Sildenafil Citrate Liposomes for Pulmonary Delivery by Ultrasonic Nebulization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
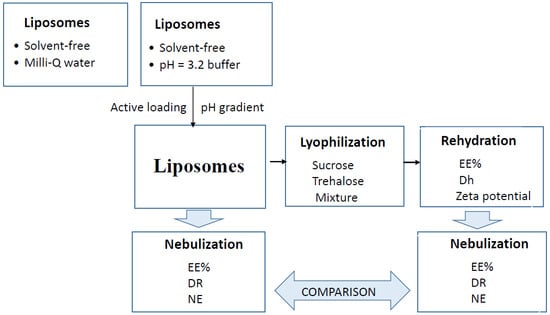
2.2. Preparation of Liposomes
2.3. Lyophilization
2.4. Nebulization
2.5. Characterization of Liposomes
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barst, R.J.; Ertel, S.I.; Beghetti, M.; Ivy, D.D. Pulmonary arterial hypertension: A comparison between children and adults. Eur. Respir. J. 2011, 37, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Frumkin, L.R. The Pharmacological Treatment of Pulmonary Arterial Hypertension. Pharmacol. Rev. 2012, 64, 583–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghofrani, H.A.; Osterloh, I.H.; Grimminger, F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat. Rev. Drug Discov. 2006, 5, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Simonca, L.; Tulloh, R. Sildenafil in Infants and Children. Children 2017, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.D.; Sampson, M.R.; Li, J.S.; Tunks, R.D.; Schulman, S.R.; Cohen-Wolkowiez, M. Pharmacokinetics of intravenous sildenafil in children with palliated single ventricle heart defects: Effect of elevated hepatic pressures. Cardiol. Young 2016, 26, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Weerateerangkul, P.; Palee, S.; Chinda, K.; Chattipakorn, S.C.; Chattipakorn, N. Effects of Kaempferia parviflora Wall. Ex. Baker and sildenafil citrate on cGMP level, cardiac function, and intracellular Ca2+ regulation in rat hearts. J. Cardiovasc. Pharmacol. 2012, 60, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Corbin, J.D.; Francis, S.H. Cyclic GMP phosphodiesterase-5: Target of sildenafil. J. Biol. Chem. 1999, 274, 13729–13732. [Google Scholar] [CrossRef] [PubMed]
- Moschos, M.M.; Nitoda, E. Pathophysiology of visual disorders induced by phosphodiesterase inhibitors in the treatment of erectile dysfunction. Drug Des. Dev. Ther. 2016, 8, 3407–3413. [Google Scholar] [CrossRef] [PubMed]
- Samiee-Zafarghandy, S.; Smith, P.B.; van den Anker, J.N. Safety of Sildenafil in Infants. Pediatr. Crit. Care Med. 2014, 15, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Chono, S.; Tanino, T.; Seki, T.; Morimoto, K. Efficient drug targeting to rat alveolar macrophages by pulmonary administration of ciprofloxacin incorporated into mannosylated liposomes for treatment of respiratory intracellular parasitic infections. J. Control Release 2008, 127, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Pinto-Alphandary, H.; Andremont, A.; Couvreur, P. Targeted delivery of antibiotics using liposomes and nanoparticles: Research and applications. Int. J. Antimicrob. Agents 2000, 13, 155–168. [Google Scholar] [CrossRef]
- Kurmi, B.D.; Kayat, J.; Gajbhiye, V.; Tekade, R.K.; Jain, N.K. Micro- and nanocarrier-mediated lung targeting. Expert Opin. Drug Deliv. 2010, 7, 781–794. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Valle, M.J.; González López, F.; Domínguez-Gil Hurlé, A.; Sánchez Navarro, A. Pulmonary versus systemic delivery of antibiotics: Comparison of vancomycin dispositions in the isolated rat lung. Antimicro. Agents Chemother. 2007, 51, 3771–3774. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Valle, M.J.; Garavís González, J.; González López, F.; Sánchez Navarro, A. Pulmonary disposition of vancomycin nebulized as lipid vesicles in rats. J. Antibiot. 2013, 66, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Rudokas, M.; Najlah, M.; Alhnan, M.A.; Elhissi, A. Liposome Delivery Systems for Inhalation: A Critical Review Highlighting Formulation Issues and Anticancer Applications. Med. Princ. Pract. 2016, 25 (Suppl. 2), 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saari, M.; Vidgren, M.T.; Koskinen, M.O.; Turjanmaa, V.M.; Nieminen, M.M. Pulmonary distribution and clearance of two beclomethasone liposome formulations in healthy volunteers. Int. J. Pharm. 1999, 181, 1–9. [Google Scholar] [CrossRef]
