The Suppression Characteristics of NH4H2PO4/Red Mud Composite Powders on Methane Explosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
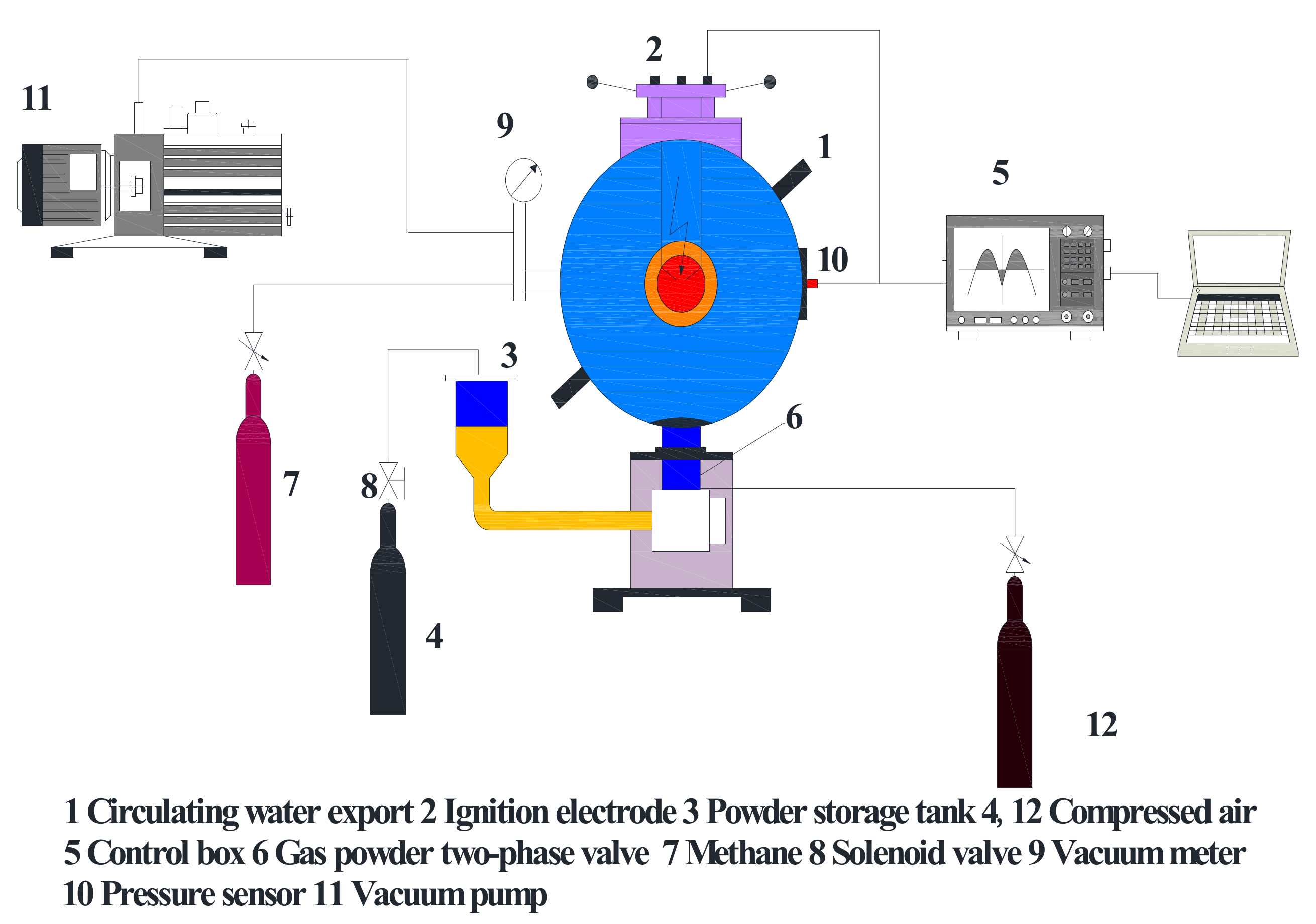
2.3. Explosion Experiment Device and Test Process
3. Results and Discussion
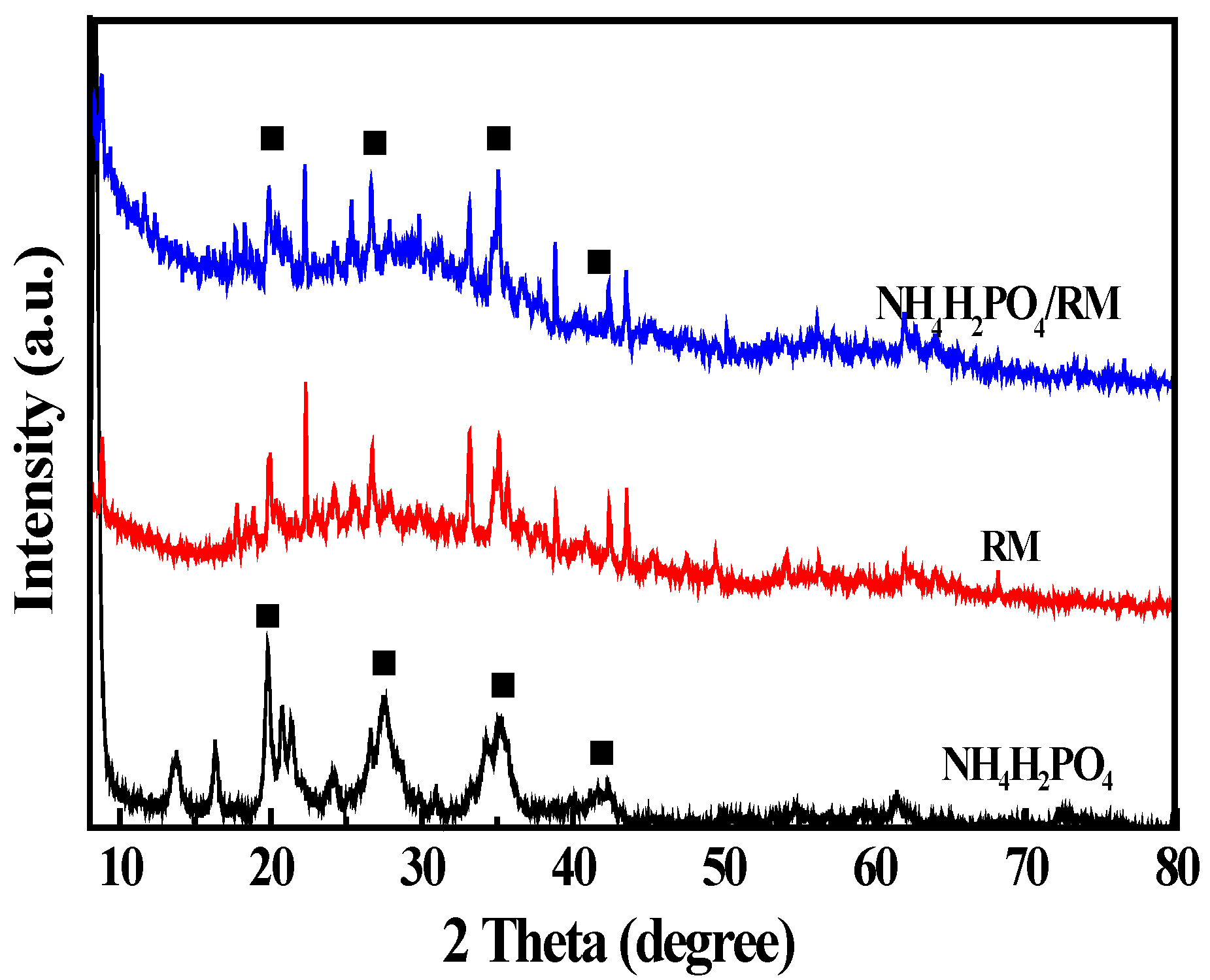
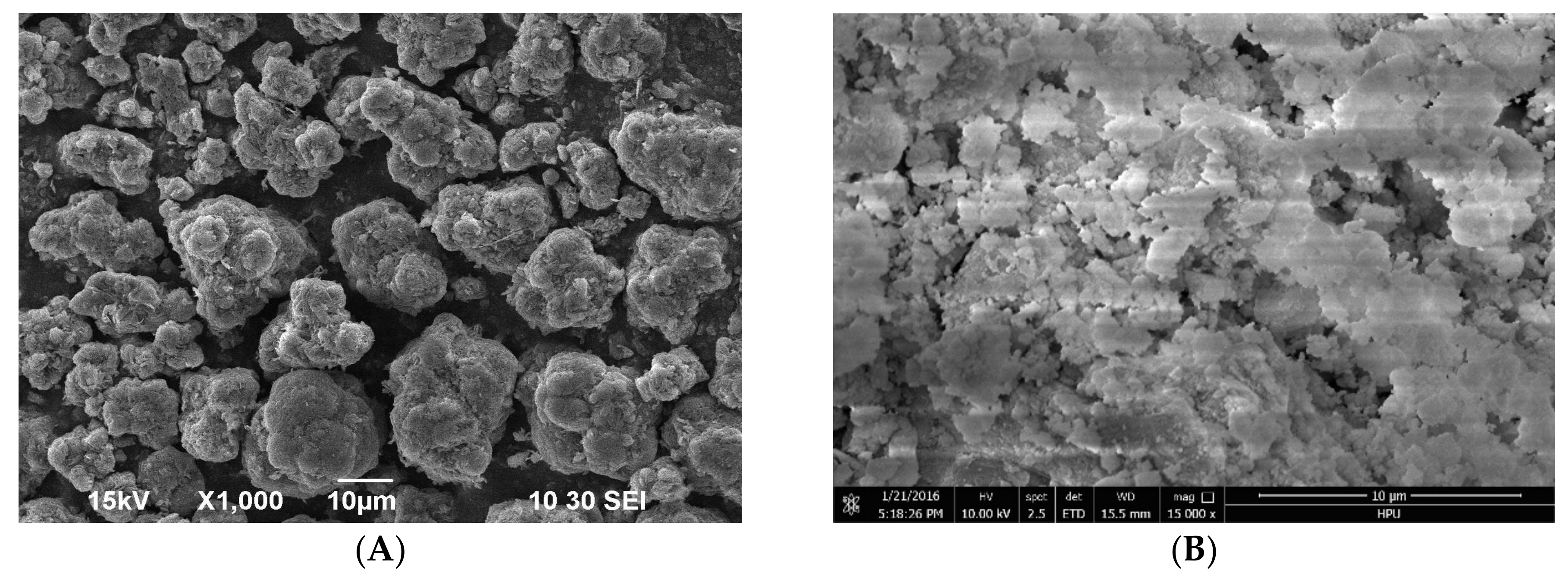
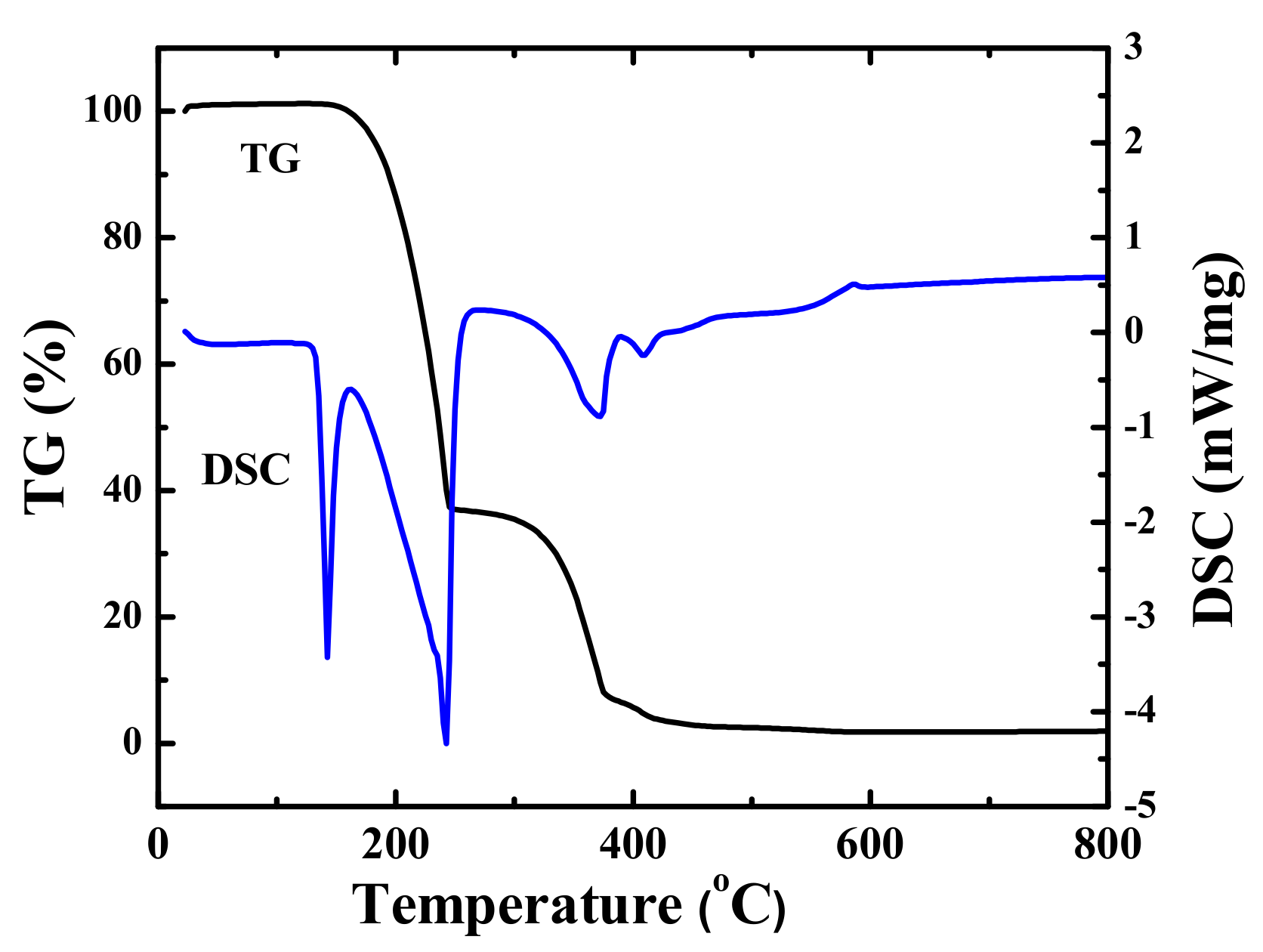
3.1. Sample Characterization
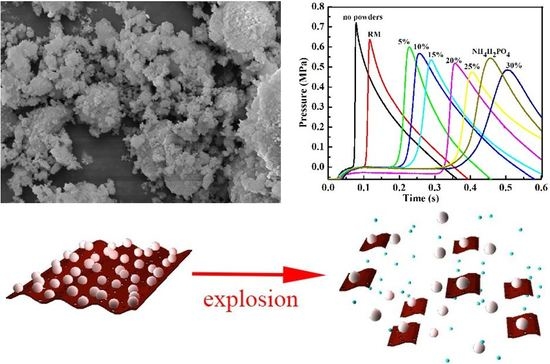
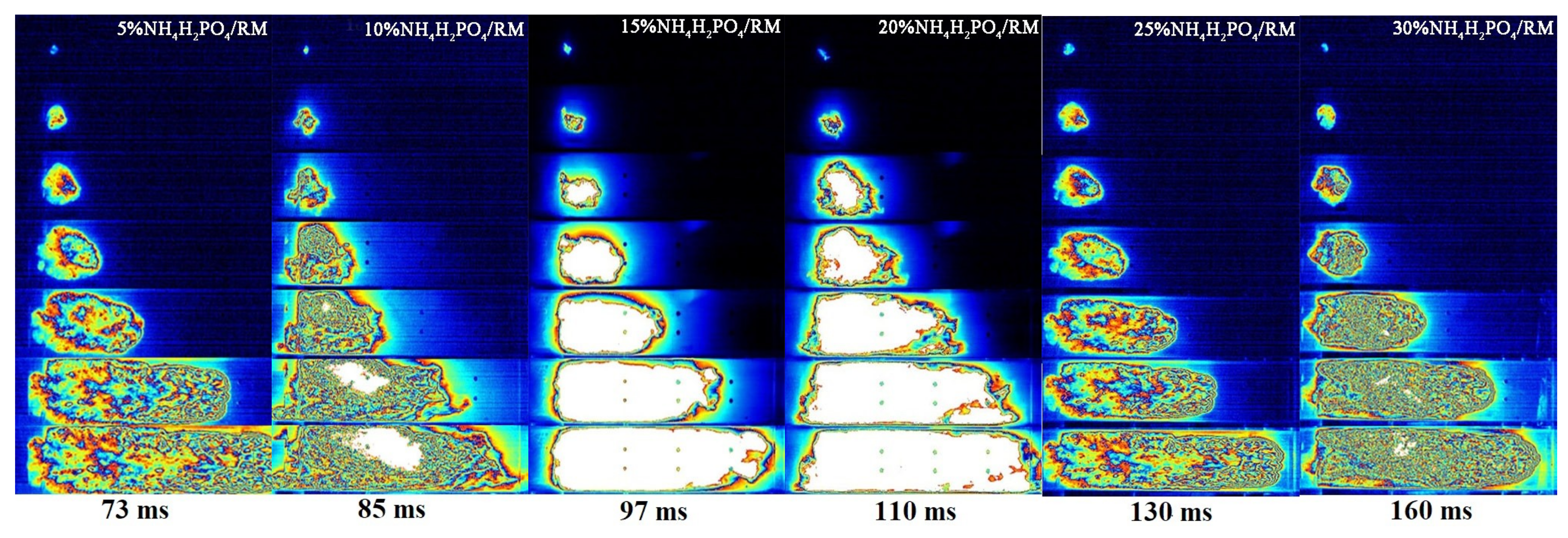
3.2. Suppression Properties of the NH4H2PO4/RM Composite Powders
3.3. Suppression Mechanism of the NH4H2PO4/RM Composite Powders
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, D.M. The construction and development of the emergency refuge system in coal mine. J. Saf. Sci. Technol. 2010, 11, 6–9. [Google Scholar]
- Mitchell, M.D. Analysis of Underground Coal Mine Refuge Shelters. Ph.D. Thesis, West Virginia University, Morgantown, WV, USA, 20 April 2008. [Google Scholar]
- Sun, J.P. The function and configuration scheme in coal mine. Ind. Mine Autom. 2010, 11, 1–4. [Google Scholar]
- Zhao, H.J.; Qian, X.M.; Li, J. Simulation analysis on structure safety of coal mine mobile refuge chamber under explosion load. Saf. Sci. 2012, 50, 674–678. [Google Scholar] [CrossRef]
