Synthesis and Catalytic Activity of Activated Carbon Supported Sulfonated Cobalt Phthalocyanine in the Preparation of Dimethyl Disulfide
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
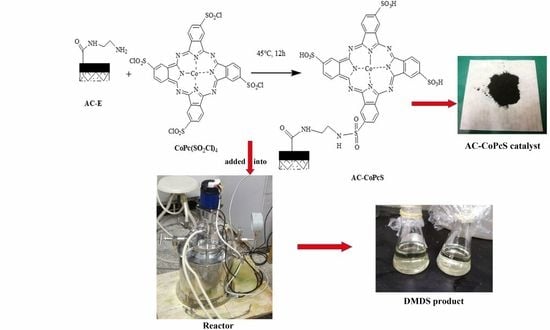
2.2. Synthesis of AC-Supported CoPcS (AC-CoPcS)
2.2.1. Synthesis of Modified AC by Ethylene Diamine (AC-E)
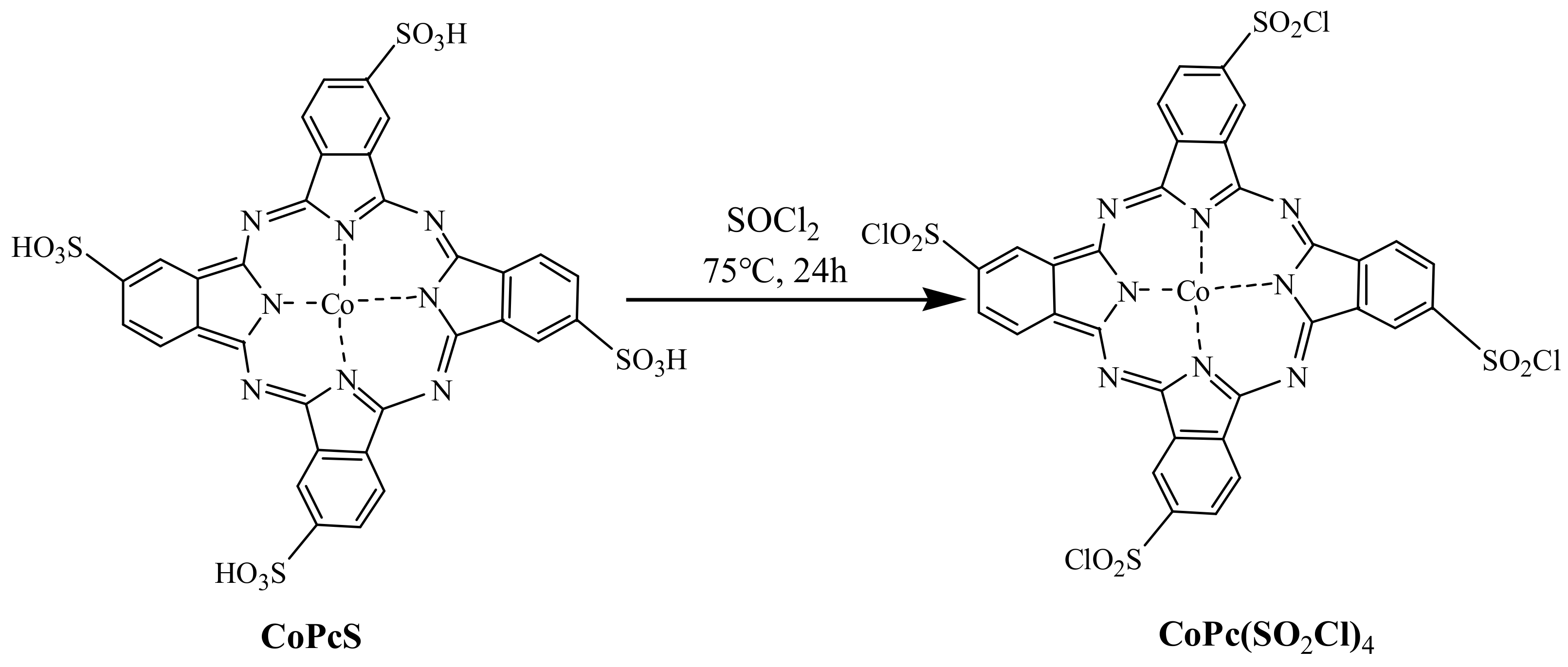
2.2.2. Synthesis of Modified CoPcS by Sulfoxide Chloride (CoPc(SO2Cl)4)
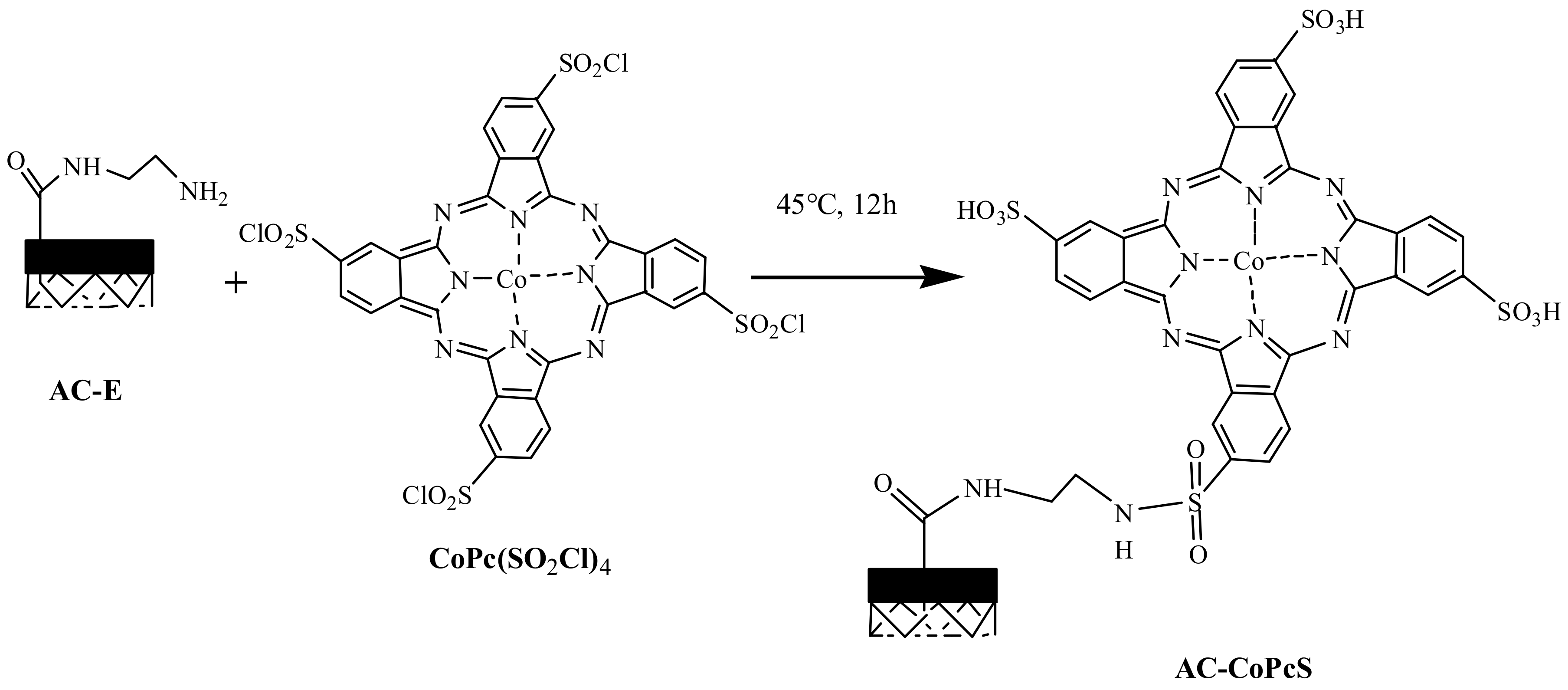
2.2.3. Synthesis of the AC-Supported CoPcS (AC-CoPcS)
2.3. Characterization Methods
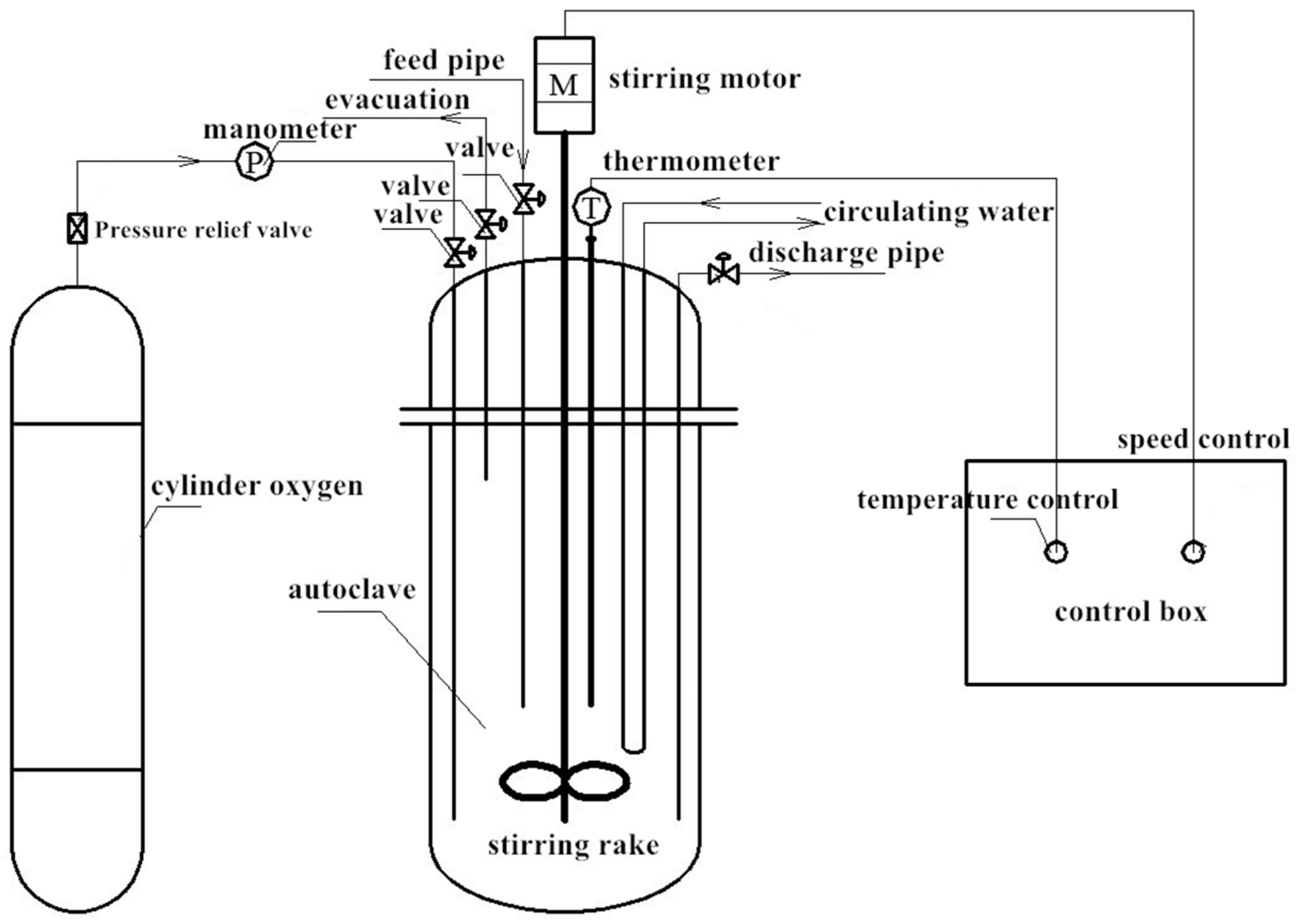
2.4. Catalytic Experiment of AC-CoPcS Catalyst for the Preparation of DMDS
2.5. The Determination of Purity of DMDS
2.6. Calculate Method of CPPSMM and YieldDMDS
3. Results and Discussion
3.1. Characterization of CoPcS and AC-CoPcS Catalysts
3.2. The Effect of Operation Parameters on AC-CoPcS Catalytic Performance
3.2.1. The Performance Comparison between Free CoPcS and AC-CoPcS Catalysts
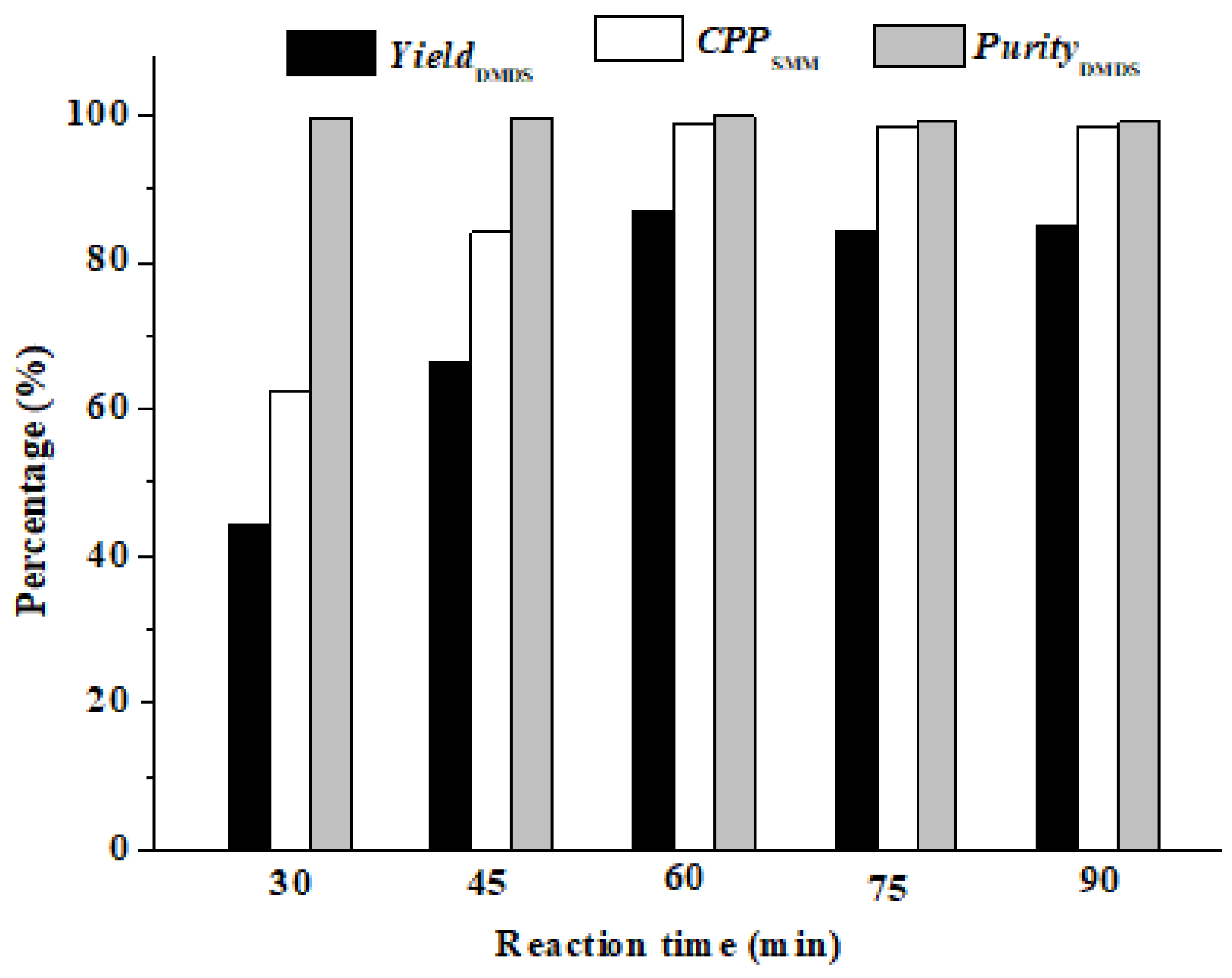
3.2.2. The Effect of Reaction Time (t)
3.2.3. The Effect of AC-CoPcS Catalyst Dosage (Cca)
3.2.4. The Effect of Reaction Temperature (Tre)
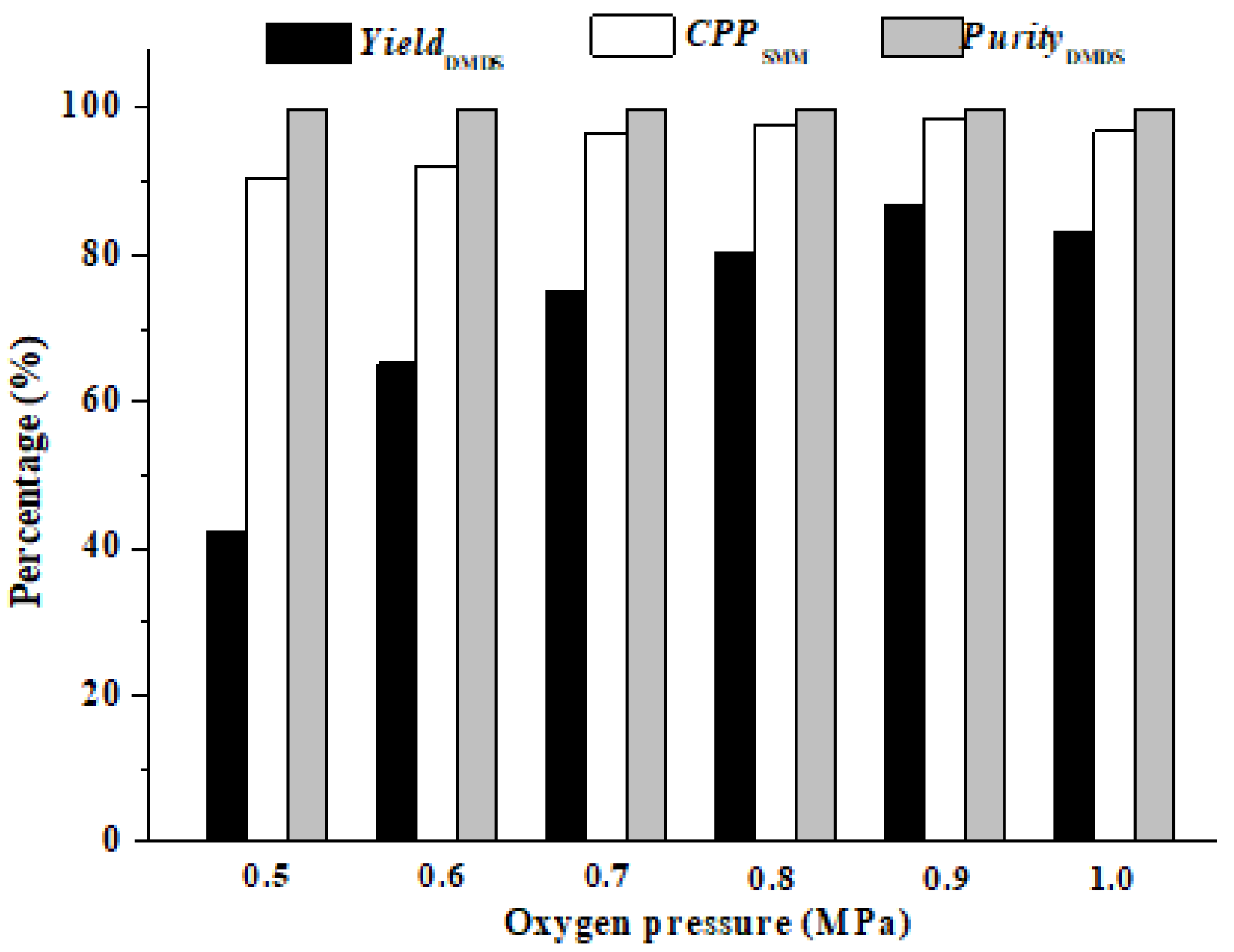
3.2.5. The Effect of Oxygen Pressure (P(O2))
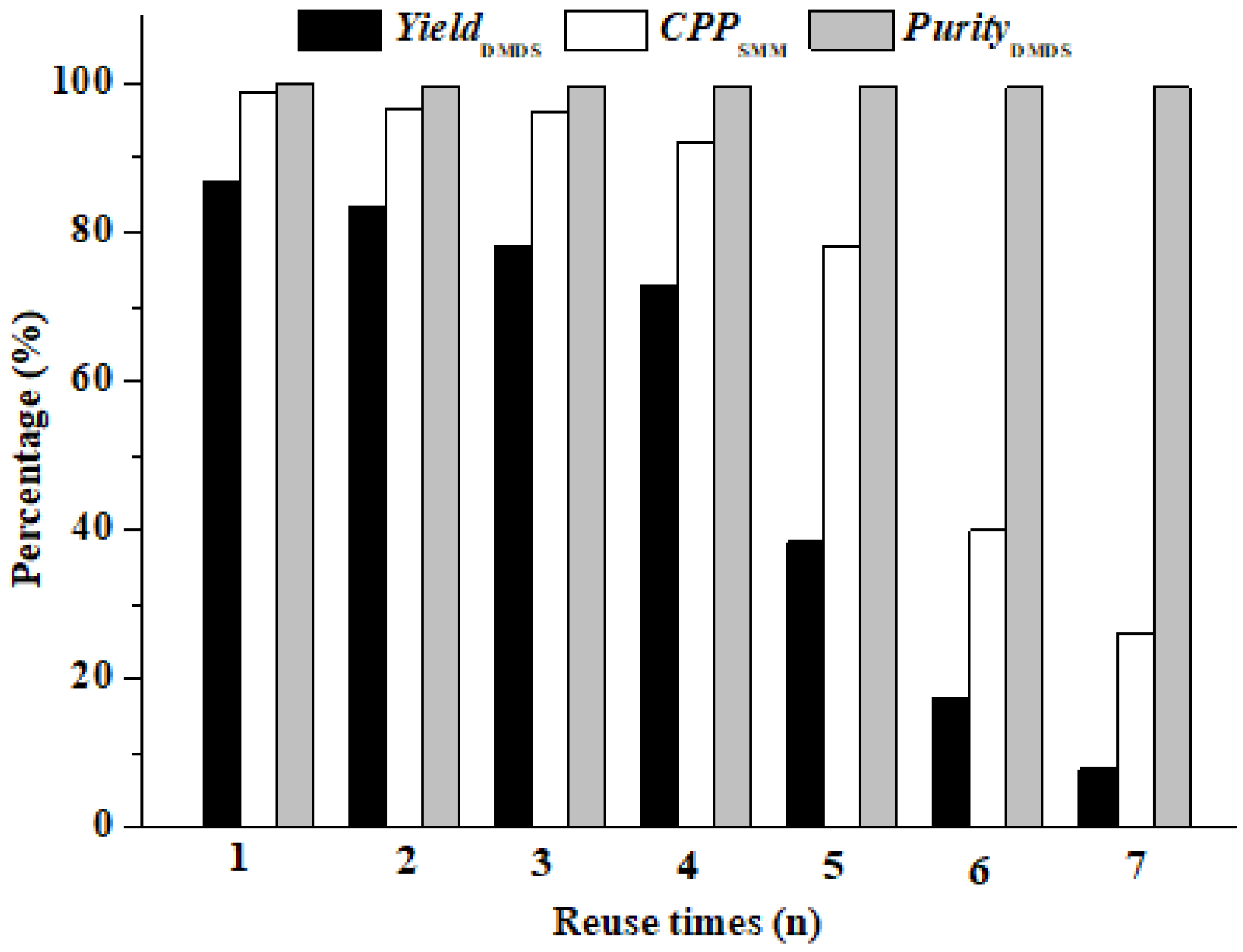