- Clancy, J.P.; Dupont, L.; Konstan, M.W.; Billings, J.; Fustik, S.; Goss, C.H.; Lymp, J.; Minic, P.; Quittner, A.L.; Rubenstein, R.C.; et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas aeruginosa infection. Thorax 2013, 68, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhan, S.; Liu, Q.; Su, H.; Dai, X.; Wang, H.; Beng, H.; Tan, W. Preparation of a Sustained-Release Nebulized Aerosol of R-terbutaline Hydrochloride Liposome and Evaluation of Its Anti-asthmatic Effects via Pulmonary Delivery in Guinea Pigs. AAPS Pharm. Sci. Tech. 2018, 19, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Makled, S.; Nafee, N.; Boraie, N. Nebulized solid lipid nanoparticles for the potential treatment of pulmonary hypertension via targeted delivery of phosphodiesterase-5-inhibitor. Int. J. Pharm. 2017, 517, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Paranjpea, M.; Finkea, J.H.; Richterc, C.; Gothschb, T.; Kwadeb, A.; Büttgenbachc, S.; Müller-Goymanna, C.C. Physicochemical characterization of sildenafil-loaded solid lipid nanoparticle dispersions (SLN) for pulmonary application. Int. J. Pharm. 2014, 476, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Schmehl, T.; Gessler, T.; Seeger, W.; Kissel, T. Development of a biodegradable nanoparticle platform for sildenafil: Formulation optimization by factorial design analysis combined with application of charge-modified branched polyesters. J. Control. Release 2012, 157, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Kleimann, P.; Gessler, T.; Seeger, W.; Kissel, T.; Schmehl, T. Nebulization performance of biodegradable sildenafil-loaded nanoparticles using the Aeroneb Pro: Formulation aspects and nanoparticle stability to nebulization. Int. J. Pharm. 2012, 422, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Hecker, A.; Kosanovic, D.; Schmehl, T.; Gessler, T.; Weissmann, N.; Ghofrani, H.A.; Kissel, T.; Seeger, W.; Schermuly, R.T. Prolonged vasodilatory response to nanoencapsulated sildenafil in pulmonary hypertension. Nanomedicine 2016, 12, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Beck-Broichsitter, M.; Stoisiek, K.; Bohr, A.; Aragão-Santiago, L.; Gessler, T.; Seeger, W.; Kissel, T. Potential of the isolated lung technique for the examination of sildenafil absorption from lung-delivered poly(lactide-co-glycolide) microparticles. J. Control Release 2016, 226, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ghasemian, E.; Vatanara, A.; Rouini, M.R.; Rouholamini Najafabadi, A.; Gilani, K.; Lavasani, H.; Mohajel, N. Inhaled sildenafil nanocomposites: Lung accumulation and pulmonary pharmacokinetics. Pharm. Dev. Technol. 2016, 21, 961–971. [Google Scholar] [CrossRef] [PubMed]
- Refai, H.; Hassan, D.; Abdelmonem, R. Development and characterization of polymer coated liposomes for vaginal delivery of sildenafil citrate. Drug Deliv. 2017, 24, 278–288. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Valle, M.J.; de la Cuesta Melgar, E.; Martín Rebellado, S.; López Díaz, D.; Velázquez Salicio, M.; Sánchez Navarro, A. Sildenafil citrate-loaded liposomes and albusomes as drug carriers for pulmonary delivery. Stability after nebulization. In Proceedings of the 11th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology, Granada, Spain, 19–22 March 2018. [Google Scholar]
- Elhissi, A. Liposomes for Pulmonary Drug Delivery: The Role of Formulation and Inhalation Device Design. Curr. Pharm. Des. 2017, 23, 362–372. [Google Scholar] [PubMed]