- Li, F.W.; Jin, L.Z.; Zhan, Z.N. Numerical simulation study of air distribution law of air pressure system in the mine refuge chamber. J. Theor. Appl. Inf. Technol. 2012, 45, 205–211. [Google Scholar]
- Zhang, B.Y.; Zhao, W.; Wang, W.; Zhang, X.H. Pressure characteristics and dynamic respond of coal mine refuge chamber with underground gas explosion. J. Loss Prev. Process Ind. 2014, 30, 37–46. [Google Scholar] [CrossRef]
- Jia, Z.W.; Jing, G.X.; Cheng, L. Study on propagation regulation about shock wave of gas explosion at laneway area break. China Saf. Sci. J. 2007, 17, 92–94. [Google Scholar]
- Zhang, B.Y.; Zhai, D.X.; Wang, W. Failure mode analysis and dynamic response of a coal mine refuge chamber with a gas explosion. Appl. Sci. 2016, 6, 145. [Google Scholar] [CrossRef]
- Yu, M.G.; Wan, S.J.; Xu, Y.L.; Zheng, K.; Liang, D.L. The influence of the charge-to-mass ratio of the charged water mist on a methane explosion. J. Loss Prev. Process Ind. 2016, 41, 68–76. [Google Scholar] [CrossRef]
- Cao, X.Y.; Ren, J.J.; Zhou, Y.H.; Wang, Q.J. Suppression of methane explosion by ultrafine water mist containing sodium chloride additive. J. Hazard. Mater. 2015, 285, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Y.; Ren, J.J.; Bi, M.S.; Zhou, Y.H.; Li, Y.M. Experimental research on the characteristics of methane explosion affected by ultrafine water mist. J. Hazard. Mater. 