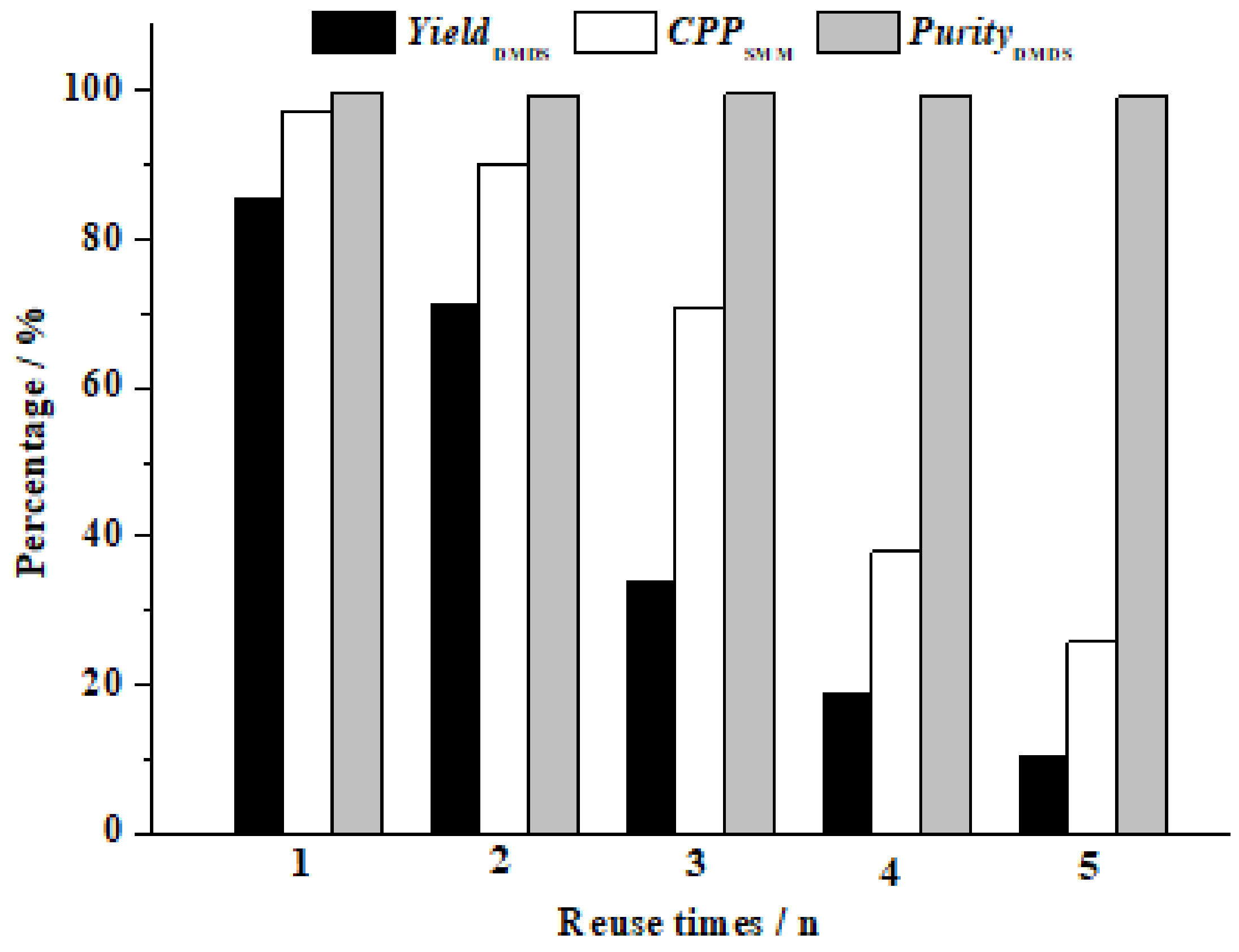
3.3. The Performance Comparison between Commercial and Newly Prepared AC-CoPcS Catalysts
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Ajwa, H.; Ntow, W.J.; Qin, R.; Gao, S. Chapter 9—Properties of Soil Fumigants and Their Fate in the Environment. In Hayes’ Handbook of Pesticide Toxicology, 3rd ed.; Krieger, R., Ed.; Academic Press: New York, NY, USA, 2010; pp. 315–330. 4p. [Google Scholar]
- Cabrera, J.A.; Wang, D.; Gerik, J.S.; Gan, J. Spot drip application of dimethyl disulfide as a post-plant treatment for the control of plant parasitic nematodes and soilborne pathogens in grape production. Pest Manag. Sci. 2014, 70, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Pecchia, S.; Franceschini, A.; Santori, A.; Vannacci, G.; Myrta, A. Efficacy of dimethyl disulfide (DMDS) for the control of chrysanthemum Verticillium wilt in Italy. Crop Prot. 2017, 93, 28–32. [Google Scholar] [CrossRef]
- Stevens, M.; Freeman, J. Efficacy of dimethyl disulfide and metam sodium combinations for the control of nutsedge species. Crop Prot. 2018, 110, 131–134. [Google Scholar] [CrossRef]
- Gereben, O.; Kohara, S.; Pusztai, L. The liquid structure of some food aromas: Joint X-ray diffraction, all-atom molecular dynamics and reverse Monte Carlo investigations of dimethyl sulfide, dimethyl disulfide and dimethyl trisulfide. J. Mol. Liq. 2012, 169, 63–73. [Google Scholar] [CrossRef]
