Plasma-Derived Reactive Species Shape a Differentiation Profile in Human Monocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Preparation
2.2. Cold Physical Plasma and Treatment of Cells
2.3. Cytosolic and Mitochondrial Oxidation
2.4. Metabolic Activity and Cell Counts
2.5. Live Cell Imaging
2.6. Cell Surface Marker Analysis
2.7. Cell Culture Supernatant Analysis
2.8. Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Plasma Treatment Oxidized THP-1 Monocytes while Being of Modest Toxicity
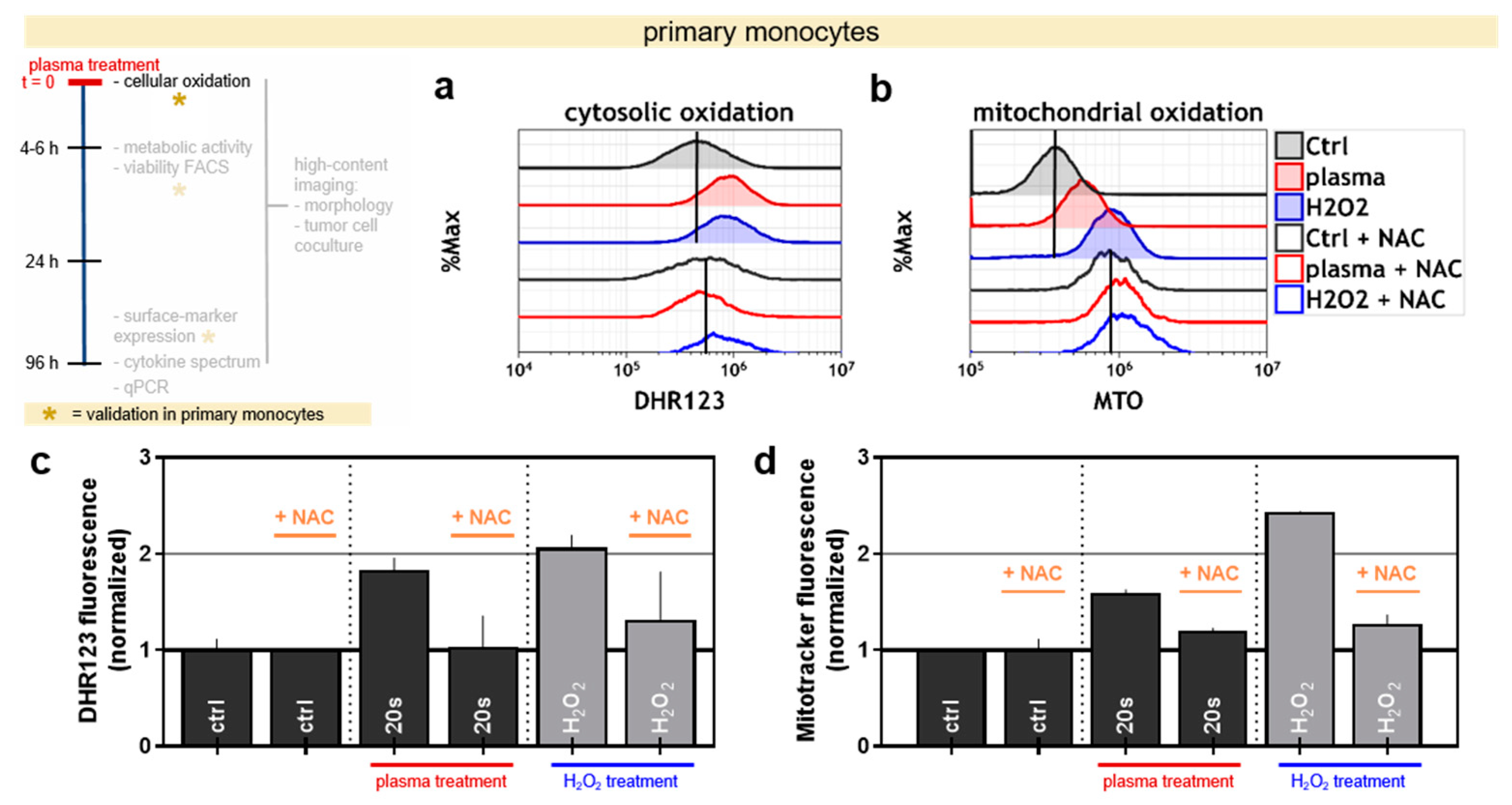
3.2. Plasma Treatment Oxidized Primary Human Monocytes and the Effect Can be Quenched via Antioxidants
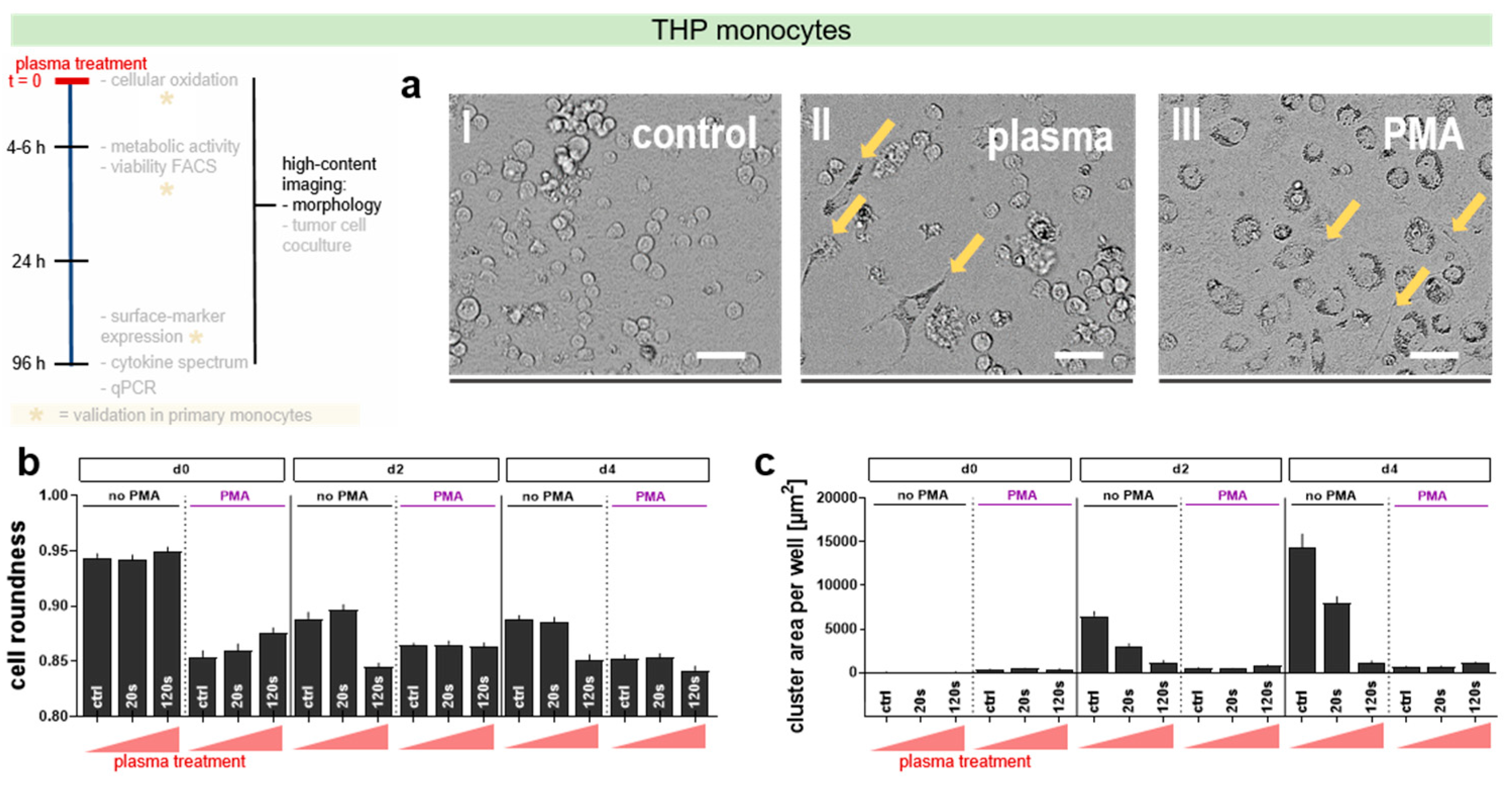
3.3. Plasma Treatment Induced Morphological Changes in THP-1 Monocytes
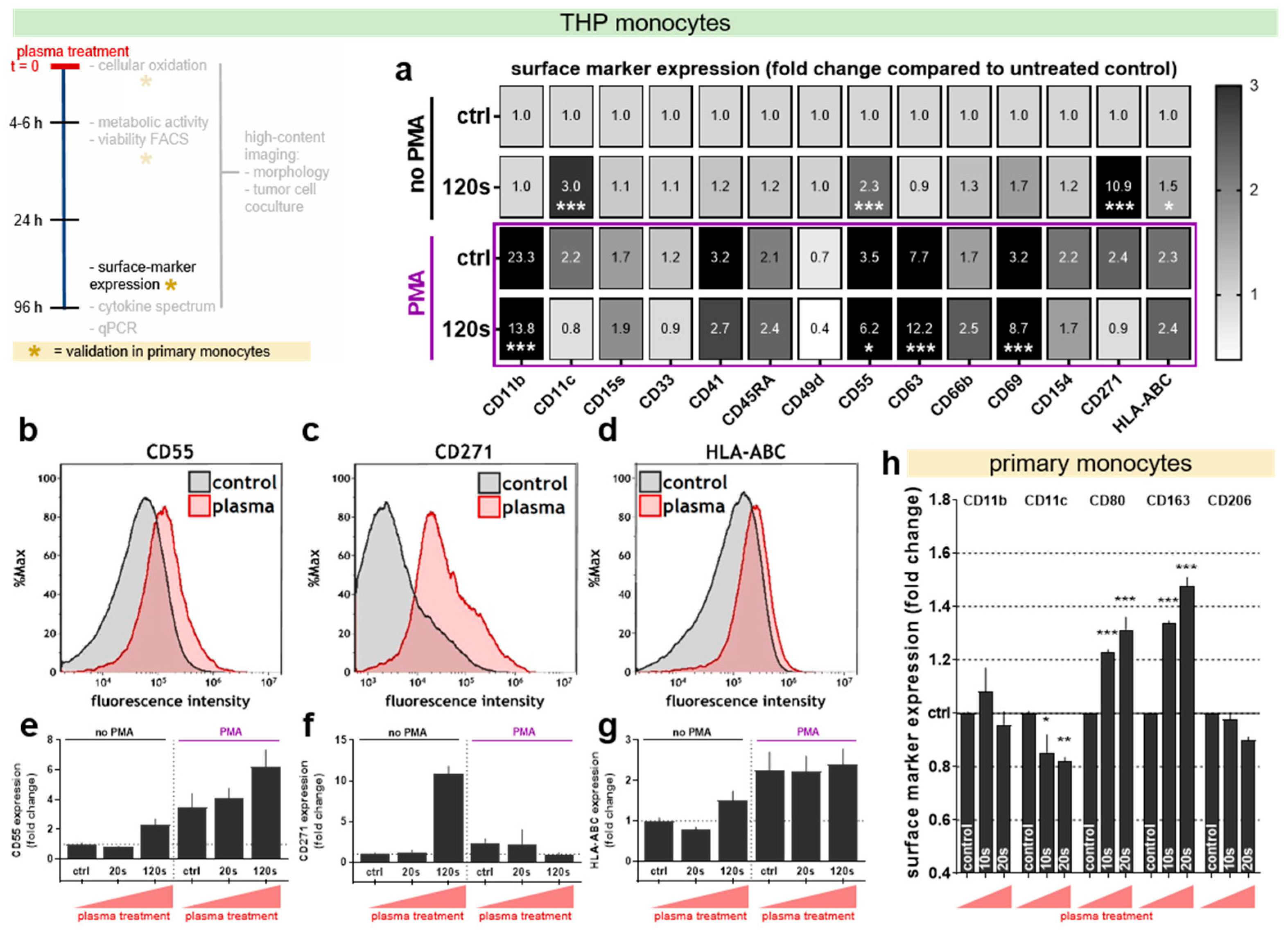
3.4. Plasma Treatment Altered Surface Marker Expression in THP-1 Cells and Primary Monocytes
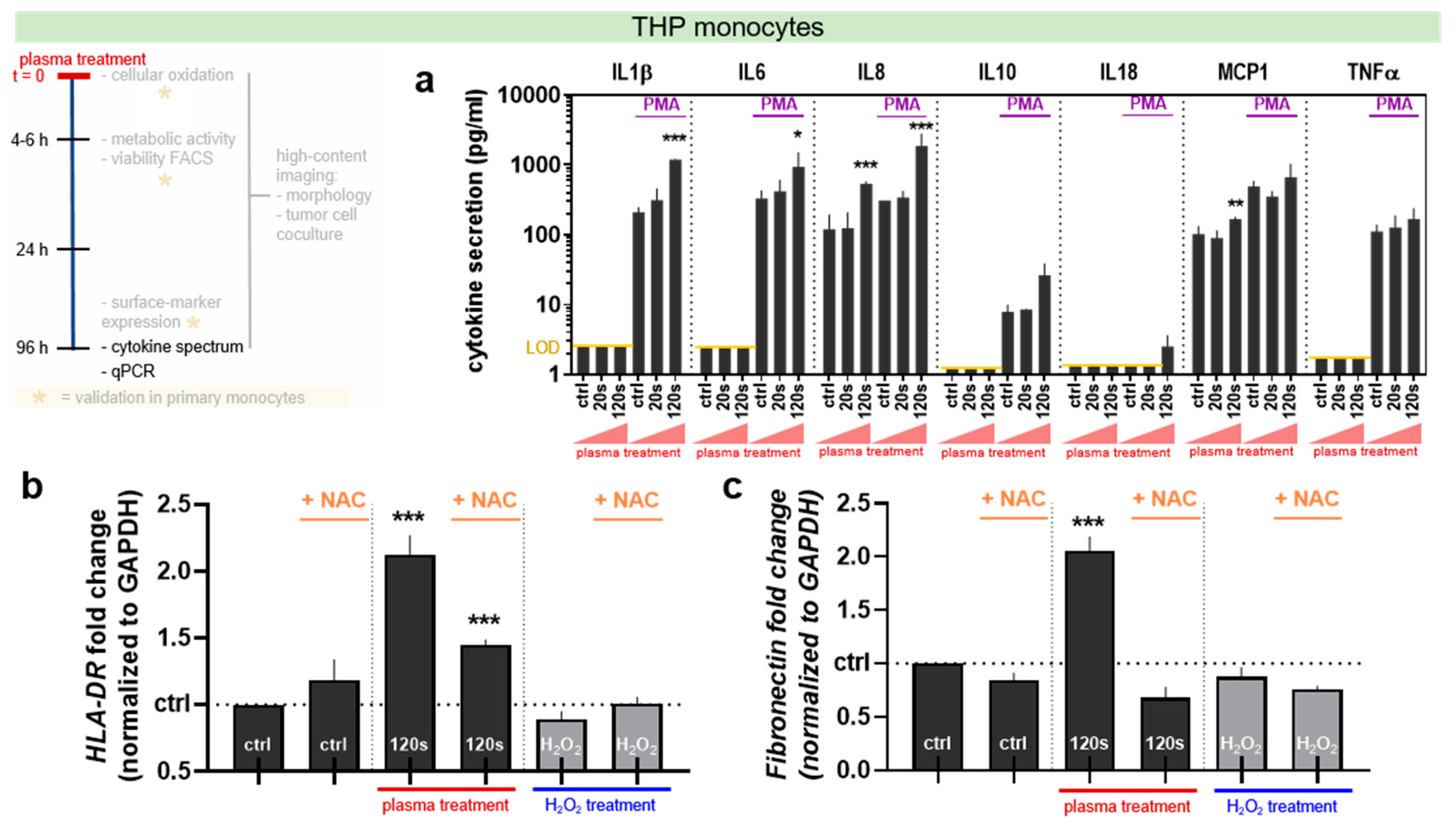
3.5. Plasma Treatment Altered Cytokine and Gene Expression Profile in THP-1 Monocytes
3.6. Plasma Treatment Increased Tumor-Toxic Activity in THP-1 Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Berckelaer, C.; Rypens, C.; Van Dam, P.; Pouillon, L.; Parizel, M.; Schats, K.; Kockx, M.; Tjalma, W.; Vermeulen, P.; van Laere, S. Infiltrating stromal immune cells in inflammatory breast cancer are associated with an improved outcome and increased PD-L1 expression. Breast Cancer Res. 2019, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Leivonen, S.-K.; Pollari, M.; Brück, O.; Pellinen, T.; Autio, M.; Karjalainen-Lindsberg, M.-L.; Mannisto, S.; Kellokumpu-Lehtinen, P.-L.; Kallioniemi, O.; Mustjoki, S. T-cell inflamed tumor microenvironment predicts favorable prognosis in primary testicular lymphoma. Haematologica 2019, 104, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Eubank, T.D.; Doseff, A.I. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J. Innate Immun. 2010, 2, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Caso, R.; Miller, G. Role of tumor associated macrophages in regulating pancreatic cancer progression. World J. Immunol. 2016, 6. [Google Scholar] [CrossRef]
- Mantovani, A.; Schioppa, T.; Porta, C.; Allavena, P.; Sica, A. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 2006, 25, 315–322. [Google Scholar] [CrossRef]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71. [Google Scholar] [CrossRef]
- Menen, R.S.; Hassanein, M.K.; Momiyama, M.; Suetsugu, A.; Moossa, A.R.; Hoffman, R.M.; Bouvet, M. Tumor-educated macrophages promote tumor growth and peritoneal metastasis in an orthotopic nude mouse model of human pancreatic cancer. In Vivo 2012, 26, 565–569. [Google Scholar]
