Scholar] [CrossRef]
- Schievano, A.; Colombo, A.; Grattieri, M.; Trasatti, S.P.; Liberale, A.; Tremolada, P.; Pino, C.; Cristiani, P. Floating microbial fuel cells as energy harvesters for signal transmission from natural water bodies. J. Power Sources 2017, 340, 80–88. [Google Scholar] [CrossRef]
- Oliveira, V.B.; Simoes, M.; Melo, L.F.; Pinto, A. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013, 73, 53–64. [Google Scholar] [CrossRef]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
- Jadhav, G.S.; Ghangrekar, M.M. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour. Technol. 2009, 100, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Savadogo, O.; Guiot, S.R.; Tartakovsky, B. The influence of operational conditions on the performance of a microbial fuel cell seeded with mesophilic anaerobic sludge. Biochem. Eng. J. 2010, 51, 132–139. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Larios, A.L.; Rios-Leal, E.; Solorza-Feria, O.; Poggi-Varaldo, H.M. Effect of the temperature on two types microbial fuel cells performance. J. Biotechnol. 2010, 150, S145–S146. [Google Scholar] [CrossRef]
- del Campo, A.G.; Lobato, J.; Canizares, P.; Rodrigo, M.A.; Morales, F.J.F. Short-term effects of temperature and COD in a microbial fuel cell. Appl. Energy 2013, 101, 213–217. [Google Scholar] [CrossRef]
- Min, B.; Roman, O.B.; Angelidaki, I. Importance of temperature and anodic medium composition on microbial fuel cell (MFC) performance. Biotechnol. Lett. 2008, 30, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.J.; Lee, H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci. Technol. 2008, 57, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Chang, I.S.; Choi, Y.S.; Chung, T.H. Experimental evaluation of influential factors for electricity harvesting from sediment using microbial fuel cell. Bioresour. Technol. 2009, 100, 3029–3035. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.A.; Harnisch, F.; Koch, C.; Hubschmann, T.; Fetzer, I.; Carmona-Martinez, A.A.; Muller, S.; Schroder, U. Electroactive mixed culture derived biofilms in microbial bioelectrochemical systems: The role of pH on biofilm formation, performance and composition. Bioresour. Technol. 2011, 102, 9683–9690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Climent, V.; Berna, A.; Feliu, J.M. Effect of Temperature on the Catalytic Ability of Electrochemically Active Biofilm as Anode Catalyst in Microbial Fuel Cells. Electroanalysis 2011, 23, 387–394. [Google Scholar] [CrossRef]
- Behera, M.; Murthy, S.S.R.; Ghangrekar, M.M. Effect of operating temperature on performance of microbial fuel cell. Water Sci. Technol. 2011, 64, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wu, C.; Zhang, J.Q.; Chi, Q.L.; Tian, S.S. Acclimation stage on the performance of microbial fuel cells subjected to variation in COD, temperature, and electron acceptor. Adv. Mater. Res. 2011, 183–185, 2346–2350. [Google Scholar] [CrossRef]
- Michie, I.S.; Kim, J.R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Operational temperature regulates anodic biofilm growth and the development of electrogenic activity. Appl. Microbiol. Biotechnol. 2011, 92, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.L.; He, Y.T.; Yu, P.F.; Sun, H.; Fu, J.X. Effect of temperature on electricity generation of single-chamber microbial fuel cells with proton exchange membrane. Biotechnol. Chem. Mater. Eng. 2012, 393–395, 1169–1172. [Google Scholar] [CrossRef]
- Michie, I.S.; Kim, J.R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. The influence of psychrophilic and mesophilic start-up temperature on microbial fuel cell system performance. Energy Environ. Sci. 2011, 4, 1011–1019. [Google Scholar] [CrossRef]