- Reid, E.E. Organic Chemistry of Bivalent Sulfur. Chemical Publishing, Co.: New York, NY, USA, 1960; Volume 3. [Google Scholar]
- Wang, P. Manufacturing Process of Dimethyl Disulfide. CN 1031838A, 22 March 1989. [Google Scholar]
- Custer, R.S.; Haines, P.G. Preparation of Dialkyl Disulfides. Eur. Patent 0171092A2, 12 February 1986. [Google Scholar]
- Buchholz, B.; Dzierza, E.J.; Baltrus, J.R. Process for the Manufacture of Dialkyl Disulfides. Eur. Patent 0202420A1, 26 November 1986. [Google Scholar]
- Pau, E.A. Process for the Manufacture of Dimethyl Disulphide. U.S. Patent US 5,312,993, 17 May 1994. [Google Scholar]
- Fremy, G.; Jean-Michel, R. Process for Preparing Dialkyl Disulphides. U.S. Patent US 8,987,519,B2, 24 March 2015. [Google Scholar]
- Roberts, J.S.; Hunt, H.R.; Drake, C.A. Continuous Process for the Production of Disulfudes. U.S. Patent US 5,202,494, 13 April 1993. [Google Scholar]
- Liu, Y.; Chen, X.; Yao, Y.; Zheng, D. A New Method for Preparation of Dimethyl Disulfide. CN 1,059,243,72B, 22 December 2017. [Google Scholar]
- Wang, J.; Fang, X.; Tian, Y.; Yang, J.; Ao, H.; Li, X.; Yi, X. A Method and Device of Methanthiol Solution Oxidation for Preparation of Dimethyl Disulfide. CN 105175296A, 23 December 2015. [Google Scholar]