- Lehofer, B.; Bloder, F.; Jain, P.P.; Marsh, L.M.; Leitinger, G.; Olschewski, H.; Leber, R.; Olschewski, A.; Prassl, R. Impact of atomization technique on the stability and transport efficiency of nebulized liposomes harbouring different surface characteristics. Eur. J. Pharm. Biopharm. 2014, 88, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Bangham, J.A.; Lea, E.J. The interaction of detergents with bilayer lipid membranes. Biochim. Biophys. Acta 1978, 551, 388–396. [Google Scholar] [CrossRef]
- Wang, T.; Wang, N.; Wang, T.; Sun, W.; Li, T. Preparation of submicron liposomes exhibiting efficient entrapment of drugs by freeze-drying water-in-oil emulsions. Chem. Phys. Lipids 2011, 164, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Zawada, Z.H. Liposomes from hydrogenated soya lecithin formed in sintered glass pores. Acta Pol. Pharm. 2012, 69, 107–111. [Google Scholar] [PubMed]
- Mozafari, M.R. Liposomes: an overview of manufacturing techniques. Cell Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Tang, S.; Hao, J.; Gao, D.; Duan, J.; Liu, Z. Preparation and characterization of oleanolic acid nanoparticles. Curr. Pharm. Anal. 2013, 9, 177–182. [Google Scholar] [CrossRef]
- Otake, K.; Shimomura, T.; Goto, T.; Imura, T.; Furuya, T.; Furuya, T.; Yoda, S.; Takebayashi, Y.; Sakai, H.; Abe, M. Preparation of liposomes using an improved supercritical reverse phase evaporation method. Langmuir 2006, 22, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Salba, Z.; Navarro, I.; Troconiz, I.F.; Tros de Llarduya, C.; Garrido, M.J. Application of different methods to formulate PEGliposomes of oxaliplatin: Evaluation in vitro and in vivo. Eur. J. Pharm. Biopharm. 2012, 81, 273–280. [Google Scholar]
- Geho, W.B.; Geho, H.C.; Lau, J.R.; Gana, T.J. Hepatic-Directed Vesicle Insulin: A Review of Formulation Development and Preclinical Evaluation. J. Diabetes Sci. Technol. 2009, 3, 1451–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjan Karn, P.; Cho, W.; Park, H.-J.; Hwang, S.-J. Characterization and stability studies of a novel liposomal cyclosporine A prepared using the supercritical fluid method: Comparison with the modified conventional Bangham method. Int. J. Nanomedicine 2013, 8, 365–377. [Google Scholar]
- De Jesús Valle, M.J.; Sánchez Navarro, A. Liposomes prepared in absence of organic solvents: Sonication versus lipid film hydration method. Curr. Pharm. Anal. 2015, 11, 86–91. [Google Scholar] [CrossRef]
- Wang, Y.; Chow, M.S.; Zuo, Z. Mechanistic analysis of pH dependent solubility and trans-membrane permeability of anphoreric compounds: application to sildenafil. Int. J. Pharm. 2008, 352, 217–224. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Valle, M.J.; López Díaz, D.; Velazquez, M.; Sánchez Navarro, A. Development and In Vitro Evaluation of a Novel Drug Delivery System (Albumin Microspheres Containing Liposomes) Applied to Vancomycin. J. Pharm. Sci. 2016, 105, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- De Jesús Valle, M.J.; Maderuelo Martín, C.; Zarzuelo Castañeda, A.; Sánchez Navarro, A. Albumin micro/nanoparticles entrapping liposomes for itraconazole green formulation. Eur. J. Pharm. Sci. 2017, 106, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control Release 2010, 142, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.Y.; Friend, J.R.; McIntosh, M.P.; Meeusen, E.N.; Morton, D.A. Ultrasonic nebulization platforms for pulmonary drug delivery. Expert Opin. Drug Deliv. 2010, 7, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.; von Hollen, D.; Pritchard, J.N.; Nikander, K.; Hardaker, L.E.; Hatley, R.H. Factors to consider when selecting a nebulizer for a new inhaled drug product development program. Expert Opin. Drug Deliv. 2015, 12, 1375–1387. [Google Scholar] [CrossRef] [PubMed]