2017, 324, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Wang, L.Y.; Yu, M.G.; Wan, S.J. Study on the characteristics of gas explosion affected by induction charged water mist in confined space. J. Loss Prev. Process Ind. 2016, 40, 227–233. [Google Scholar] [CrossRef]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Inert gas influence on the laminar burning velocity of methane-air mixtures. J. Hazard. Mater. 2017, 321, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Mitu, M.; Giurcan, V.; Razus, D.; Oancea, D. Influence of inert gas addition on propagation indices of methane–air deflagrations. Process. Saf. Environ. 2016, 102, 513–522. [Google Scholar] [CrossRef]
- Wang, Z.R.; Ni, L.; Liu, X.; Jiang, J.C.; Wang, R. Effects of N2/CO2 on explosion characteristics of methane and air mixture. J. Loss Prev. Process Ind. 2014, 31, 10–15. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Liu, Z.G.; Tang, M.Y.; Yang, K.; Lv, P.; Lin, B.Q. Active suppression of premixed methaneair explosion propagation by non-premixed suppressant with nitrogen and ABC powder in a semiconfined duct. J. Nat. Gas Sci. Eng. 2016, 29, 141–149. [Google Scholar] [CrossRef]
- Luo, Z.M.; Wang, T.; Tian, Z.H.; Cheng, F.M.; Deng, J.; Zhang, Y.T. Experimental study on the suppression of gas explosion using the gas-solid suppressant of CO2/ABC powder. J. Loss Prev. Process Ind. 2014, 30, 17–23. [Google Scholar] [CrossRef]