- Guo, K.; Guo, M.; Huang, Y.; Huang, F. A Method and Device for the Preparation of Dimethyl Disulfide. CN 105085338A, 25 November 2015. [Google Scholar]
- Quan, X.; Dai, M.; Zheng, D.; Li, S.; Liu, Y.; Cheng, Z.; Yao, Y. Activated Carbon-Loaded Tetraamino Cobalt Phthalocyanine and Application Thereof in Preparing Dimethyl Disulfide as Catalyst. CN 106984361A, 28 July 2017. [Google Scholar]
- Gleim, W.; Urban, P. Treatment of Hydrocarbon Distillates. U.S. Patent US 2988500, 13 June 1961. [Google Scholar]
- Jiang, D.-e.; Zhao, B.; Huang, H.; Xie, Y.; Pan, G.; Ran, G.; Min, E. Dispersion of cobalt (II) phthalocyaninetetrasulfonate on active carbon. Appl. Catal. A Gen. 2000, 192, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Fang, Y.; Lu, W.; Li, N.; Chen, W. Oxidative removal of sulfa antibiotics by introduction of activated carbon fiber to enhance the catalytic activity of iron phthalocyanine. Microporous Mesoporous Mater. 2018, 261, 98–104. [Google Scholar] [CrossRef]
- Huang, Z.; Bao, H.; Yao, Y.; Lu, W.; Chen, W. Novel green activation processes and mechanism of peroxymonosulfate based on supported cobalt phthalocyanine catalyst. Appl. Catal. B Environ. 2014, 154–155, 36–43. [Google Scholar] [CrossRef]