- Baj-Krzyworzeka, M.; Mytar, B.; Szatanek, R.; Surmiak, M.; Weglarczyk, K.; Baran, J.; Siedlar, M. Colorectal cancer-derived microvesicles modulate differentiation of human monocytes to macrophages. J. Transl. Med. 2016, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Garlanda, C.; Borrello, M.G.; Sica, A.; Mantovani, A. Pathways connecting inflammation and cancer. Curr. Opin. Genet. Dev. 2008, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Kitamura, T. Macrophage targeting: Opening new possibilities for cancer immunotherapy. Immunology 2018, 155, 285–293. [Google Scholar] [CrossRef]
- Sevenich, L. Turning ‘cold’ into ‘hot’ tumors—Opportunities and challenges for radio-immunotherapy against primary and metastatic brain cancers. Front. Oncol. 2019, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Binnewies, M.; Roberts, E.W.; Kersten, K.; Chan, V.; Fearon, D.F.; Merad, M.; Coussens, L.M.; Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Hedrick, C.C.; et al. Understanding the tumor immune microenvironment (time) for effective therapy. Nat. Med. 2018, 24, 541–550. [Google Scholar] [CrossRef]
- Covarrubias, A.; Byles, V.; Horng, T. Ros sets the stage for macrophage differentiation. Cell Res. 2013, 23, 984–985. [Google Scholar] [CrossRef]
- Laskin, D.L.; Sunil, V.R.; Gardner, C.R.; Laskin, J.D. Macrophages and tissue injury: Agents of defense or destruction? Annu. Rev. Pharmacol. Toxicol. 2011, 51, 267–288. [Google Scholar] [CrossRef]
- Albina, J.E.; Henry, W.L., Jr.; Mastrofrancesco, B.; Martin, B.A.; Reichner, J.S. Macrophage activation by culture in an anoxic environment. J. Immunol. 1995, 155, 4391–4396. [Google Scholar]
- Brune, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Jyrkkanen, H.K.; Levonen, A.L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012, 52, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Zhao, J.; Shi, J.; Wang, M.; Qiu, S.; Hu, Y.; Xu, Y.; Cui, Y.; Liu, C. The specific inhibition of sod1 selectively promotes apoptosis of cancer cells via regulation of the ros signaling network. Oxid. Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Arjunan, K.P.; Clyne, A.M. A nitric oxide producing pin-to-hole spark discharge plasma enhances endothelial cell proliferation and migration. Plasma Med. 2011, 1, 279–293. [Google Scholar] [CrossRef]
- Uchiyama, H.; Zhao, Q.L.; Hassan, M.A.; Andocs, G.; Nojima, N.; Takeda, K.; Ishikawa, K.; Hori, M.; Kondo, T. Epr-spin trapping and flow cytometric studies of free radicals generated using cold atmospheric argon plasma and x-ray irradiation in aqueous solutions and intracellular milieu. PLoS ONE 2015, 10, e0136956. [Google Scholar] [CrossRef]
