Small-Scale Bioreactor for Sterile Hydroponics and Hairy Roots: Metabolic Diversity and Salicylic Acid Exudation by Hairy Roots of Hyoscyamus niger
Abstract
:1. Introduction
2. Materials and Methods
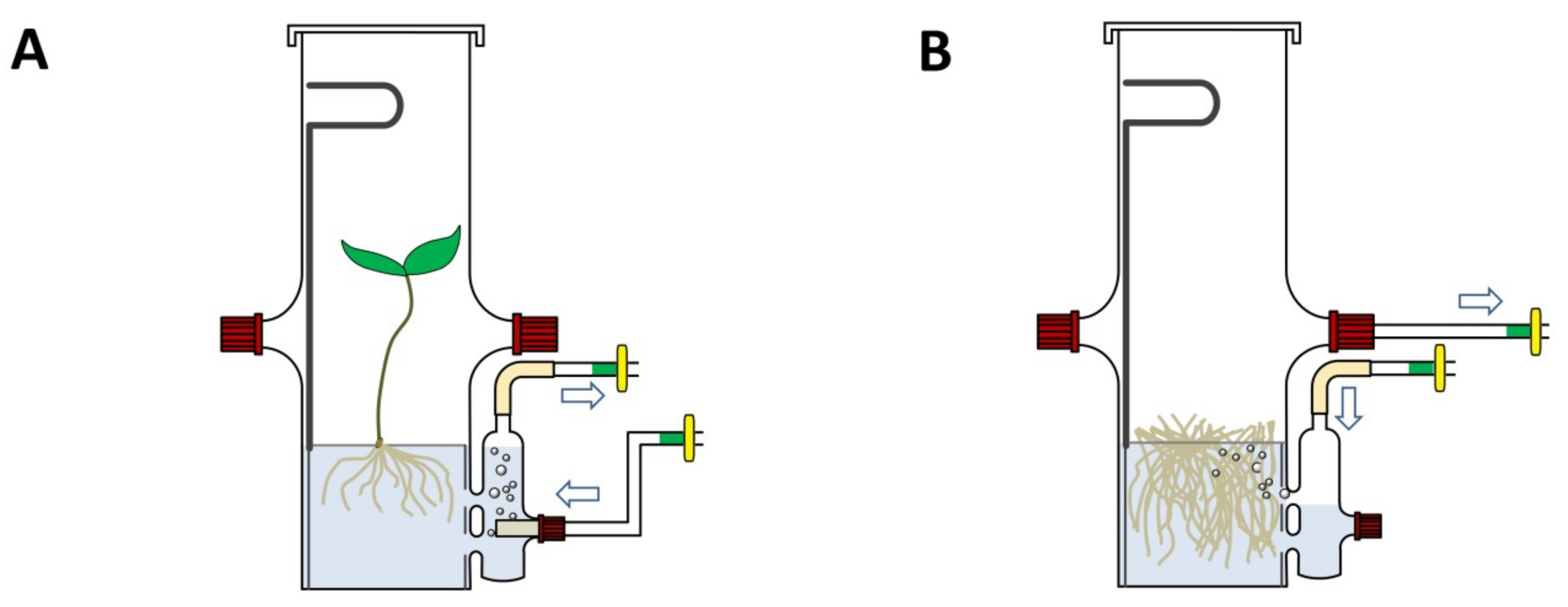
2.1. Bioreactor Design
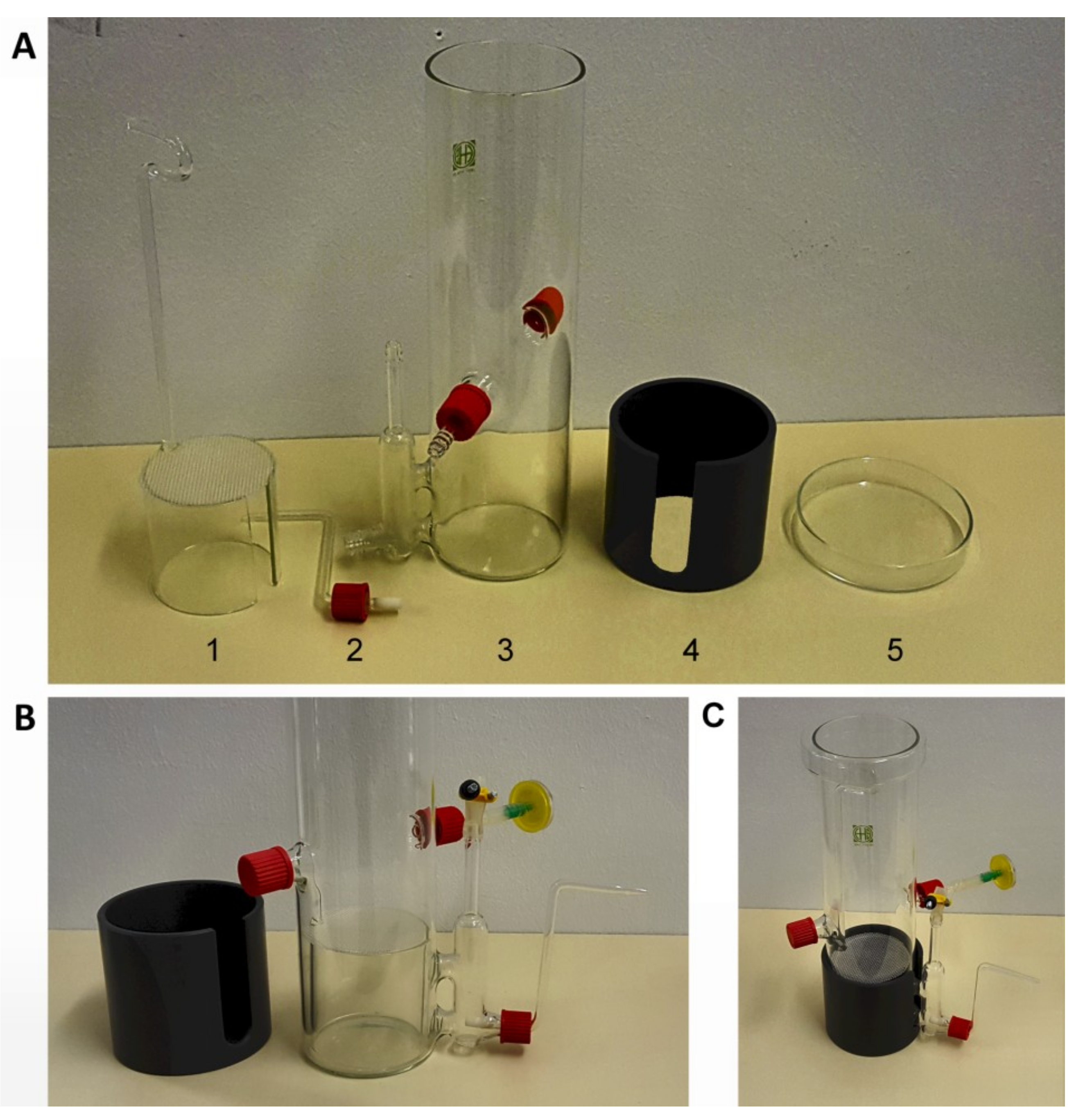
2.2. Fabrication of the Bioreactor
2.3. Operation of the Bioreactor