- Cheng, S.A.; Xing, D.F.; Logan, B.E. Electricity generation of single-chamber microbial fuel cells at low temperatures. Biosens. Bioelectron. 2011, 26, 1913–1917. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Mohan, S.V.; Reddy, M.V.; Mohanakrishna, G.; Sarma, P.N. Behavior of single chambered mediatorless microbial fuel cell (MFC) at acidophilic, neutral and alkaline microenvironments during chemical wastewater treatment. Int. J. Hydrogen Energy 2009, 34, 7547–7554. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Ho, G.; Cord-Ruwisch, R. Anodophilic Biofilm Catalyzes Cathodic Oxygen Reduction. Environ. Sci. Technol. 2010, 44, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Raghavulu, S.V.; Mohan, S.V.; Goud, R.K.; Sarma, P.N. Effect of anodic pH microenvironment on microbial fuel cell (MFC) performance in concurrence with aerated and ferricyanide catholytes. Electrochem. Commun. 2009, 11, 371–375. [Google Scholar] [CrossRef]
- Erable, B.; Etcheverry, L.; Bergel, A. Increased power from a two-chamber microbial fuel cell with a low-pH air-cathode compartment. Electrochem. Commun. 2009, 11, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Behera, M.; Ghangrekar, M.M. Performance of microbial fuel cell in response to change in sludge loading rate at different anodic feed pH. Bioresour. Technol. 2009, 100, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Serra, M.; Coma, M.; Cabre, M.; Balaguer, M.D.; Colprim, J. Effect of pH on nutrient dynamics and electricity production using microbial fuel cells. Bioresour. Technol. 2010, 101, 9594–9599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Li, C.; Ding, L.L.; Xu, K.; Ren, H.Q. Influences of initial pH on performance and anodic microbes of fed-batch microbial fuel cells. J. Chem. Technol. Biotechnol. 2011, 86, 1226–1232. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, B.; Zhou, S.G.; Zhong, S.K.; Zhuang, L. Electrocatalytic activity of anodic biofilm responses to pH changes in microbial fuel cells. Bioresour. Technol. 2011, 102, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Mench, M.M.; Regan, J.M. Impedance Characteristics and Polarization Behavior of a Microbial Fuel Cell in Response to Short-Term Changes in Medium pH. Environ. Sci. Technol. 2011, 45, 9069–9074. [Google Scholar] [CrossRef] [PubMed]
- Nimje, V.R.; Chen, C.Y.; Chen, C.C.; Tsai, J.Y.; Chen, H.R.; Huang, Y.M.; Jean, J.S.; Chang, Y.F.; Shih, R.C. Microbial fuel cell of Enterobacter cloacae: Effect of anodic pH microenvironment on current, power density, internal resistance and electrochemical losses. Int. J. Hydrogen Energy 2011, 36, 11093–11101. [Google Scholar] [CrossRef]
- He, Z.; Huang, Y.L.; Manohar, A.K.; Mansfeld, F. Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry 2008, 74, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Z.; Hu, H.Q.; Liu, H. Sustainable power generation in microbial fuel cells using bicarbonate buffer and proton transfer mechanisms. Environ. Sci. Technol. 2007, 41, 8154–8158. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, S.H.; Li, M.; Wei, Y. Simultaneous pollutant removal and electricity generation in denitrifying microbial fuel cell with boric acid-borate buffer solution. Water Sci. Technol. 2015, 71, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Kim, H.W.; Lim, K.H.; Shin, H.S.; Logan, B.E. Variation of power generation at different buffer types and conductivities in single chamber microbial fuel cells. Biosens. Bioelectron. 2010, 25, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Fornero, J.J.; Rosenbaum, M.; Cotta, M.A.; Angenent, L.T. Carbon Dioxide Addition to Microbial Fuel Cell Cathodes Maintains Sustainable Catholyte pH and Improves Anolyte pH, Alkalinity, and Conductivity. Environ. Sci. Technol. 2010, 44, 2728–2734. [Google Scholar] [CrossRef] [PubMed]
- Biffinger, J.C.; Pietron, J.; Bretschger, O.; Nadeau, L.J.; Johnson, G.R.; Williams, C.C.; Nealson, K.H.; Ringeisen, B.R. The influence of acidity on microbial fuel cells containing Shewanella oneidensis. Biosens. Bioelectron. 2008, 24, 900–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, L.; Zhou, S.G.; Li, Y.T.; Yuan, Y. Enhanced performance of air-cathode two-chamber microbial fuel cells with high-pH anode and low-pH cathode. Bioresour. Technol. 2010, 101, 3514–3519. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, J.; Ye, D.D.; Zhang, L.; Zhu, X.; Liao, Q. A hybrid microbial fuel cell stack based on single and double chamber microbial fuel cells for self-sustaining pH control. J. Power Sources 2016, 306, 685–691. [Google Scholar] [CrossRef]