- Sousa, J.S.; Niemi, K.; Cox, L.J.; Algwari, Q.T.; Gans, T.; O’Connell, D. Cold atmospheric pressure plasma jets as sources of singlet delta oxygen for biomedical applications. J. Appl. Phys. 2011, 109, 123302. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kinpen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, M.; Chen, H.; Wu, Z.; Xu, L.; Li, J.; Cao, J.; Yang, Y.; Xiao, X.; Lian, X.; et al. Non-thermal plasma suppresses bacterial colonization on skin wound and promotes wound healing in mice. J. Huazhong Univ. Sci. Technol. Med. Sci. 2011, 31, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Bekeschus, S.; Wende, K.; Vollmar, B.; von Woedtke, T. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp. Dermatol. 2017, 26, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ngo Thi, M.-H.; Shao, P.-L.; Liao, J.-D.; Lin, C.-C.K.; Yip, H.-K. Enhancement of angiogenesis and epithelialization processes in mice with burn wounds through ros/rns signals generated by non-thermal n2/ar micro-plasma. Plasma Process. Polym. 2014, 11, 1076–1088. [Google Scholar] [CrossRef]
- Hartwig, S.; Doll, C.; Voss, J.O.; Hertel, M.; Preissner, S.; Raguse, J.D. Treatment of wound healing disorders of radial forearm free flap donor sites using cold atmospheric plasma: A proof of concept. J. Oral Maxillofac. Surg. 2017, 75, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Heinlin, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.; Schmidt, H.U.; Monetti, R.; Steffes, B.; Bunk, W.; Li, Y.; et al. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Br. J. Dermatol. 2012, 167, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, F.; Haenssle, H.A.; Daeschlein, G.; Ahmed, R.; Pfeiffer, S.; Gorlitz, A.; Simon, D.; Schon, M.P.; Wandke, D.; Emmert, S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (plasmaderm((r)) vu-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial (nct01415622). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Brulle, L.; Vandamme, M.; Ries, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.M.; Le Pape, A. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a mia paca2-luc orthotopic pancreatic carcinoma model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Lutze, S.; Arnold, A.; von Podewils, S.; Kiefer, T.; Tueting, T.; Hardt, O.; Haase, H.; Grisk, O.; et al. Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Exp. Dermatol. 2013, 22, 582–586. [Google Scholar] [CrossRef]
