3.1. Adsorbents Characterization
XRD patterns obtained for the natural and Si-PILC clays and their main fractions are shown in
Figure 1A. The obtained XRD pattern for the NC exhibits a basal distance (d
001) of 1.26 nm, which is typical of natural sodic montmorillonite. The Si-PILC diffractograms did not show any defined peaks in the range in which the basal distance should be present. Furthermore, there are no other structural changes in the PILC in reference to the raw material [
37,
38]. In order to evaluate the existence of an increase in the basal distance (d
001), XRD at low angle was obtained for the Si-PILC 75 and is shown in
Figure 1B. A basal distance of 4.32 nm was found for this material, which evidences the presence of silica oxide species within the interlayer clay suggesting an effective pillarization process. This result agrees with the values reported by other authors [
26]. Si-PILC 25 and 50 were not analyzed at low angle but a similar behavior could expect for them.
The FTIR spectra obtained for the four materials and their main bands are shown in
Figure 2. The natural clay spectrum is in accordance with previous reports, where the absorption bands at 3630, 3454, and 1640 cm
−1 are typical for smectites and associated to tension vibrations of the O–H bond in hydroxyl groups of dioctahedral structures and H–O–H vibrations of water molecules weakly hydrogen bonded to the Si–O surface, respectively [
26,
39]. In addition, the bands found at 1040, 800, 524, and 470 cm
−1 are attributed to Si–O stretching vibrations, Si–O–Al (octahedral Al) and Si–O–Si bending vibrations, respectively. The spectra obtained for the Si-PILC show a considerable reduction of the bands related to water presence and O–H vibrations, which could be due to the acid treatment used during the synthesis of these materials [
26,
39]. Additionally, the spectra of the Si-PILC show increments in the bands of 1200 and 800 cm
−1 which evidences the increase of Si–O–Si bonds in their structures as a consequence of the pillaring process.
Nitrogen adsorption–desorption isotherms at −196 °C obtained from the materials are shown in
Figure 3. The natural clay isotherm is classified as type IIb with an H3 hysteresis loop according to the International Pure and Applied Chemistry (IUPAC) classification which is associated with mesoporous materials with aggregates of plate-like like the montmorillonites [
27,
40]. The isotherms obtained for Si-PILC can be classified as a combination between type I and IIb. The first one is due to the high amount adsorbed at low relative pressures related to the presence of microporosity in the PILC structures generated after the pillaring process. The second one is associated with the growth of the adsorption in the mono-multilayer region.
Hysteresis loops were observed for the three Si-PILC demonstrating the presence of mesoporosity in their structures. The type of hysteresis loop is classified as type H4 according to the IUPAC and it is associated with slit-like pores as well as the pores generated within the interlayer of the clay minerals [
27,
40]. As can be seen, all the pillared clays present higher adsorption at low relative pressures than the natural clay, which is associated with their microporosity and evidences a successful pillaring process. However, among the pillared clays Si-PILC 50 showed the highest adsorption at low relative pressure suggesting there is not a correlation between the adsorption increase observed and the Si/clay ratio used during the synthesis process. This could be due to the amount of siliceous present in the interlayer for the Si-PILC 75 which could generate higher pillars density than the Si-PILC 50 implicating a lower microporosity.
Textural properties for all materials were obtained from the N
2 adsorption–desorption isotherms and are summarized in
Table 2. Significant increases in the specific surface areas (S
BET) for the Si-PILC with regard to the natural clay are observed, reaching nine times the NC value for the Si-PILC 50. This last is related to the increase in the microporosity of these materials resulting in 57, 65, and 61% for the Si-PILC 25, 50, and 75, respectively. The S
BET and micropores volume (V
μp) values increased from Si-PILC 25 to Si-PILC 75 and Si-PILC 50, respectively, and it is associated with the amount of micropores present in their structures. The fact that Si-PILC 75 showed lower values than Si-PILC 50 could be due to the amount of pillaring agent added. The results suggest that Si/clay ratio higher than 50 mol Si.g
−1 could generate more pillars density within the clay interlayer which during the heating treatment could collapse, decreasing the microporosity. The V
T values supported the behavior of the isotherms at high relative pressures obtaining the highest value for the Si-PILC 50.
The PSD for the PILC and NC were studied in the micropores region (pores size below 2 nm) and are shown in
Figure 4. As can be observed, the microporosity in the natural clay is considerably lower than that obtained for the pillared clay, which is in accordance with the textural results. All pillared clays have micropore sizes between 0.5 and 2 nm, whereas Si-PILC 25 and 50 showed similar distributions with micropore sizes approximately 0.75 nm. Si-PILC 75 shows contribution of smaller pore sizes (near to 0.7 nm) than the other PILC, suggesting the greater pillar density within the interlayer due to the high Si/clay ratio.