- Mohan, S.V.; Raghavulu, S.V.; Srikanth, S.; Sarma, P.N. Bioelectricity production by mediatorless microbial fuel cell under acidophilic condition using wastewater as substrate: Influence of substrate loading rate. Curr. Sci. 2007, 92, 1720–1726. [Google Scholar]
- Aelterman, P.; Versichele, M.; Marzorati, M.; Boon, N.; Verstraete, W. Loading rate and external resistance control the electricity generation of microbial fuel cells with different three-dimensional anodes. Bioresour. Technol. 2008, 99, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Raghavulu, S.V.; Peri, D.; Sarma, P.N. Integrated function of microbial fuel cell (MFC) as bio-electrochemical treatment system associated with bioelectricity generation under higher substrate load. Biosens. Bioelectron. 2009, 24, 2021–2027. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Scott, K.; Curtis, T.P.; Head, I.M. Effect of increasing anode surface area on the performance of a single chamber microbial fuel cell. Chem. Eng. J. 2010, 156, 40–48. [Google Scholar] [CrossRef]
- Reddy, M.V.; Srikanth, S.; Mohan, S.V.; Sarma, P.N. Phosphatase and dehydrogenase activities in anodic chamber of single chamber microbial fuel cell (MFC) at variable substrate loading conditions. Bioelectrochemistry 2010, 77, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Premier, G.C.; Hawkes, F.R.; Rodriguez, J.; Dinsdale, R.M.; Guwy, A.J. Modular tubular microbial fuel cells for energy recovery during sucrose wastewater treatment at low organic loading rate. Bioresour. Technol. 2010, 101, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.Y.; Kim, H.W.; Lim, K.H.; Shin, H.S. Effects of organic loading rates on the continuous electricity generation from fermented wastewater using a single-chamber microbial fuel cell. Bioresour. Technol. 2010, 101, S33–S37. [Google Scholar] [CrossRef] [PubMed]
- Juang, D.F.; Yang, P.C.; Chou, H.Y.; Chiu, L.J. Effects of microbial species, organic loading and substrate degradation rate on the power generation capability of microbial fuel cells. Biotechnol. Lett. 2011, 33, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Goud, R.K.; Babu, P.S.; Mohan, S.V. Canteen based composite food waste as potential anodic fuel for bioelectricity generation in single chambered microbial fuel cell (MFC): Bio-electrochemical evaluation under increasing substrate loading condition. Int. J. Hydrogen Energy 2011, 36, 6210–6218. [Google Scholar] [CrossRef]
- Velvizhi, G.; Mohan, S.V. Electrogenic activity and electron losses under increasing organic load of recalcitrant pharmaceutical wastewater. Int. J. Hydrogen Energy 2012, 37, 5969–5978. [Google Scholar] [CrossRef]
- Fan, Y.Z.; Sharbrough, E.; Liu, H. Quantification of the Internal Resistance Distribution of Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- HaoYu, E.; Cheng, S.; Scott, K.; Logan, B. Microbial fuel cell performance with non-Pt cathode catalysts. J. Power Sources 2007, 171, 275–281. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, P.; Zhang, C.Y.; Bian, Y.H.; Yang, X.F.; Huang, X.; Girguis, P.R. Enhancing the response of microbial fuel cell based toxicity sensors to Cu(II) with the applying of flow-through electrodes and controlled anode potentials. Bioresour. Technol. 2015, 190, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Modin, O.; Wilen, B.M. A novel bioelectrochemical BOD sensor operating with voltage input. Water Res. 2012, 46, 6113–6120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jouanneau, S.; Recoules, L.; Durand, M.J.; Boukabache, A.; Picot, V.; Primault, Y.; Lakel, A.; Sengelin, M.; Barillon, B.; Thouand, G. Methods for assessing biochemical oxygen demand (BOD): A review. Water Res. 2014, 49, 62–82. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; de Alda, M.J.L.; Barcelo, D. Biosensors as useful tools for environmental analysis and monitoring. Anal. Bioanal. Chem. 2006, 386, 1025–1041. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Z.; Kingori, G.P.; Si, R.W.; Zhai, D.D.; Liao, Z.H.; Sun, D.Z.; Zheng, T.; Yong, Y.C. Microbial fuel cell-based biosensors for environmental monitoring: A review. Water Sci. Technol. 2015, 71, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Jeong, C.K.; Hwang, G.T.; Lee, K.J. Self-powered flexible inorganic electronic system. Nano Energy 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Cheng, L.; Yuan, M.M.; Gu, L.; Wang, Z.; Qin, Y.; Jing, T.; Wang, Z.L. Wireless, power-free and implantable nanosystem for resistance-based biodetection. Nano Energy 2015, 15, 598–606. [Google Scholar] [CrossRef]
- Kougianos, E.; Mohanty, S.P. Biosensors: A tutorial review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, W.; Mulchandani, A. Microbial biosensors. Anal. Chim. Acta 2006, 568, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Mashkour, M.; Rahimnejad, M. Effect of various carbon-based cathode electrodes on the performance of microbial fuel cell. Biofuel Res. J. BRJ 2015, 2, 296–300. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.H.; Chang, I.S.; Gil, G.C.; Park, H.S.; Kim, H.J. Novel BOD (biological oxygen demand) sensor using mediator-less microbial fuel cell. Biotechnol. Lett. 2003, 25, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.; Min, B.; Martins, G.; Brito, A.G.; Kroff, P.; Parpot, P.; Angelidaki, I.; Nogueira, R. In situ microbial fuel cell-based biosensor for organic carbon. Bioelectrochemistry 2011, 81, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Ayyaru, S.; Dharmalingam, S. Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal. Chim. Acta 2014, 818, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21. [Google Scholar] [CrossRef] [PubMed]
- Consortium, C.T.A. Design concepts for the Cherenkov Telescope Array CTA: An advanced facility for ground-based high-energy gamma-ray astronomy. Exp. Astron. 2011, 32, 193–316. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Han, J.I. Fast detection and quantification of Escherichia coli using the base principle of the microbial fuel cell. J. Environ. Manag. 2013, 130, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Chang, I.S.; Kang, K.H.; Jang, J.K.; Kim, B.H. Improving the dynamic response of a mediator-less microbial fuel cell as a biochemical oxygen demand (BOD) sensor. Biotechnol. Lett. 2004, 26, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Gil, G.C.; Chang, I.S.; Kim, B.H.; Kim, M.; Jang, J.K.; Park, H.S.; Kim, H.J. Operational parameters affecting the performance of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef]
- Moon, H.; Chang, I.S.; Jang, J.K.; Kim, K.S.; Lee, J.; Lovitt, R.W.; Kim, B.H. On-line monitoring of low biochemical oxygen demand through continuous operation of a mediator-less microbial fuel cell. J. Microbiol. Biotechnol. 2005, 15, 192–196. [Google Scholar]
- Kang, K.H.; Jang, J.K.; Pham, T.H.; Moon, H.; Chang, I.S.; Kim, B.H. A microbial fuel cell with improved cathode reaction as a low biochemical oxygen demand sensor. Biotechnol. Lett. 2003, 25, 1357–1361. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hyun, M.S.; Gadd, G.M.; Kim, H.J. A novel biomonitoring system using microbial fuel cells. J. Environ. Monit. 2007, 9, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Stein, N.E.; Hamelers, H.M.V.; van Straten, G.; Keesman, K.J. On-line detection of toxic components using a microbial fuel cell-based biosensor. J. Process Control 2012, 22, 1755–1761. [Google Scholar] [CrossRef]
