Damage Mechanisms of Polymer Impregnated Carbon Textiles Used as Anode Material for Cathodic Protection
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials
3. Investigations
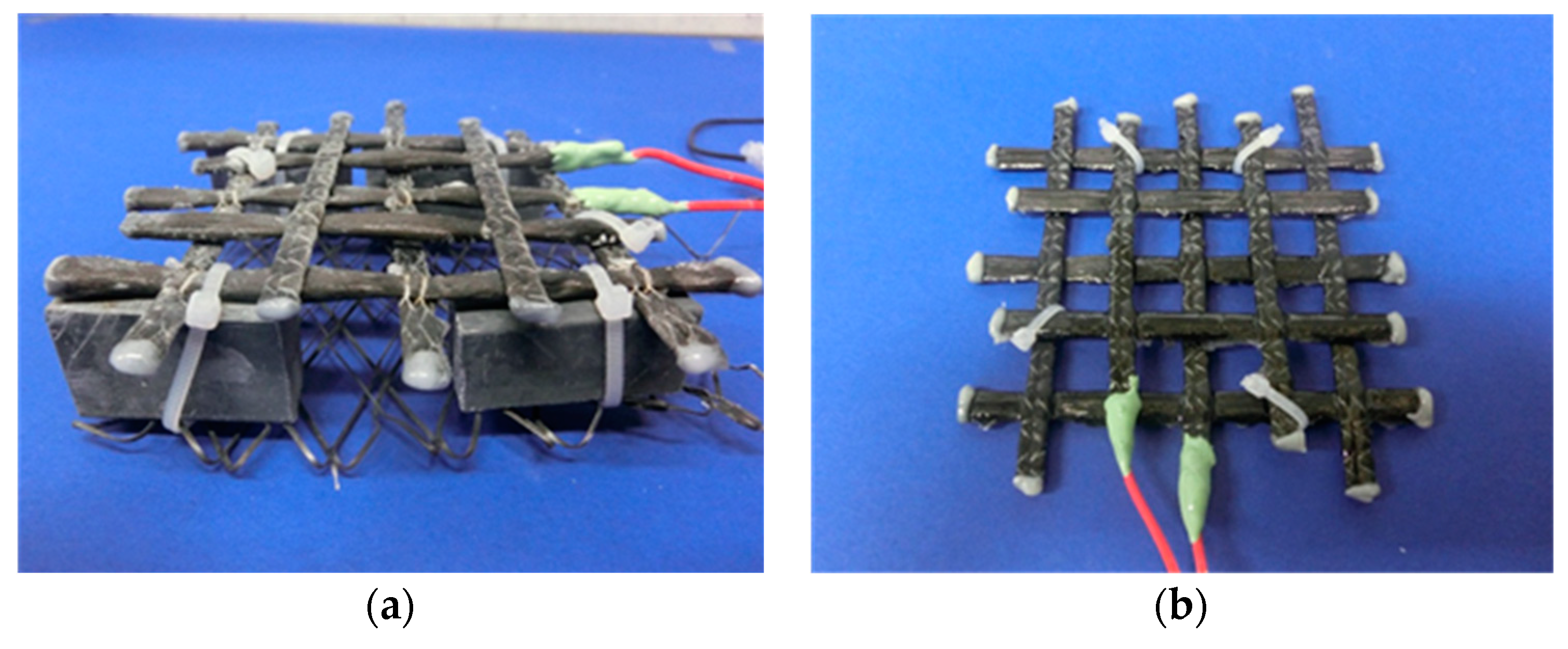