3.2. Effect of the pH Media on the Adsorption
The presence of protonable groups in the antibiotics molecules generates different species according to the media pH. Thus, the species present in the aqueous solution and their solubility depend on the pH; this also influences their interaction with the adsorbent surfaces. In a previous work, the strong correlation between the solubility in water and the adsorption on clay of CPX with the pH of the media was shown [
16]. The tetracycline solubility curves in water with its distribution of species as a function of the media pH are shown in
Figure 5. The solubility behavior can be explained considering the species present within each pH range. As was mentioned above, the TC molecule has three pK
a values which implicate the existence of four possible species in solution. At the lowest pH values the soluble cationic TC species (
) is present and its percentage decreases from pH 2 to 3.3 (pK
a1) as well as its solubility. In the pH range from 3.3 to 7.7 (pK
a2) three TC species exist, where the zwitterion form (
) is the predominant one, reaching the lowest solubility due to its neutral charge. Later increases in the media pH generate the increase in solubility because TC turns into its anionic forms,
(between pH values of 7.7 and 9.7) and
(pH values higher than 9.7). Thus, analogously to the results for CPX, TC is more soluble when the ionic forms are present in the media and shows the lowest solubility around a pH value of 4.8 because at this pH it has the highest zwitterion percentage. Additionally, it is known that due to the existence of asymmetric carbon atoms in the TC molecule, its species in solution can adopt two possible conformations called the extended and twisted conformation [
41]. The first one is predominant in basic media, whereas the second one in neutral or acid media. This must be taken into consideration because according to the conformation the molecular size can suffer changes.
The studies of antibiotic adsorption on NC and Si-PILC 75 at different pH values were assessed under the above mentioned conditions with an initial fixed concentration of 110 and 480 mg.L
−1 for CPX and TC, respectively. The pH of the initial solution was adjusted to values between 3 and 10 using HCl or NaOH solutions. These values were chosen based on the antibiotics solubility curves and considering the species present in each pH range [
16]. The adsorption capacity of CPX and TC on NC and Si-PILC 75 at different pH values are shown in
Figure 6. In the case of TC, its adsorption on NC showed a behavior similar to the reported by other authors, where the highest adsorption occurs at low pH values, when the
species is present and the adsorption is governed mainly by electrostatic interactions [
42]. The cationic form of TC is adsorbed on the negative charge surface of the NC mainly by cation exchange for the natural cation within the montmorillonite interlayer [
42,
43]. After that, at pH values greater than 4 the adsorption decreases with increases in pH because the TC transitions into zwitterionic and anionic forms. At these pH values, the species could be adsorbed on the mineral negative surface by other adsorption mechanisms like hydrophobic interactions, hydrogen bonding, or inner sphere complexes. However, it is important to consider that at pH values lower than 10,
and
species have net charges of zero and one, respectively, but the amine group is protonated in their structures. This could favor the adsorption of these species on the negatively charged surface of the natural clay. Other authors proposed that when these species are adsorbed within the interlayer clay, they arrange at the surface so that the positively charged group is located close to the surface sites and their negatively charged part is driven as far away as possible from the surface [
42,
43]. The lowest TC adsorption was obtained at pH values higher than 9.7, where the main species was the TC
2− and it was probably because of the repulsion with the negatively charged surface of the NC. The TC adsorption on Si-PILC 75 was considerably lower than that obtained for the NC in all the pH range except to values up to 8.5. No greater differences in the amount of TC adsorbed on pillared clay were observed in the whole pH range. This could suggest that the interactions between the TC species and the pillared clay surface are low, or that the porous structure of this material could be avoiding the molecule access to the adsorption sites. The adsorption of CPX on natural clay showed a similar behavior to the TC adsorption, were the highest adsorptions were obtained at low pH values with a maximum at pH 6; decreasing when the pH increases. These results can be explained similarly to those above; at low pH values the CPX
+ is present and is adsorbed on the negative surface of the natural clay by cation exchange. After that, at pH values of 7.5, CPX
± and CPX
− are present in the solution, decreasing the adsorption and resulting in other adsorption mechanisms [
16]. Results shown for the adsorption of CPX on Si-PILC 75 evidenced that there are no variations in the amount adsorbed at different pH values, decreasing with an increase in the pH. However, the adsorption for this material under basic conditions (up to 7.5 pH) was higher than for the natural clay, suggesting that Si-PILC interacts with the CPX species by another mechanism, like the formation of an inner sphere complex [
25,
44].