- Chen, W.; Lu, W.; Yao, Y.; Xu, M. Highly Efficient Decomposition of Organic Dyes by Aqueous-Fiber Phase Transfer and in Situ Catalytic Oxidation Using Fiber-Supported Cobalt Phthalocyanine. Environ. Sci. Technol. 2007, 41, 6240–6245. [Google Scholar] [CrossRef]
- Mirzaeian, M.; Rashidi, A.M.; Zare, M.; Ghabezi, R.; Lotfi, R. Mercaptan removal from natural gas using carbon nanotube supported cobalt phthalocyanine nanocatalyst. J. Nat. Gas Sci. Eng. 2014, 18, 439–445. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Huang, H.; Zhang, Q.; Guo, Q. Graphene Oxide–Supported Cobalt Phthalocyanine as Heterogeneous Catalyst to Activate Peroxymonosulfate for Efficient Degradation of Norfloxacin Antibiotics. J. Environ. Eng. 2018, 144, 04018052. [Google Scholar] [CrossRef]
- Sorokin, A.B.; Tuel, A. Metallophthalocyanine functionalized silicas: catalysts for the selective oxidation of aromatic compounds. Catal. Today 2000, 57, 45–59. [Google Scholar] [CrossRef]
- Wöhrle, D.; Hündorf, U.; Schulz-Ekloff, G.; Ignatzek, E. Phthalocyanines on Mineral Carriers, 2 Synthesis of Cobalt(II) and Copper(II)-phthalocyanines on γ-Al2O3 and SiO2. Z. Naturforsch. B 1986, 41, 179. [Google Scholar] [CrossRef]