- Lupu, A.R.; Georgescu, N.; Calugaru, A.; Cremer, L.; Szegli, G.; Kerek, F. The effects of cold atmospheric plasma jets on b16 and colo320 tumoral cells. Roum. Arch. Microbiol. Immunol. 2009, 68, 136–144. [Google Scholar]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of indirect nonequilibrium atmospheric pressure plasma on anti-proliferative activity against chronic chemo-resistant ovarian cancer cells in vitro and in vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef]
- Yajima, I.; Iida, M.; Kumasaka, M.Y.; Omata, Y.; Ohgami, N.; Chang, J.; Ichihara, S.; Hori, M.; Kato, M. Non-equilibrium atmospheric pressure plasmas modulate cell cycle-related gene expressions in melanocytic tumors of ret-transgenic mice. Exp. Dermatol. 2014, 23, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.-R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.-M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in pma-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; von Woedtke, T.; Weltmann, K.-D. The kinpen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51. [Google Scholar] [CrossRef]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Kluge, S.; Bekeschus, S.; Bender, C.; Benkhai, H.; Sckell, A.; Below, H.; Stope, M.B.; Kramer, A. Investigating the mutagenicity of a cold argon-plasma jet in an het-mn model. PLoS ONE 2016, 11, e0160667. [Google Scholar] [CrossRef]
- Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One year follow-up risk assessment in skh-1 mice and wounds treated with an argon plasma jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef]
- Terstappen, L.W.; Nguyen, M.; Lazarus, H.M.; Medof, M.E. Expression of the DAF (CD55) and CD59 antigens during normal hematopoietic cell differentiation. J. Leukoc. Biol. 1992, 52, 652–660. [Google Scholar] [CrossRef]
- Das, B.; Kashino, S.S.; Pulu, I.; Kalita, D.; Swami, V.; Yeger, H.; Felsher, D.W.; Campos-Neto, A. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant mycobacterium tuberculosis. Sci. Transl. Med. 2013, 5, 170ra113. [Google Scholar] [CrossRef]
- Battula, V.L.; Le, P.M.; Sun, J.C.; Nguyen, K.; Yuan, B.; Zhou, X.; Sonnylal, S.; McQueen, T.; Ruvolo, V.; Michel, K.A.; et al. Aml-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Naglah, A.M.; Shinwari, Z.; Bhat, M.A.; Al-Tahhan, M.; Al-Omar, M.A.; Al-Dhfyan, A. Targeting leukemic side population cells by isatin derivatives of nicotinic acid amide. J. Biol. Regul. Homeost. Agents 2016, 30, 353–363. [Google Scholar] [PubMed]
- Huang, S.D.; Yuan, Y.; Tang, H.; Liu, X.H.; Fu, C.G.; Cheng, H.Z.; Bi, J.W.; Yu, Y.W.; Gong, D.J.; Zhang, W.; et al. Tumor cells positive and negative for the common cancer stem cell markers are capable of initiating tumor growth and generating both progenies. PLoS ONE 2013, 8, e54579. [Google Scholar] [CrossRef] [PubMed]