3.3. Adsorption Kinetics
Adsorption kinetics of both antibiotics on NC and Si-PILC 75 were evaluated at pH values of 8.5 and 10 for TC and CPX, respectively, so that the species in solution were anionic in order to evaluate their interactions with the surfaces materials. The experiments were performed using the same initial and fixed concentrations than above, 110 and 480 mg.L
−1 for CPX and TC with contact times varying between 0.5 and 24 h. Adsorption kinetic curves obtained for the Si-PILC 75 and their fitting to the pseudo-first and pseudo-second order are shown in
Figure 7. For the adsorption on NC, the results showed that the adsorption equilibrium is rapidly reached for TC and is lower for CPX (around 4 h). These results could be due to the species present in each case. For the TC at a pH value of 8.5, the species present is TCH
− and its structure still has a positively charged group. This last favors the adsorption on the negative surface of the NC by cation exchange, implicating a fast adsorption. In the case of CPX at a pH value of 10, the species present is anionic and the higher time to reach the equilibrium could be due to the fact that the adsorption mechanism is not cation exchange. No significant differences were obtained among the data, indicating that the equilibrium of the systems was reached, and therefore the kinetic studies on NC were not fitted to the models. The TC adsorption on Si-PILC 75 reached equilibrium at around 7 h, what could be due to the fact that the pillared clay porous structure is limiting the access of the TC molecule to the interlayer region and delaying the adsorption. This implicates that the adsorption mechanism in the Si-PILC is different from the one proposed for the NC. The results obtained for CPX on Si-PILC 75 are similar to those obtained for TC, the system reaches the equilibrium at a contact time of 8 h. This supports the fact that in the pillared clay its microporous structure could be avoiding the access of the molecules to the interlayer region generating a slower adsorption process.
The adsorption kinetic parameters were estimated by nonlinear regression of pseudo-first and pseudo-second order equations and for two materials [
32,
45]. The obtained values are in good agreement with the previously shown results obtained for the adsorption vs. pH curves and are summarized in
Table 3. As can be seen from the table, all the regression coefficients are higher than 0.90. However, if the studied systems are considered, the second-order model is more appropriate due to the fact that it is based on the sorption capacity of the solid and it considers that the rate controlling step is the chemical or electrostatic change generated in the system [
32,
44,
45]. In addition, the
k2 values agree with the fact that natural clay requires lower time than the pillared clay to reach equilibrium in both systems. For the kinetics adsorption results shown, an equilibrium time of 24 h was chosen to perform the adsorption isotherms.
In order to acquire more information regarding the adsorption process on the pillared clay, the intraparticle model was used and the results obtained are shown in
Figure 8. As can be seen from this figure, the plots resulted in two different straight lines with regressions that do not pass through the origin, indicating that intraparticle diffusion was not the only rate controlling step. This could be related to the existence of an initial external mass transfer or a chemical reaction between the antibiotic molecule and the adsorbent surface [
33]. The slopes of the linear regressions (k
`3 and k
``3 in
Table 3) indicate the rate of the adsorption process. The values obtained for the intraparticle diffusion parameters indicate the decrease of the diffusion rates with the increase of the contact time for both antibiotics. These results evidenced that as the antibiotic molecules diffused into the pillared clay structure, the pores aviable for diffusion decreased, limiting the adsorption. Thus, the first section is related to the stage in which the antibiotics are rapidly adsorbed on the PILC surface, which could be related to the microporous structure. The second section is related to the adsorption on the external surface, which contains the mesoporous structure. Similar results were reported for the adsorption of dyes on pillared clays [
18].