| Influence of the Transmembrane pH Gradient on the Characteristics of the Liposomes | ||||||
|---|---|---|---|---|---|---|
| Liposomes | Dh (nm) | PDI | Zeta Potential (mV) | EE (%) | DL (mg/g lipid) | |
| Without Vit E TPGS | pH = 6.7 | - | - | - | <18% | <11 |
| pH = 3.2 | 304.3 | 0.413 | −2.10 | 49.47 ± 9.78 | 29.71 ± 5.87 | |
| pH gradient | 209.7 | 0.537 | −20.90 | 89.77 ± 7.64 | 53.92 ± 4.59 | |
| With Vit E TPGS | pH = 6.7 | - | - | - | <15% | <9 |
| pH = 3.2 | 303.2 | 0.452 | −2.05 | 22.67 ± 11.32 | 13.62 ± 11.00 | |
| pH gradient | 219.8 | 0.534 | −21.30 | 80.30 ± 11.03 | 48.23 ± 6.62 | |
| Influence of Lyophilization on the Characteristics of the Liposomes | ||||||
|---|---|---|---|---|---|---|
| Liposomes | Dh (nm) | PDI | Zeta Potential (mV) | EE% | DL (mg/g Lipid) | |
| Without Vit E TPGS | Fresh | 209.7 | 0.537 | −20.90 | 89.77 ± 7.64 | 53.32 ± 4.59 |
| S | 408.4 | 0.572 | −21.0 | 89.65 ± 1.02 | 53.84 ± 0.61 | |
| T | 433.1 | 0.577 | −46.6 | 89.95 ± 2.15 | 54.02 ± 1.29 | |
| S + T | 471.7 | 0.701 | −38.9 | 88.95 ± 0.30 | 53.42 ± 0.18 | |
 | ||||||
| Liposomes | Dh (nm) | PDI | Zeta Potential (mV) | EE% | DL (mg/g Lipid) | |
| With Vit E TPGS | Fresh | 219.8 | 0.534 | −21.30 | 80.30 ± 11.03 | 48.23 ± 0.13 |
| S | 367.5 | 0.642 | −34.0 | 88.77 ± 0.22 | 53.32 ± 0.35 | |
| T | 826.9 | 0.642 | −36.6 | 88.57 ± 0.59 | 53.20 ± 0.35 | |
| S + T | 688.4 | 0.682 | −31.2 | 88.39 ± 0.25 | 53.09 ± 0.15 | |
 | ||||||
| Influence of Nebulization on the Liposomes Stability (EE%) | |||||
|---|---|---|---|---|---|
| Liposomes | 0 min | 5 min | 10 min | 15 min | |
| Without Vit E TPGS | Fresh | 89.77 ± 7.64 | 85.10 ± 0.64 | 83.56 ± 3.04 | 77.78 ± 0.89 |
| S | 89.65 ± 1.02 | 87.35 ± 0.88 | 82.73 ± 1.28 | 79.47 ± 1.02 | |
| T | 89.95 ± 2.15 | 86.90 ± 0.50 | 81.23 ± 4.29 | 78.15 ± 1.91 | |
| S + T | 88.95 ± 0.30 | 87.06 ± 0.39 | 83.04 ± 0.88 | 79.89 ± 1.79 | |
| With Vit E TPGS | Fresh | 80.30 ± 11.03 | 76.76 ± 0.52 | 69.34 ± 1.16 | 59.24 ± 1.61 |
| S | 88.77 ± 0.22 | 85.84 ± 0.92 | 84.06 ± 0.97 | 82.20 ± 1.13 | |
| T | 88.57 ± 0.59 | 85.94 ± 0.43 | 82.86 ± 0.53 | 81.12 ± 1.43 | |
| S + T | 88.39 ± 0.25 | 86.05 ± 1.05 | 84.15 ± 1.04 | 81.82 ± 0.89 | |
| Nebulizer Performance | |||||
|---|---|---|---|---|---|
| Liposomes | DR (mL/min) | NE (%) | η (mm2/s) | ρ (g/mL) | |
| Without Vit E TPGS | Fresh | 0.21 ± 0.01 | 50.51 ± 2.47 | 1.13 ± 0.04 | 1.01 ± 0.01 |
| S | 0.17 ± 0.01 | 65.19 ± 2.98 | 1.27 ± 0.03 | 1.02 ± 0.01 | |
| T | 0.19 ± 0.02 | 75.22 ± 6.08 | 1.24 ± 0.01 | 1.01 ± 0.01 | |
| S + T | 0.19 ± 0.02 | 68.00 ± 2.48 | 1.27 ± 0.01 | 1.02 ± 0.01 | |
| With Vit E TPGS | Fresh | 0.20 ± 0.01 | 50.87 ± 1.90 | 1.18 ± 0.06 | 1.01 ± 0.01 |
| S | 0.16 ± 0.02 | 76.99 ± 2.69 | 1.30 ± 0.04 | 1.02 ± 0.01 | |
| T | 0.18 ± 0.01 | 78.41 ± 0.22 | 1.28 ± 0.03 | 1.00 ± 0.00 | |
| S + T | 0.16 ± 0.01 | 81.24 ± 2.12 | 1.26 ± 0.04 | 1.02 ± 0.01 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesús Valle, M.J.; Gil González, P.; Prata Ribeiro, M.; Araujo, A.R.T.S.; Sánchez Navarro, A. Sildenafil Citrate Liposomes for Pulmonary Delivery by Ultrasonic Nebulization. Appl. Sci. 2018, 8, 1291. https://doi.org/10.3390/app8081291
De Jesús Valle MJ, Gil González P, Prata Ribeiro M, Araujo ARTS, Sánchez Navarro A. Sildenafil Citrate Liposomes for Pulmonary Delivery by Ultrasonic Nebulization. Applied Sciences. 2018; 8(8):1291. https://doi.org/10.3390/app8081291
Chicago/Turabian StyleDe Jesús Valle, María José, Pablo Gil González, Maximiano Prata Ribeiro, André R. T. S. Araujo, and Amparo Sánchez Navarro. 2018. "Sildenafil Citrate Liposomes for Pulmonary Delivery by Ultrasonic Nebulization" Applied Sciences 8, no. 8: 1291. https://doi.org/10.3390/app8081291