- Ngo, M.; Han, A.; Lakatos, A.; Sahoo, D.; Hachey, S.J.; Weiskopf, K.; Beck, A.H.; Weissman, I.L.; Boiko, A.D. Antibody therapy targeting cd47 and cd271 effectively suppresses melanoma metastasis in patient-derived xenografts. Cell Rep. 2016, 16, 1701–1716. [Google Scholar] [CrossRef] [PubMed]
- Caroleo, M.C.; Costa, N.; Bracci-Laudiero, L.; Aloe, L. Human monocyte/macrophages activate by exposure to lps overexpress ngf and ngf receptors. J. Neuroimmunol. 2001, 113, 193–201. [Google Scholar] [CrossRef]
- Czernek, L.; Chworos, A.; Duechler, M. The uptake of extracellular vesicles is affected by the differentiation status of myeloid cells. Scand. J. Immunol. 2015, 82, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Mitani, H.; Katayama, N.; Araki, H.; Ohishi, K.; Kobayashi, K.; Suzuki, H.; Nishii, K.; Masuya, M.; Yasukawa, K.; Minami, N.; et al. Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br. J. Haematol. 2000, 109, 288–295. [Google Scholar] [CrossRef]
- Woltman, A.M. Rapamycin induces apoptosis in monocyte- and cd34-derived dendritic cells but not in monocytes and macrophages. Blood 2001, 98, 174–180. [Google Scholar] [CrossRef]
- Rey-Giraud, F.; Hafner, M.; Ries, C.H. In vitro generation of monocyte-derived macrophages under serum-free conditions improves their tumor promoting functions. PLoS ONE 2012, 7, e42656. [Google Scholar] [CrossRef]
- Lu, R.; Pitha, P.M. Monocyte differentiation to macrophage requires interferon regulatory factor 7. J. Biol. Chem. 2001, 276, 45491–45496. [Google Scholar] [CrossRef]
- Riddy, D.M.; Goy, E.; Delerive, P.; Summers, R.J.; Sexton, P.M.; Langmead, C.J. Comparative genotypic and phenotypic analysis of human peripheral blood monocytes and surrogate monocyte-like cell lines commonly used in metabolic disease research. PLoS ONE 2018, 13, e0197177. [Google Scholar] [CrossRef]
- Baek, Y.S.; Haas, S.; Hackstein, H.; Bein, G.; Hernandez-Santana, M.; Lehrach, H.; Sauer, S.; Seitz, H. Identification of novel transcriptional regulators involved in macrophage differentiation and activation in u937 cells. BMC Immunol. 2009, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, H.; Aikawa, M.; Hill, C.C.; Weiss, D.; Taylor, W.R.; Libby, P.; Lee, R.T. Biomechanical strain induces class a scavenger receptor expression in human monocyte/macrophages and THP-1 cells. Circulation 2001, 104, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Jung, H.S.; Yang, H.I.; Yoo, M.C.; Kim, C.; Kim, K.S. Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm. Res. 2007, 56, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Schildberger, A.; Rossmanith, E.; Eichhorn, T.; Strassl, K.; Weber, V. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediat. Inflamm. 2013, 2013, 697972. [Google Scholar] [CrossRef] [PubMed]