- Wöhrle, D.; Buck, T.; Hündorf, U.; Schulz-Ekloff, G.; Andreev, A. Phthalocyanines on mineral carriers, 4. Low-molecular-weight and polymeric phthalocyanines on SiO2, γ-Al2O3 and active charcoal as catalysts for the oxidation of 2-mercaptoethanol. Die Makromol. Chem. 1989, 190, 961–974. [Google Scholar] [CrossRef]
- Schubert, U.; Lornez, A.; Kundo, N.; Stuchinskaya, T.; Gogina, L.; Salanov, A.; Zalkovskii, V.; Maizlish, V.; Shaposhmikov, G.P. Cobalt Phthalocyanine Derivatives Supported on TiO2 by Sol-Gel Processing. Part 1: Preparation and Microstructure. Chem. Ber. 1997, 130, 1585–1589. [Google Scholar] [CrossRef]
- Stuchinskaya, T.; Kundo, N.; Gogina, L.; Schubert, U.; Lorenz, A.; Maizlish, V. Cobalt phthalocyanine derivatives supported on TiO2 by sol–gel processing: Part 2. Activity in sulfide and ethanethiol oxidation. J. Mol. Catal. A Chem. 1999, 140, 235–240. [Google Scholar] [CrossRef]
- Zanjanchi, M.A.; Ebrahimian, A.; Arvand, M. Sulphonated cobalt phthalocyanine–MCM-41: An active photocatalyst for degradation of 2,4-dichlorophenol. J. Hazard. Mater. 2010, 175, 992–1000. [Google Scholar] [CrossRef]
- Quan, X.; Dai, M.; Zheng, D.; Li, S.; Liu, Y.; Cheng, Z.; Yao, Y. Activated Carbon-Loaded Tetrasulfo Cobalt Phthalocyanine and Application Thereof in Preparing Dimethyl Disulfide as Catalyst. CN 106984362A, 28 July 2017. [Google Scholar]