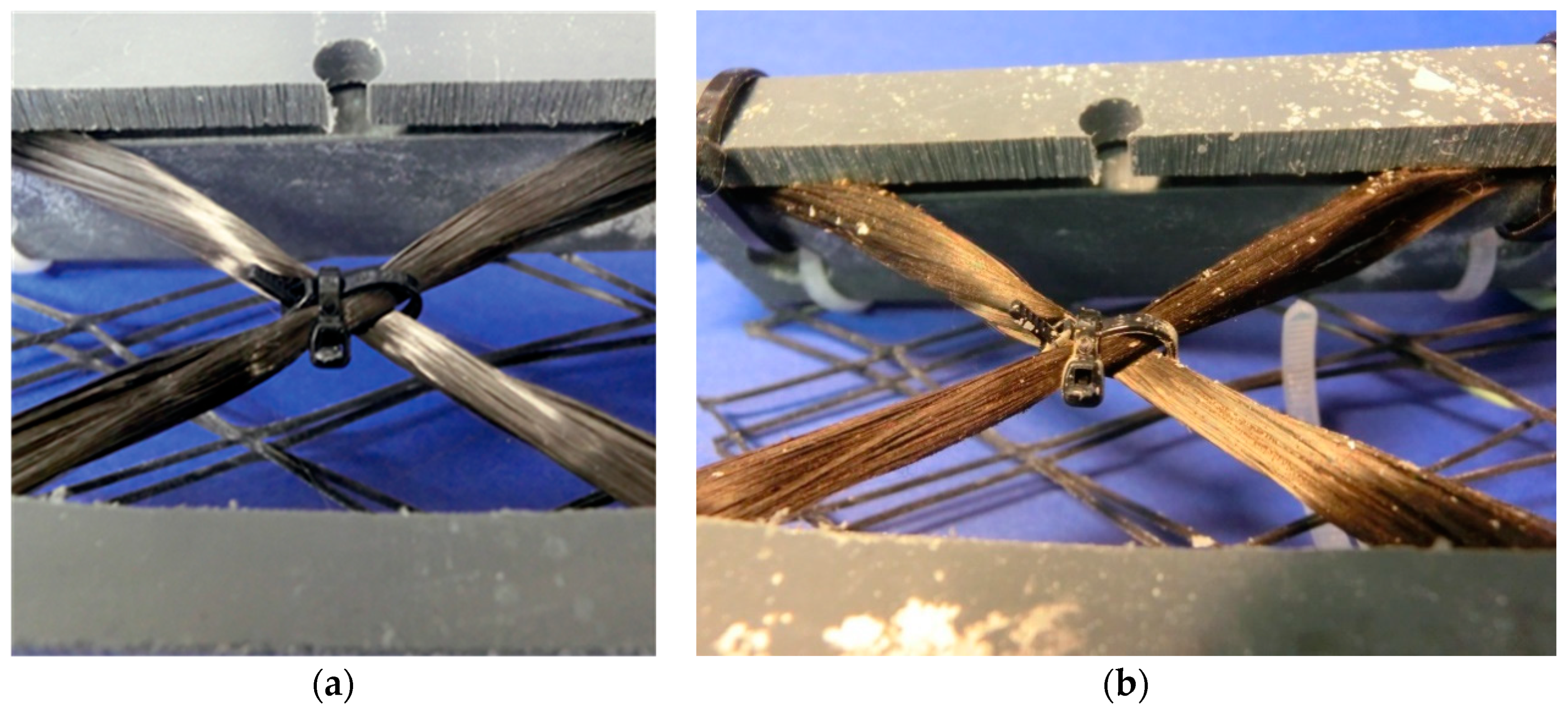
3.1. Specimen Preparation
3.2. Potentiodynamic Polarization
3.3. Potentiostatic Polarization
4. Results and Discussion
4.1. Potentiodynamic Tests
4.2. Potentiostatic Tests
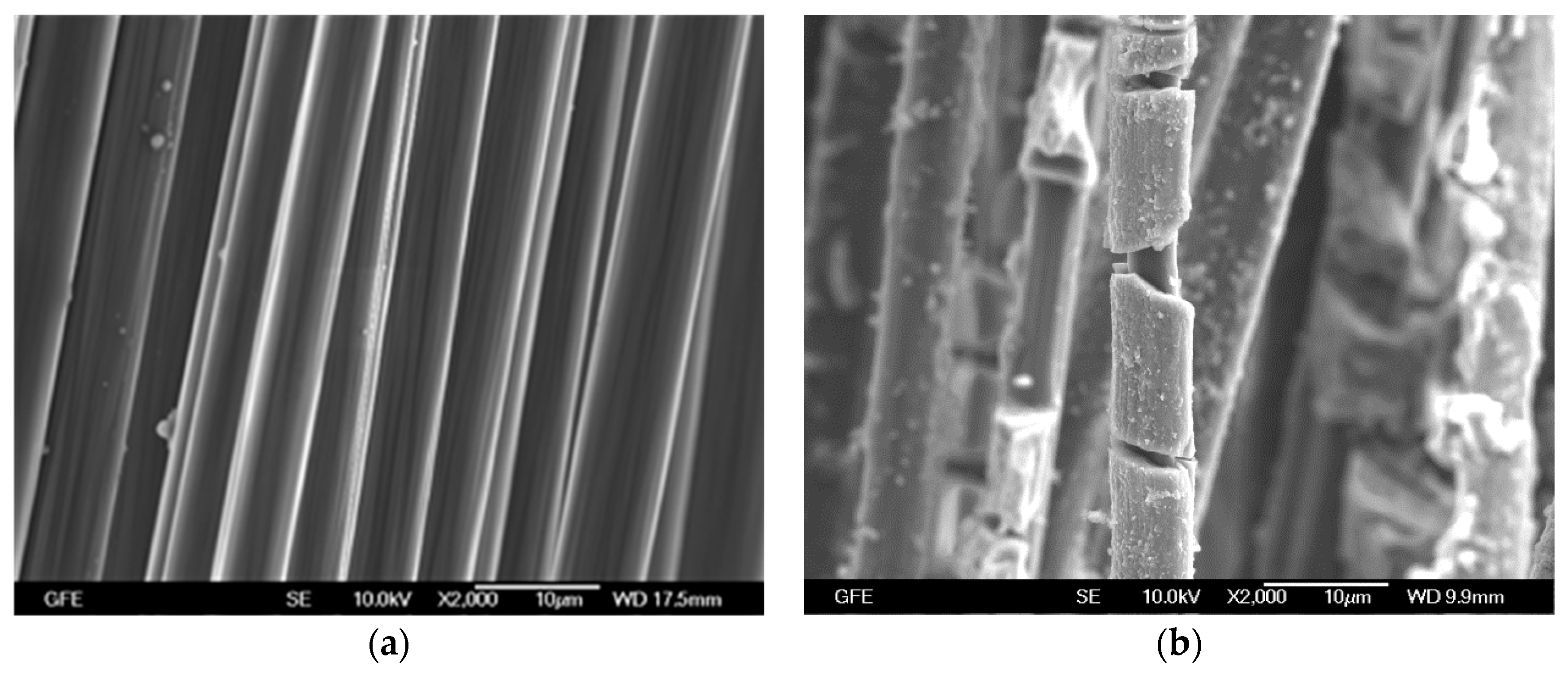