3.4. Adsorption Isotherms
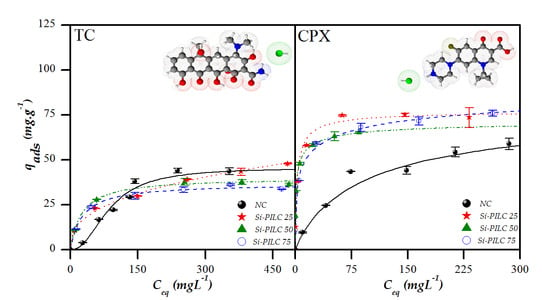
The batch adsorption experiments were performed in the conditions mentioned above varying the initial concentrations between 40–610 mg.L
−1 for the TC and 18–500 mg.L
−1 for the CPX. The contact time was set to a period of 24 h and the contact solutions were set at pH 8.5 and 10 for TC and CPX, respectively. These values were chosen based on the results previously shown and in order to evaluate the behavior of the clays adsorption when the species with a net charge of -1 are present (CPX
− and TCH
−). The adsorption isotherms obtained for the antibiotics on four materials and their best adjustments are shown in
Figure 9. Taking into account the Giles et al. classification [
46], different behaviors can be associated with the isotherm shapes obtained. For the adsorption of TC on the natural clay, the isotherm can be classified as sigmoidal type (S type) which is considered to be a result of two opposite mechanism. In first place the TCH
− species has a low affinity towards the negative surface of the clay but once some molecules are adsorbed, they can become new adsorption sites favoring subsequent adsorptions. This phenomenon is called cooperative adsorption and the isotherm saturation point occurs when no vacant sites remain [
46,
47]. The isotherms obtained for the adsorption of TC on pillared clays showed some differences among them. The Si-PILC 50 and 75 isotherms can be classified as high affinity type (H-type) and the one obtained for the Si-PILC 25 was a Langmuir type (L-type). Both types of isotherms are obtained when the saturation of solid sites is progressive until reaching its limited adsorption capacity [
46,
47]. The H-type isotherm is usually associated with the adsorption of ionic solutes, where the competition between adsorptive and solvent molecules is low. This could be the result of the higher hydrophobicity exhibited by the pillared clays in contrast with the natural clay. Also, these results evidenced that pillared clays have more affinity for the TCH
− species present in solution than natural clay. The high affinity shown suggests that the adsorption occurs by a different mechanism and it could be due to the presence of new adsorption sites on the surface of the pillared clays associated with the pillars. Additionally, Si-PILC 25 was the pillared clay with the lowest affinity but the highest adsorption capacity and this could be explained considering that it was the material with the lowest ratio Si/clay. This could implicate that the Si-PILC 25 interlayer region has probably lower pillars density resulting in a porous structure that favors the interaction between the TC species and the pillars. The adsorption isotherms obtained for CPX can be classified as H-type for Si-PILC 50 and 25 and L-type for Si-PILC 75 and NC. Their analysis is similar to the one done for TC adsorption, all the pillared clays showed a higher affinity towards the CPX
− than the natural clay. The natural clay isotherm suggests a lower affinity of the anionic CPX species present towards the more negatively charged clay than the one observed for the PILC. As in the case of the TC adsorption, this last could be due to a combination of the adsorption mechanism and the hydrophobicity of these materials.
As can be seen in
Figure 9, all the materials showed higher CPX adsorption capacities than those obtained for TC adsorption. The adsorption results obtained for the NC evidences that it has higher affinity and adsorption capacity for the CPX
− than for the TCH
−, even when these species still have a protonated group in their structure. These results may suggest that these two species are adsorbed in different ways on the natural clay, where TCH
− is probably adsorbed within the interlayer region, whereas the CPX
− interacts with adsorption sites on the clay surface. Further work is required to stablish this idea.
In the case of the Si-PILC, the results evidenced that these materials have a considerably higher affinity and adsorption capacities for the CPX species than for the TC which could be related to the access that the molecules have to the porous structures. In a previous work, the adsorption of CPX on different pillared clays in alkaline media was proposed as result of a combination of two effects. The first one was the porous structure of the PILC limiting the molecule access to the pillared structure, and the other one was the adsorption of the anionic species by inner sphere complex formation with the pillars’ adsorption sites [
25]. Taking into account the criteria proposed in that work about the porous size needed for the molecules to access to the pillared structure, and considering that the molecules diffuse into the porous structure of the adsorbents lengthwise, the adsorption results could be explained. At alkaline pH, TC shows its extended conformation with dimensions of 1.2 × 0.8 × 1.3 nm which implicates that the size needed to be adsorbed is 1.4 nm, whereas for the CPX, the size is 1.3 nm. There are no significant differences between these two values but considering the dimensions of CPX (1.2 × 0.3 × 0.7 nm), its highest adsorption could be explained. Also, this may indicate that the CPX molecule has more access to the pillar structure that favors its interaction with the pillars.