- Stein, N.E.; Hamelers, H.V.M.; Buisman, C.N.J. Stabilizing the baseline current of a microbial fuel cell-based biosensor through overpotential control under non-toxic conditions. Bioelectrochemistry 2010, 78, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, N.S.J.; Zhou, Q.X. Concentration responses of toxicity sensor with Shewanella oneidensis MR-1 growing in bioelectrochemical systems. Biosens. Bioelectron. 2013, 43, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Angelidaki, I. Submersible Microbial Fuel Cell Sensor for Monitoring Microbial Activity and BOD in Groundwater: Focusing on Impact of Anodic Biofilm on Sensor Applicability. Biotechnol. Bioeng. 2011, 108, 2339–2347. [Google Scholar] [CrossRef] [PubMed]
- Tront, J.M.; Fortner, J.D.; Plotze, M.; Hughes, J.B.; Puzrin, A.M. Microbial fuel cell biosensor for in situ assessment of microbial activity. Biosens. Bioelectron. 2008, 24, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Angelidaki, I. A simple and rapid method for monitoring dissolved oxygen in water with a submersible microbial fuel cell (SBMFC). Biosens. Bioelectron. 2012, 38, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Vishwanathan, A.S.; Rao, G.; Sai, S.S.S. A novel minimally invasive method for monitoring oxygen in microbial fuel cells. Biotechnol. Lett. 2013, 35, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Min, B.; Logan, B.E. Cathode performance as a factor in electricity generation in microbial fuel cells. Environ. Sci. Technol. 2004, 38, 4900–4904. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kim, J.R.; Michie, I.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. ; Sustainable Environment Research Centre (SERC). Microbial fuel cell type biosensor for specific volatile fatty acids using acclimated bacterial communities. Biosens. Bioelectron. 2013, 47, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Liu, J.; Zhang, S.P.; Xing, X.H.; Su, Z.G. Microbial fuel cell based biosensor for in situ monitoring of anaerobic digestion process. Bioresour. Technol. 2011, 102, 10221–10229. [Google Scholar] [CrossRef] [PubMed]
- Reuther, C.G. Water Environment Federation. Environ. Health Perspect. 2000, 108, A63. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. (Eds.) Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Liu, J.; Olsson, G.; Mattiasson, B. Short-term BOD (BODst) as a parameter for on-line monitoring of biological treatment process Part 1. A novel design of BOD biosensor for easy renewal of bio-receptor. Biosens. Bioelectron. 2004, 20, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.H.; Kayode, O.; Harper, W.F. Using microbial fuel cell output metrics and nonlinear modeling techniques for smart biosensing. Sci. Total Environ. 2013, 449, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Chae, K.J.; Choi, M.J.; Lee, J.W.; Kim, K.Y.; Kim, I.S. Effect of different substrates on the performance, bacterial diversity, and bacterial viability in microbial fuel cells. Bioresour. Technol. 2009, 100, 3518–3525. [Google Scholar] [CrossRef] [PubMed]
- Abrevaya, X.C.; Sacco, N.J.; Bonetto, M.C.; Hilding-Ohlsson, A.; Corton, E. Analytical applications of microbial fuel cells. Part I: Biochemical oxygen demand. Biosens. Bioelectron. 2015, 63, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, N.; Buning, C.; Pfeifer, F.; Dohrmann, A.B.; Tebbe, C.C.; Nijenhuis, I.; Kastner, M.; Richnow, H.H. In situ microcosms to evaluate natural attenuation potentials in contaminated aquifers. Org. Geochem. 2006, 37, 1394–1410. [Google Scholar] [CrossRef]
- Fossing, H.; Jorgensen, B.B. Oxidation and reduction of radiolabeled inorganic sulfur-compounds in an estuarine sediment, kysing fjord, Denmark. Geochim. Cosmochim. Acta 1990, 54, 2731–2742. [Google Scholar] [CrossRef]
- Kharkwal, S.; Tan, Y.C.; Lu, M.; Ng, H.Y. Development and Long-Term Stability of a Novel Microbial Fuel Cell BOD Sensor with MnO2 Catalyst. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.J.; Lefebvre, O.; Tan, Z.; Ng, H.Y. Microbial fuel-cell-based toxicity sensor for fast monitoring of acidic toxicity. Water Sci. Technol. 2012, 65, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Clean Water Act Analytical Methods. Available online: https://www.epa.gov/cwa-methods/ (accessed on 17 July 2018).