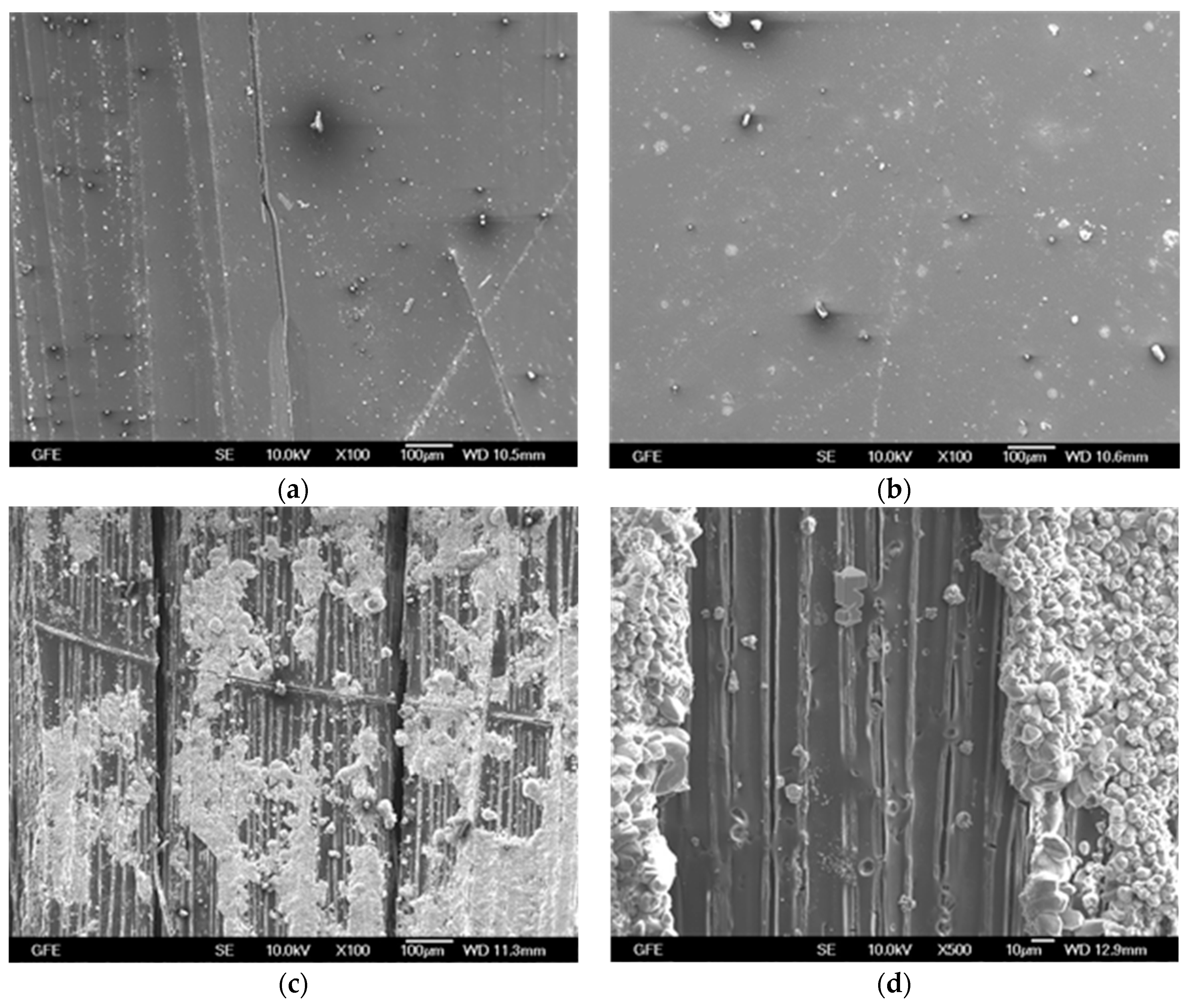
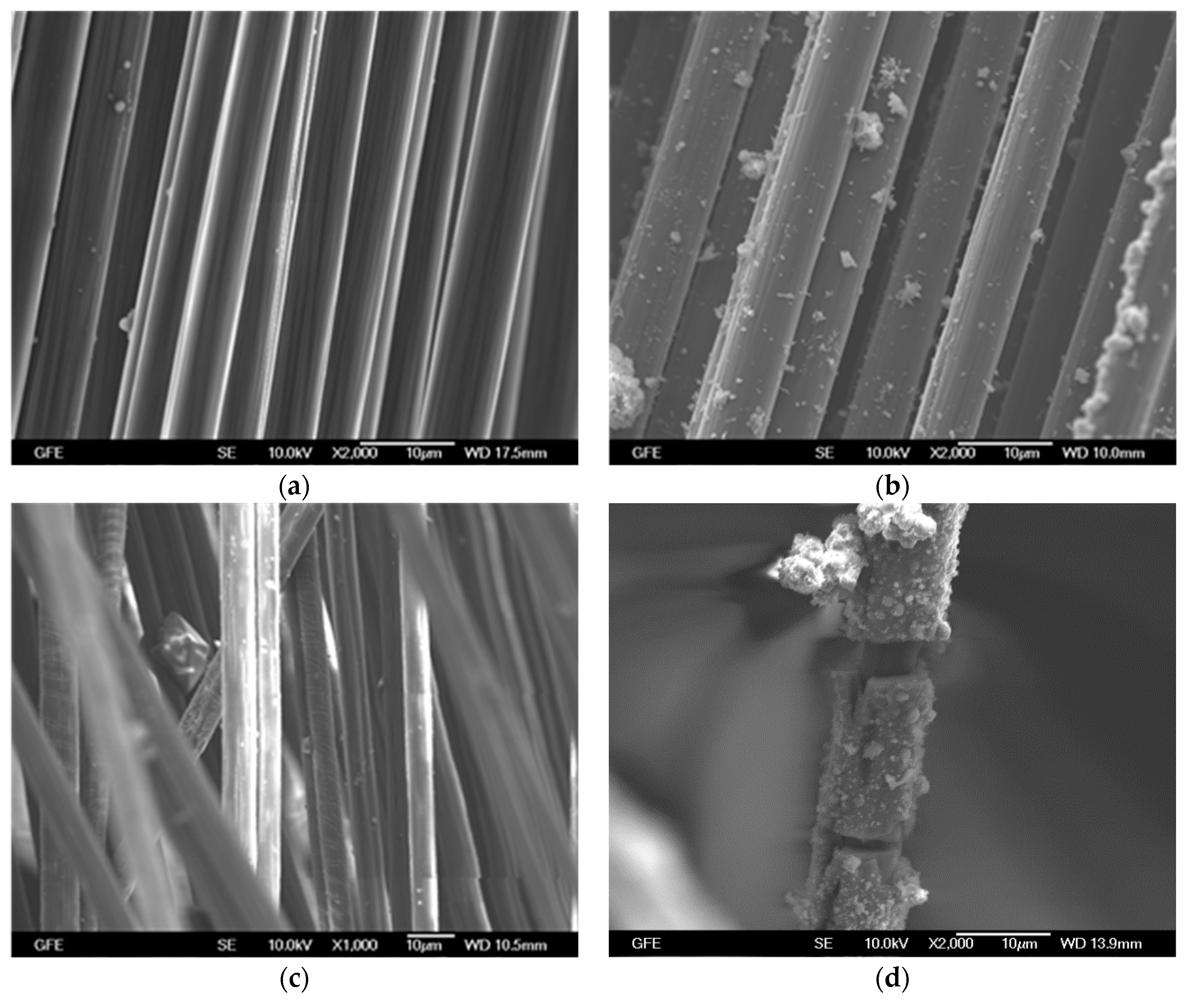
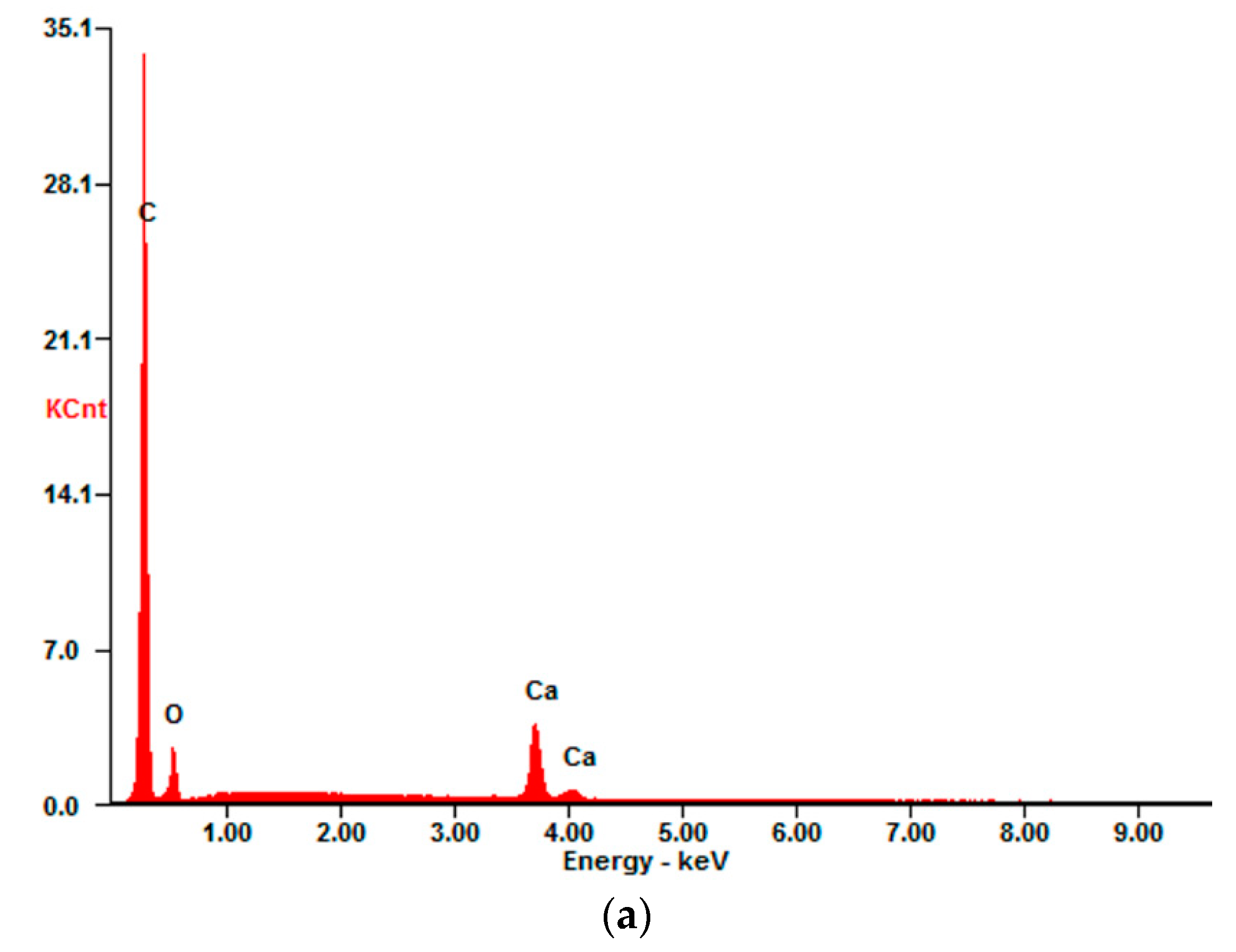
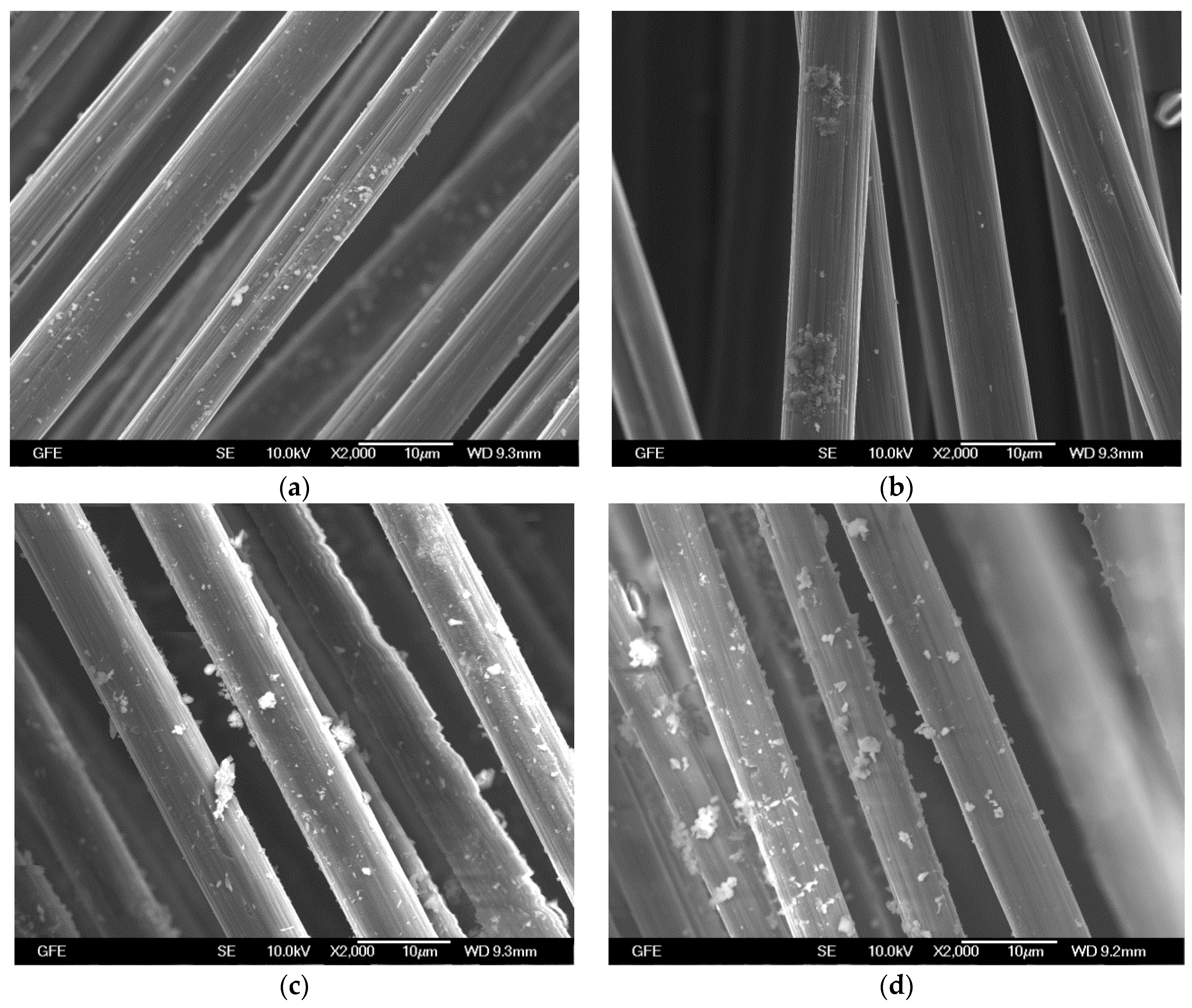
4.2.1. Epoxy Impregnated Carbon (E4)
4.2.2. Carbon Coated with SBR-based Material (S4)
4.2.3. Unimpregnated Carbon with Sizing (UC)
4.2.4. Unimpregnated Carbon without Sizing (UC_WS)
5. Conclusions and Outlooks
- The investigations in solution have shown that CP with investigated carbon anodes up to 2200 mV versus NHE is possible
- The Carbon filaments within the Carbon textiles as CP anode have not been destroyed up to the investigated potentials up to 2200 mV versus NHE.
- With SBR impregnated samples, the impregnation is destroyed right from the start during polarization.
- The sizing is destroyed at a potential of about 900 mV versus NHE.
- Epoxy impregnation started to destroy between 1050 and 1150 mV versus NHE.