All the adsorption data obtained were fitted to Freundlich, Langmuir, and Sips models and their fitting parameters are summarized in
Table 4. Considering the results obtained for TC adsorption on NC and Si-PILC 25, Langmuir and Sips models, respectively, are not shown because they have a higher percentage error associated, suggesting that the adsorption does not agree with the assumptions proposed in those models. As can be seen from the
Table 4, the best fittings were obtained for the Sips model in all the systems under study. These results are likely related to the fact that there are more heterogeneous adsorption systems which could be generated for the different adsorption sites on the solid surfaces, the adsorptive species or, a combination of them. Regarding the adsorption on the NC, the heterogeneity could be associated to the hydrophobic effect of the solvent towards the antibiotics molecules generating the adsorption as result of its repulsion against the solvent from the solution [
25]. This is in accordance with the obtained values for the
b parameter for NC systems which suggest that these systems have the lowest affinity of the adsorbates towards the surface. It could be explained for the species present for each antibiotic which are negatively charged, and its adsorption probably occurs by different adsorption short-range forces such as hydrophobic bonding, hydrogen bridges, steric, or orientation effects. These results are according to those mentioned above. In the case of the antibiotics adsorption on Si-PILC, the hydrophobic effect possibly influences in the same way of the NC, but with the highest
n values, suggesting more heterogeneous systems. This higher heterogeneity for the PILC compared to the NC could be related to the new adsorption sites generated by the pillars presence and their porous structures. The results showed that for all the PILC materials, the systems for CPX have higher adsorption capacities than for TC and are also more heterogeneous. This can be related to the molecule size, due to the fact that the CPX size is lower than the TC size, allowing more access for CPX to the porous structure and resulting in higher adsorptions and affinities than the TC species. Finally, regarding the adsorption of TC on Si-PILC, the highest adsorption capacity was obtained for Si-PILC 25, and the best fit was obtained with the Freundlich model, suggesting that for this material the adsorption occurs on heterogeneous sites. This last result could be related to the lower amount of micropores in the Si-PILC 25 structure which could allow more access of the TCH
− species to the adsorption sites.
The Scatchard plots obtained for all the materials and both antibiotics are shown in
Figure 10. From these plots, the
R2 and binding constant (
kd) values can be obtained. The first could be related to the presence of nonspecific or multitype interactions, while the second indicates the adsorbate affinity for the adsorption sites. The
R2 values obtained for the TC adsorption on four materials were 0.04, 0.78, 0.99, and 0.94 for the NC, Si-PILC 25, 50, and 75, respectively. These values suggest that the nonspecific interactions in the adsorption of TC on pillared clays are considerably higher than for the NC. Similarly, the
kd values obtained were 0.001, 0.02, 0.04, and 0.03 for the NC, Si-PILC 25, 50, and 75, respectively. These values evidence a poor affinity of the TCH
− species for the adsorption sites in the four materials; the lowest affinity was obtained for the NC. In the case of CPX adsorption the values obtained were 0.91, 0.97, 0.93, and 0.74 (
R2) and 0.015, 0.45, 1.36, and 3.07 (
kd) for NC, Si-PILC 25, 50, and 75, respectively. As it can be observed, the presence of multitype interactions is lower for this molecule than for the TC and the
kd values obtained are higher than those obtained for adsorption of TC on all the materials. This could be due to the lower molecular size of CPX favors the interaction with the pillar for the PILC materials.
Additionally, the Scatchard plot shapes can be related to different adsorption mechanisms. Two different types of plots were obtained for the CPX adsorption, a straight line for the NC and concave curves for the three pillared clays. The first one suggests that there is one type of site for CPX adsorption on the adsorbent surface, whereas the curves acquired for the pillared clays are related to a negative cooperative phenomenon. This last is proposed when two independent sets of data that individually arrange in a linear combination are obtained. Each of them could be associated to two types of affinities of CPX to the surface, one being the consequence of a strong interaction like the formation of inner sphere complexes (high affinity sites) and the other resulting from low affinity interactions like the hydrophobic effect, van der Waals interactions, or hydrogen bridges [
25,
34,
35]. On other hand, Scatchard plots obtained for TC adsorption showed three different shapes. The plot shown for NC resulted in a convex curve suggesting a positive cooperative phenomenon, similar to that proposed for its adsorption isotherms [
34,
35]. Scatchard plots obtained for the pillared clays showed that Si-PILC 25 has a different behavior than other materials. The plot obtained for TCH
− adsorption on Si-PILC 25 showed a similar behavior that the obtained for the CPX
− on pillared clays, where a negative cooperative phenomenon was proposed as the result of the presence of two different adsorption sites for the adsorbate. However, for Si-PILC 50 and 75, straight lines were obtained suggesting that the adsorption of TCH
− on these materials occurs in the same type of sites. This last could be due to the fact that TC species only has access to one type of adsorption site because the porous structure denies it access to another one. All the results obtained suggest that the adsorption capacity of Si-PILC depends on their porous structure and the adsorbate molecular sizes.