- Mugadza, T.; Nyokong, T. Synthesis and Characterization of Electrocatalytic Conjugates of Tetraamino Cobalt (II) Phthalocyanine and Single Wall Carbon Nanotubes. Electrochim. Acta 2009, 54, 6347–6353. [Google Scholar] [CrossRef]
- Su, Y.; Wang, J.; Chen, G. Study on the enhancement of electrochemiluminescence of luminol–H2O2 system by sulphonated cobalt(II) phthalocyanine. Anal. Chim. Acta 2005, 551, 79–84. [Google Scholar] [CrossRef]
- Seoudi, R.; El-Bahy, G.S.; El Sayed, Z.A. FTIR, TGA and DC electrical conductivity studies of phthalocyanine and its complexes. J. Mol. Struct. 2005, 753, 119–126. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Fu, Q.; Xu, Z.; Li, K.; Wei, J. One-Step Solvothermal Synthesis of Tetranitro-Cobalt Phthalocyanine/Reduced Graphene Oxide Composite. Nano 2015, 10, 1550045. [Google Scholar] [CrossRef]
- Leitão, A.; Rodrigues, A. Studies on the Merox process: kinetics of N-butyl mercaptan oxidation. Chem. Eng. Sci. 1989, 44, 1245–1253. [Google Scholar] [CrossRef]








| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| AC | 704.8 | 1.30 | 2.50 |
| AC-CoPcS (commercial) | 265.4 | 0.46 | 4.16 |
| AC-CoPcS (new) | 301.3 | 0.51 | 3.95 |
| Catalysts | Cca | YieldDMDS | PurityDMDS | Post-Treatment of DMDS |
|---|---|---|---|---|
| CoPcS | 160 ppm | 81.4% | 98.7% | AC adsorption |
| AC-CoPcS | 888.9 ppm | 86.8% | 99.8% | None |