Author Contributions
Funding
Conflicts of Interest
References
- Gao, S.L.; Mäder, E.; Plonka, R. Nanostructured coatings of glass fibres: Improvement of alkali resistance and mechanical properties. Acta Mater. 2007, 55, 1043–1052. [Google Scholar] [CrossRef]
- Zhang, R.L.; Huang, Y.D.; Liu, L.; Tang, Y.R.; Su, D.; Xu, L.W. Effect of emulsifier content of sizing agent on the surface of carbon fibres and interface of its composites. Appl. Surf. Sci. 2011, 257, 3519–3523. [Google Scholar] [CrossRef]
- Lesko, J.J.; Swain, R.E.; Cartwright, J.W.; Chin, J.W.; Reifsnider, K.L.; Dillard, D.A.; Wightman, J.P. Interphases developed from fibre sizings and their chemical-structural relationship to composite performance. J. Adhes. 1994, 45, 43–57. [Google Scholar] [CrossRef]
- Xu, L. Interfacial Engineering of the Interphase between Carbon Fibers and Vinyl Ester Resin. Ph.D. Thesis, Department of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI, USA, 2003. [Google Scholar]
- Broyles, N.S.; Verghese, K.N.E.; Davis, S.V.; Li, H.; Davis, R.M.; Lesko, J.J.; Riffle, J.S. Fatigue performance of carbon fibre/vinyl ester composites: The effect of two dissimilar polymeric sizing agents. Polymer 1998, 39, 3417–3424. [Google Scholar] [CrossRef]
- Wetjen, D. Wechselwirkung von Carbonfasern, Schlichte und Epoxidbasierter Polymerer Matrix in Carbonfaserverstärkten Kunststoffen. Ph.D. Thesis, Mathematisch-Naturwissenschaftlich-Technische Fakultät, Universität Augsburg, Augsburg, Germany, 2016. [Google Scholar]
- Poltavtseva, M.; Ebell, G.; Mietz, J. Electrochemical investigations of carbon-based conductive coatings for application as anodes in ICCP systems of reinforced concrete structures. Mater. Corros. 2015, 66, 627–634. [Google Scholar] [CrossRef]
- Rueffer, M.; Bejan, D.; Bunce, N.J. Graphite. An active or an inactive anode? Electrochim. Acta 2011, 56, 2246–2253. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Asgharzadeh, A.; Raupach, M.; Koch, D. Investigations on the Suitability of Technical Textiles for Cathodic Corrosion Protection. In Proceedings of the 4th International Conference on Concrete Repair, Rehabilitation and Retrofitting (ICCRRR), Leipzig, Germany, 5–7 October 2015. [Google Scholar]
- Asgharzadeh, A.; Raupach, M. Development of a test Method for the Durability of Carbon Textiles under Anodic Polarisation. In Service Life and Durability of Reinforced Concrete Structures (RILEM Book Series); Springer International Publishing: Basel, Switzerland, 2018; pp. 143–158. [Google Scholar]
- Asgharzadeh, A.; Raupach, M. Durability behaviour of polymer impregnated carbon textiles in alkaline solution as CP anode. Mater. Corros. 2018, 1–12. [Google Scholar] [CrossRef]