- Zheng, L.G.; Li, G.; Wang, Y.L.; Zhu, X.C.; Pan, R.K.; Wang, Y. Effect of blockage ratios on the characteristics of methane/air explosion suppressed by BC powder. J. Hazard. Mater. 2018, 355, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.S.; Yang, L.L.; Ge, B.Q.; Wang, J.W.; Li, X.C. Chemical kinetic characteristics of methane mixture explosion and its affecting factors. J. Loss Prev. Process Ind. 2017, 49, 675–682. [Google Scholar] [CrossRef]
- Cheng, W.M.; Hu, X.M.; Xie, J.; Zhao, Y.Y. An intelligent gel designed to control the spontaneous combustion of coal: Fire prevention and extinguishing properties. Fuel 2017, 210, 826–835. [Google Scholar] [CrossRef]
- Jiang, H.P.; Bi, M.S.; Gao, W.; Gan, B.; Zhang, D.W.; Zhang, Q. Inhibition of aluminum dust explosion by NaHCO3 with different particle size distributions. J. Hazard. Mater. 2018, 344, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.L.; Sapko, M.J.; Zlochower, I.A.; Perera, I.E.; Weiss, E.S. Particle size and surface area effects on explosibility using a 20-L chamber. J. Loss Prev. Process Ind. 2015, 37, 33–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, X.M.; Kuang, K.Q.; Yang, D.L.; Jin, X.; Liao, G.X. A new type of fire suppressant powder of NaHCO3/zeolite nanocomposites with core–shell structure. Fire Saf. J. 2009, 44, 968–975. [Google Scholar] [CrossRef]