- Davila, D.; Esquivel, J.P.; Sabate, N.; Mas, J. Silicon-based microfabricated microbial fuel cell toxicity sensor. Biosens. Bioelectron. 2011, 26, 2426–2430. [Google Scholar] [CrossRef] [PubMed]



| Parameter Measured | Power, Voltage, or Current | Detection Range | Reference |
|---|---|---|---|
| BOD5 | 0.26–0.90 mA | 32–1280 mg L−1 | [119] |
| 0.063–0.55 mA | - | [120] | |
| 72 mW m−2 | 17–183 mg L−1 | [121] | |
| 3.7–5.2 mA | 20–200 mg L−1 | [48] | |
| 0.05–1.1 mA | 2.6–206 mg L−1 | [122] | |
| 0.7–1.9 mA | 50–100 mg L−1 | [123] | |
| 0.2–1.7 mA | - | [124] | |
| 0.05–8 µA | 2–10 mg L−1 | [125] | |
| 0.0015–0.2 mA | [126] | ||
| Organophosphorus | 0.005–0.042 mA | 1–10 mg L−1 | [127] |
| Cd(II) and Pb(II) | 0.005–0.035 mA | 0.1–1 mg L−1 | [127] |
| Ni | 0.15–2.25 mA | 10–30 mg L−1 | [128] |
| 0.022–0.132 A m−2 | 0–88 mg L−1 | [128] | |
| Na dodecyl sulfate | 0.85–1.7 mA | 10–50 mg L−1 | [128] |
| Bentazon | 0.9–1.4 mA | 1–3 mg L−1 | [128] |
| Cu | 0.7–1.5 A m−2 | - | [129] |
| Formaldehyde | 0.05 ± 0.04–0.1 ± 0.03 mA | - | [130] |
| E. Coli | 0.1–0.38 mA | - | [122] |
| Microbial activity | 0.6–12.4 A m−2 | 0–13 nmol l−1 | [131] |
| 0–0.30 mA | - | [132] | |
| Oxygen Dissolved | 5.6–462 mA m−2 | 0–8.8 mg L−1 | [133] |
| 9.5–17 mW m−3 | - | [134] | |
| 0–0.092 mW | 0–8 mg L−1 | [135] | |
| Volatile fatty acids | 0.22–1.29 mA | 0–40 mg L−1 | [136] |
| Anaerobic digestion | 0.01–0.095 mA | - | [137] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivars-Barceló, F.; Zuliani, A.; Fallah, M.; Mashkour, M.; Rahimnejad, M.; Luque, R. Novel Applications of Microbial Fuel Cells in Sensors and Biosensors. Appl. Sci. 2018, 8, 1184. https://doi.org/10.3390/app8071184
Ivars-Barceló F, Zuliani A, Fallah M, Mashkour M, Rahimnejad M, Luque R. Novel Applications of Microbial Fuel Cells in Sensors and Biosensors. Applied Sciences. 2018; 8(7):1184. https://doi.org/10.3390/app8071184
Chicago/Turabian StyleIvars-Barceló, Francisco, Alessio Zuliani, Marjan Fallah, Mehrdad Mashkour, Mostafa Rahimnejad, and Rafael Luque. 2018. "Novel Applications of Microbial Fuel Cells in Sensors and Biosensors" Applied Sciences 8, no. 7: 1184. https://doi.org/10.3390/app8071184