| Catalyst | Synthetic Method | CPPSMM | YieldDMDS | PurityDMDS | Optimum Operation Parameters | Proper Reuse Times | |||
|---|---|---|---|---|---|---|---|---|---|
| t | Cca | Tre | P(O2) | ||||||
| AC-CoPcS (new) | chemical grafting | 98.70% | 86.8% | 99.8% | 60 min | 888.9 ppm | 65 °C | 0.9 MPa | 4 |
| AC-CoPcS (commercial) | physical dipping | 97.4% | 85.6% | 99.7% | 60 min | 1333.3 ppm | 65 °C | 0.9 Mpa | 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Dai, M.; Quan, X.; Li, S.; Zheng, D.; Liu, Y.; Yao, R. Synthesis and Catalytic Activity of Activated Carbon Supported Sulfonated Cobalt Phthalocyanine in the Preparation of Dimethyl Disulfide. Appl. Sci. 2019, 9, 124. https://doi.org/10.3390/app9010124
Cheng Z, Dai M, Quan X, Li S, Zheng D, Liu Y, Yao R. Synthesis and Catalytic Activity of Activated Carbon Supported Sulfonated Cobalt Phthalocyanine in the Preparation of Dimethyl Disulfide. Applied Sciences. 2019; 9(1):124. https://doi.org/10.3390/app9010124
Chicago/Turabian StyleCheng, Zhiliang, Mingxing Dai, Xuejun Quan, Shuo Li, Daomin Zheng, Yaling Liu, and Rujie Yao. 2019. "Synthesis and Catalytic Activity of Activated Carbon Supported Sulfonated Cobalt Phthalocyanine in the Preparation of Dimethyl Disulfide" Applied Sciences 9, no. 1: 124. https://doi.org/10.3390/app9010124