- Bertolino, V.; Cavallaro, G.; Milioto, S.; Parisi, F.; Lazzara, G. Thermal Properties of Multilayer Nanocomposites Based on Halloysite Nanotubes and Biopolymers. J. Compos. Sci. 2018, 2, 41. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.S.; Yu, M.G.; Li, Y.; Cao, J.L.; Zheng, L.G.; Yi, H.W. Methane explosion suppression characteristics based on the NaHCO3/red-mud composite powders with core-shell structure. J. Hazard. Mater. 2017, 335, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Rachel, A.P.; Sara, J.C.; Graeme, J.M. Value adding red mud waste: Impact of red mud composition upon fluoride removal performance of synthesised akaganeite sorbents. J. Environ. Chem. Eng. 2018, 6, 2063–2074. [Google Scholar]
- Li, Y.C.; Min, X.B.; Ke, Y.; Chai, L.Y.; Shi, M.Q.; Tang, C.J.; Wang, Q.W.; Liang, Y.J.; Lei, J.; Liu, D.G. Utilization of red mud and Pb/Zn smelter waste for the synthesis of a red mud-based cementitious material. J. Hazard. Mater. 2018, 344, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Aswath, M.U.; Ranganath, R.V. Effect of mechanical activation of red mud on the strength of geopolymer binder. Constr. Build. Mater. 2018, 177, 91–101. [Google Scholar] [CrossRef]