- Bundscherer, L.; Bekeschus, S.; Tresp, H.; Hasse, S.; Reuter, S.; Weltmann, K.-D.; Lindequist, U.; Masur, K. Viability of human blood leukocytes compared with their respective cell lines after plasma treatment. Plasma Med. 2013, 3, 71–80. [Google Scholar] [CrossRef]
- Costantini, A.; Viola, N.; Berretta, A.; Galeazzi, R.; Matacchione, G.; Sabbatinelli, J.; Storci, G.; De Matteis, S.; Butini, L.; Rippo, M.R. Age-related m1/m2 phenotype changes in circulating monocytes from healthy/unhealthy individuals. Inflammation 2018, 14, 15. [Google Scholar] [CrossRef] [PubMed]
- Becker, S. Interferons as modulators of human monocyte-macrophage differentiation. I. Interferon-gamma increases hla-dr expression and inhibits phagocytosis of zymosan. J. Immunol. 1984, 132, 1249–1254. [Google Scholar] [PubMed]
- Gratchev, A.; Guillot, P.; Hakiy, N.; Politz, O.; Orfanos, C.E.; Schledzewski, K.; Goerdt, S. Alternatively activated macrophages differentially express fibronectin and its splice variants and the extracellular matrix protein betaig-h3. Scand. J. Immunol. 2001, 53, 386–392. [Google Scholar] [CrossRef]
- Jovanovic, D.V.; Di Battista, J.A.; Martel-Pelletier, J.; Jolicoeur, F.C.; He, Y.; Zhang, M.; Mineau, F.; Pelletier, J.P. Il-17 stimulates the production and expression of proinflammatory cytokines, il-beta and tnf-alpha, by human macrophages. J. Immunol. 1998, 160, 3513–3521. [Google Scholar] [PubMed]
- Lin, W.W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Il-18: A th1-inducing, proinflammatory cytokine and new member of the il-1 family. J. Allergy Clin. Immunol. 1999, 103, 11–24. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Yang, S.H.; Pang, J.H. Cilostazol reduces MCP-1-induced chemotaxis and adhesion of THP-1 monocytes by inhibiting CCR2 gene expression. Biochem. Biophys. Res. Commun. 2011, 411, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Green, S.R.; Han, K.H.; Chen, Y.; Almazan, F.; Charo, I.F.; Miller, Y.I.; Quehenberger, O. The CC chemokine MCP-1 stimulates surface expression of CX3CR1 and enhances the adhesion of monocytes to fractalkine/CX3CL1 via p38 MAPK. J. Immunol. 2006, 176, 7412–7420. [Google Scholar] [CrossRef]