- Bertolini, L.; Bolzoni, F.; Pastore, T.; Pedeferri, P. Effectiveness of a conductive cementitious mortar anode for cathodic protection of steel in concrete. Cem. Concr. Res. 2004, 34, 681–694. [Google Scholar] [CrossRef]
- Chini, M.; Antonsen, R.; Vennesland, Ø.; Mork, J.H.; Arntsen, B. Polarization Behavior of Carbon Fiber as an Anodic Material in Cathodic Protection. In Proceedings of the 11DBMC International Conference on Durability of Building Materials and Components, Istanbul, Turkey, 11–14 May 2008. [Google Scholar]
- Chini, M. Pan-Based Carbon Fiber as Anode Material in Cathodic Protection Systems for Concrete Structures. Avhandling–Norges Teknisk-Naturvitenskapelige Universitet, Trondheim. Ph.D. Thesis, Department of Structural Engineering, Faculty of Engineering Science and Technology, Norwegian University of Science and Technology, Trondheim, Norway, 2010. [Google Scholar]
- Zhang, E.Q.; Tang, L.; Zack, T. Carbon Fiber as Anode Material for Cathodic Prevention in Cementitious Materials. In Proceedings of the 5th International Conference on the Durability of Concrete Structures, Shenzhen, China, 30 June–1 July 2016; Xing, F., Han, N., Zhu, J.-H., Eds.; Shenzhen University: Shenzhen, China; Purdue University Press: West Lafayette, IN, USA, 2016. [Google Scholar] [Green Version]
- Van Nguyen, C.; Lambert, P.; Mangat, P.; O’Flaherty, F.; Jones, G. The Performance of Carbon Fibre Composites as ICCP Anodes for Reinforced Concrete Structures. ISRN Corros. 2012, 2012, 1–9. [Google Scholar] [CrossRef]




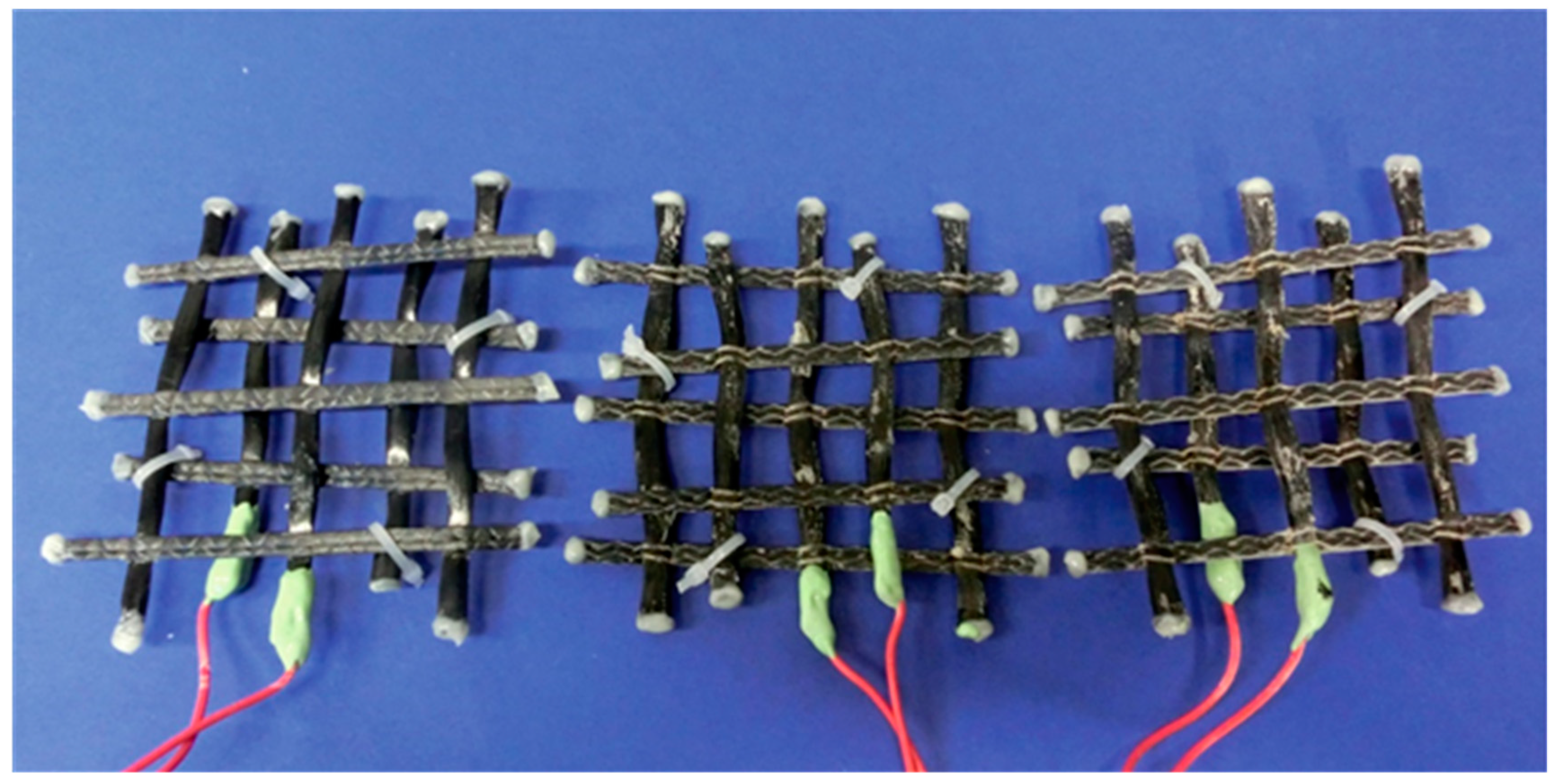





| Specimen Specification | Sizing | Impregnation Material | Mesh Size [mm/mm] 0°/90° | |
|---|---|---|---|---|
| impregnated carbon textile | E4 | yes | Epoxy | 38/38 |
| S4 | yes | SBR | 38/38 | |
| unimpregnated carbon textile | UC | yes | - | - |
| UC_WS | no | - | - |
| Material | Level 1 | Level 2 [mV] vs. NHE | Level 3 |
|---|---|---|---|
| E4 | 940 | 1490 | 1940 |
| S4 | 490 | 1490 | 1840 |
| UC | 690 | 1300 | 1940 |
| UC_WS | 690 | 1300 | 1940 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgharzadeh, A.; Raupach, M. Damage Mechanisms of Polymer Impregnated Carbon Textiles Used as Anode Material for Cathodic Protection. Appl. Sci. 2019, 9, 110. https://doi.org/10.3390/app9010110
Asgharzadeh A, Raupach M. Damage Mechanisms of Polymer Impregnated Carbon Textiles Used as Anode Material for Cathodic Protection. Applied Sciences. 2019; 9(1):110. https://doi.org/10.3390/app9010110
Chicago/Turabian StyleAsgharzadeh, Amir, and Michael Raupach. 2019. "Damage Mechanisms of Polymer Impregnated Carbon Textiles Used as Anode Material for Cathodic Protection" Applied Sciences 9, no. 1: 110. https://doi.org/10.3390/app9010110