- Toniolo, N.; Rincón, A.; Avadhut, Y.S.; Hartmann, M.; Bernardo, E.; Boccaccini, A.R. Novel geopolymers incorporating red mud and waste glass cullet. Mater. Lett. 2018, 219, 152–154. [Google Scholar] [CrossRef]
- Cao, J.L.; Yan, Z.L.; Deng, Q.F.; Yuan, Z.Y.; Wang, Y.; Sun, G.; Wang, X.D.; Hari, B.; Zhang, Z.Y. Homogeneous precipitation method preparation of modified red mud supported Ni mesoporous catalysts for ammonia decomposition. Catal. Sci. Technol. 2014, 4, 361–368. [Google Scholar] [CrossRef]
- Yu, M.G.; Kong, J.; Wang, Y.; Zheng, K.; Zheng, L.G. Experimental research on gas explosion suppression by modified red mud. J. China Coal Soc. 2014, 39, 1289–1294. [Google Scholar]
- Kordylewski, W.; Amrogowicz, J. Comparison of NaHCO3 and NH4H2PO4 Effectiveness as Dust Explosion Suppressants. Combust. Flame 1992, 90, 344–345. [Google Scholar] [CrossRef]
- Frenklach, M.; Wang, H.; Rabinowitz, M.J. Optimization and analysis of large chemical kinetic mechanisms using the solution mapping method-combustion of methane. Prog. Energy Combust. Sci. 1992, 18, 47–73. [Google Scholar] [CrossRef]





| Samples | Max Pressure (MPa) | The Rate of Max-Pressure Rise (MPa·s−1) | Time of Pressure Peak Arriving (s) | Decline Rate of Max Pressure (%) | Decline Rate of Max Pressure Rise (%) | Delay Time of Pressure Peak Arriving (s) |