- Cross, A.K.; Richardson, V.; Ali, S.A.; Palmer, I.; Taub, D.D.; Rees, R.C. Migration responses of human monocytic cell lines to alpha- and beta-chemokines. Cytokine 1997, 9, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.S.; Weiss, L. Transformation of monocytes in tissue culture into macrophages epithelioid cells and multinucleated giant cells: An electron microscope study. J. Cell Biol. 1966, 28, 303–332. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Akanuma, S.; Matsumoto-Akanuma, A.; Yamanaka, D.; Ishibashi, K.I.; Adachi, Y.; Ohno, N. Activation of macrophages by a laccase-polymerized polyphenol is dependent on phosphorylation of rac1. Biochem. Biophys. Res. Commun. 2018, 495, 2209–2213. [Google Scholar] [CrossRef]
- Reimer, M.K.; Brange, C.; Rosendahl, A. CCR8 signaling influences toll-like receptor 4 responses in human macrophages in inflammatory diseases. Clin. Vaccine Immunol. 2011, 18, 2050–2059. [Google Scholar] [CrossRef]
- Hoshino, A.; Kawamura, Y.I.; Yasuhara, M.; Toyama-Sorimachi, N.; Yamamoto, K.; Matsukawa, A.; Lira, S.A.; Dohi, T. Inhibition of CCL1-CCR8 interaction prevents aggregation of macrophages and development of peritoneal adhesions. J. Immunol. 2007, 178, 5296–5304. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Kolata, J.; Winterbourn, C.; Kramer, A.; Turner, R.; Weltmann, K.D.; Broker, B.; Masur, K. Hydrogen peroxide: A central player in physical plasma-induced oxidative stress in human blood cells. Free Radic. Res. 2014, 48, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; von Woedtke, T.; Bekeschus, S. Periodic exposure of keratinocytes to cold physical plasma: An in vitro model for redox-related diseases of the skin. Oxid. Med. Cell. Longev. 2016, 2016, 9816072. [Google Scholar] [CrossRef]
- Wende, K.; Strassenburg, S.; Haertel, B.; Harms, M.; Holtz, S.; Barton, A.; Masur, K.; von Woedtke, T.; Lindequist, U. Atmospheric pressure plasma jet treatment evokes transient oxidative stress in hacat keratinocytes and influences cell physiology. Cell Biol. Int. 2014, 38, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of n-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Ates, B.; Abraham, L.; Ercal, N. Antioxidant and free radical scavenging properties of n-acetylcysteine amide (NACA) and comparison with n-acetylcysteine (NAC). Free Radic. Res. 2008, 42, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Hoffer, E.; Baum, Y.; Nahir, A.M. N-acetylcysteine enhances the action of anti-inflammatory drugs as suppressors of prostaglandin production in monocytes. Mediators Inflamm. 2002, 11, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Nakagawara, A.; Nathan, C.F.; Cohn, Z.A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J. Clin. Investig. 1981, 68, 1243–1252. [Google Scholar] [CrossRef]