|---|---|---|---|---|---|---|
| No powders | 0.749 | 41.16 | 0.07 | 0 | 0 | 0 |
| RM | 0.651 | 21.48 | 0.11 | 13.1 | 47.8 | 0.04 |
| 5%-NH4H2PO4/RM | 0.607 | 8.79 | 0.23 | 19.9 | 78.6 | 0.16 |
| 10%-NH4H2PO4/RM | 0.571 | 6.16 | 0.26 | 23.8 | 85.1 | 0.19 |
| 15%-NH4H2PO4/RM | 0.562 | 5.17 | 0.29 | 24.9 | 87.4 | 0.22 |
| 20%-NH4H2PO4/RM | 0.519 | 4.32 | 0.36 | 30.7 | 89.5 | 0.29 |
| 25%-NH4H2PO4/RM | 0.497 | 2.54 | 0.40 | 33.6 | 93.8 | 0.33 |
| 30%-NH4H2PO4/RM | 0.486 | 1.72 | 0.50 | 35.1 | 95.8 | 0.43 |
| NH4H2PO4 | 0.545 | 2.99 | 0.45 | 27.2 | 92.8 | 0.38 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Y.; Meng, X.; Zheng, L.; Gao, J. The Suppression Characteristics of NH4H2PO4/Red Mud Composite Powders on Methane Explosion. Appl. Sci. 2018, 8, 1433. https://doi.org/10.3390/app8091433
Zhang Y, Wang Y, Meng X, Zheng L, Gao J. The Suppression Characteristics of NH4H2PO4/Red Mud Composite Powders on Methane Explosion. Applied Sciences. 2018; 8(9):1433. https://doi.org/10.3390/app8091433
Chicago/Turabian StyleZhang, Yimin, Yan Wang, Xiangqing Meng, Ligang Zheng, and Jianliang Gao. 2018. "The Suppression Characteristics of NH4H2PO4/Red Mud Composite Powders on Methane Explosion" Applied Sciences 8, no. 9: 1433. https://doi.org/10.3390/app8091433