- Schmidt, A.; Rodder, K.; Hasse, S.; Masur, K.; Toups, L.; Lillig, C.H.; von Woedtke, T.; Wende, K.; Bekeschus, S. Redox-regulation of activator protein 1 family members in blood cancer cell lines exposed to cold physical plasma-treated medium. Plasma Process. Polym. 2016, 13, 1179–1188. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Min, B.; Choi, K.H.; Hong, Y.J.; Miller, V.; Fridman, A.; Choi, E.H. Cytotoxic macrophage-released tumour necrosis factor-alpha (TNF-alpha) as a killing mechanism for cancer cell death after cold plasma activation. J. Phys. D Appl. Phys. 2016, 49, 084001. [Google Scholar] [CrossRef]
- Bourassa, B.; Monaghan, S.; Rittling, S.R. Impaired anti-tumor cytotoxicity of macrophages from osteopontin-deficient mice. Cell. Immunol. 2004, 227, 1–11. [Google Scholar] [CrossRef]
- Rossol, M.; Pierer, M.; Raulien, N.; Quandt, D.; Meusch, U.; Rothe, K.; Schubert, K.; Schoneberg, T.; Schaefer, M.; Krugel, U.; et al. Extracellular Ca2+ is a danger signal activating the nlrp3 inflammasome through g protein-coupled calcium sensing receptors. Nat. Commun. 2012, 3, 1329. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Kepp, O.; Galluzzi, L.; Kroemer, G. Inflammasomes in carcinogenesis and anticancer immune responses. Nat. Immunol. 2012, 13, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bundscherer, L.; Nagel, S.; Hasse, S.; Tresp, H.; Wende, K.; Walther, R.; Reuter, S.; Weltmann, K.D.; Masur, K.; Lindequist, U. Non-thermal plasma treatment induces mapk signaling in human monocytes. Open Chem. 2015, 13, 606–613. [Google Scholar] [CrossRef]
- Bundscherer, L.; Wende, K.; Ottmuller, K.; Barton, A.; Schmidt, A.; Bekeschus, S.; Hasse, S.; Weltmann, K.D.; Masur, K.; Lindequist, U. Impact of non-thermal plasma treatment on mapk signaling pathways of human immune cell lines. Immunobiology 2013, 218, 1248–1255. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freund, E.; Moritz, J.; Stope, M.; Seebauer, C.; Schmidt, A.; Bekeschus, S. Plasma-Derived Reactive Species Shape a Differentiation Profile in Human Monocytes. Appl. Sci. 2019, 9, 2530. https://doi.org/10.3390/app9122530
Freund E, Moritz J, Stope M, Seebauer C, Schmidt A, Bekeschus S. Plasma-Derived Reactive Species Shape a Differentiation Profile in Human Monocytes. Applied Sciences. 2019; 9(12):2530. https://doi.org/10.3390/app9122530
Chicago/Turabian StyleFreund, Eric, Juliane Moritz, Matthias Stope, Christian Seebauer, Anke Schmidt, and Sander Bekeschus. 2019. "Plasma-Derived Reactive Species Shape a Differentiation Profile in Human Monocytes" Applied Sciences 9, no. 12: 2530. https://doi.org/10.3390/app9122530