2.4. Plant Material
2.5. Analysis of Root Exudates by HPLC-DAD
2.6. Induction of Hairy Roots
2.7. Analysis of Hairy Root Exudates by HPLC-ELSD
2.8. Analysis of Salicylic Acid Content in Hairy Root Exudates by HPLC-MS
2.9. Primer Design and Sequence Analysis
3. Application Examples
3.1. Growing Oilseed Rape (Brassica napus L.) in Sterile Hydroponics
3.2. Growing Rose Plants (Rosa canina L.) in Sterile Hydroponics
3.3. Collection and Analysis of Root Exudates of Rose (Rosa canina L.) by HPLC-DAD
3.4. Growing Hairy Roots of Hyoscyamus niger and Sesamum indicum
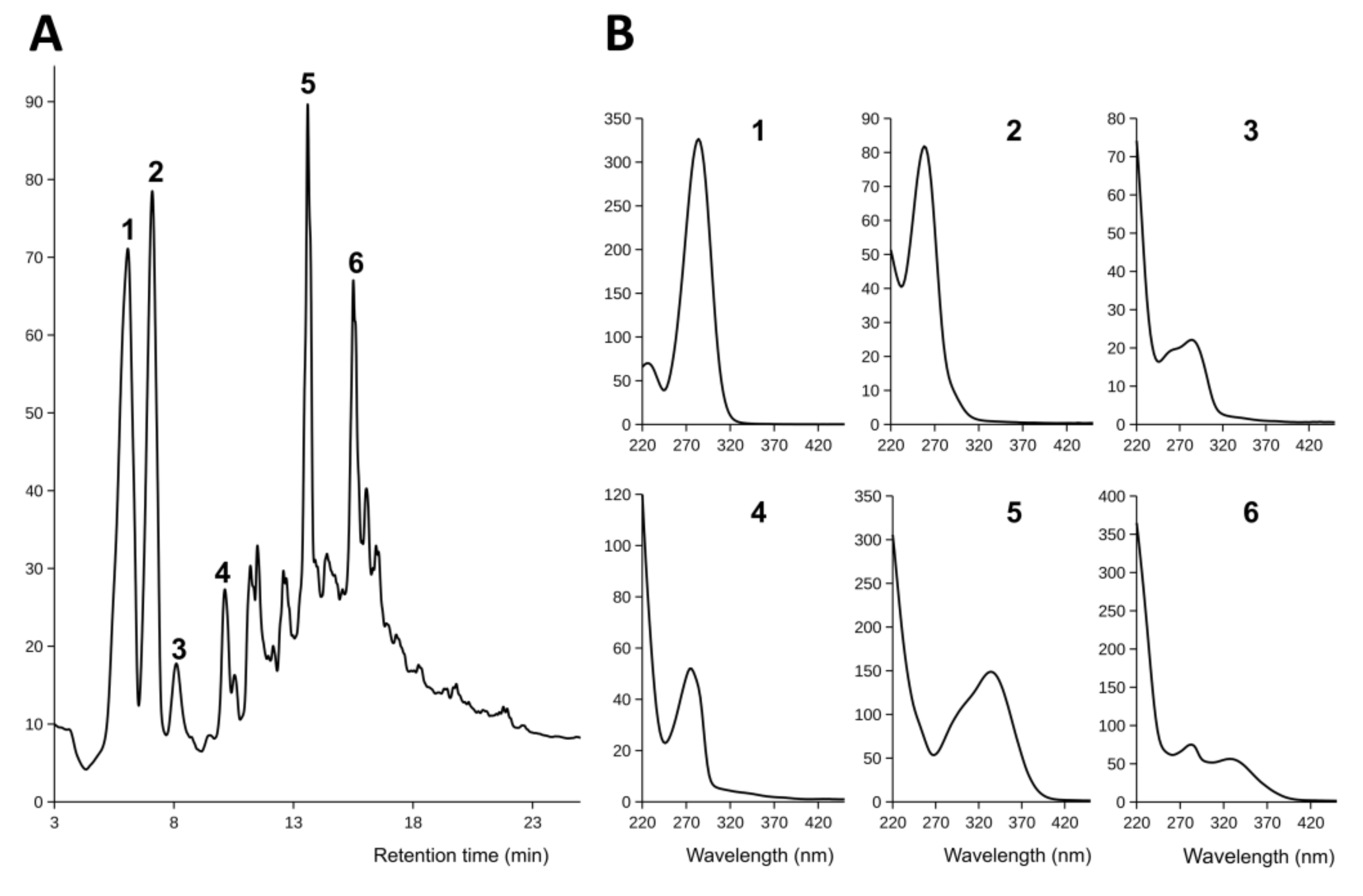
3.5. Collection and Nontargeted Analysis of Hairy Root Exudates of Sesamum indicum
3.6. Analysis of Salicylic Acid Exudation by Hairy Roots of Hyoscyamus niger
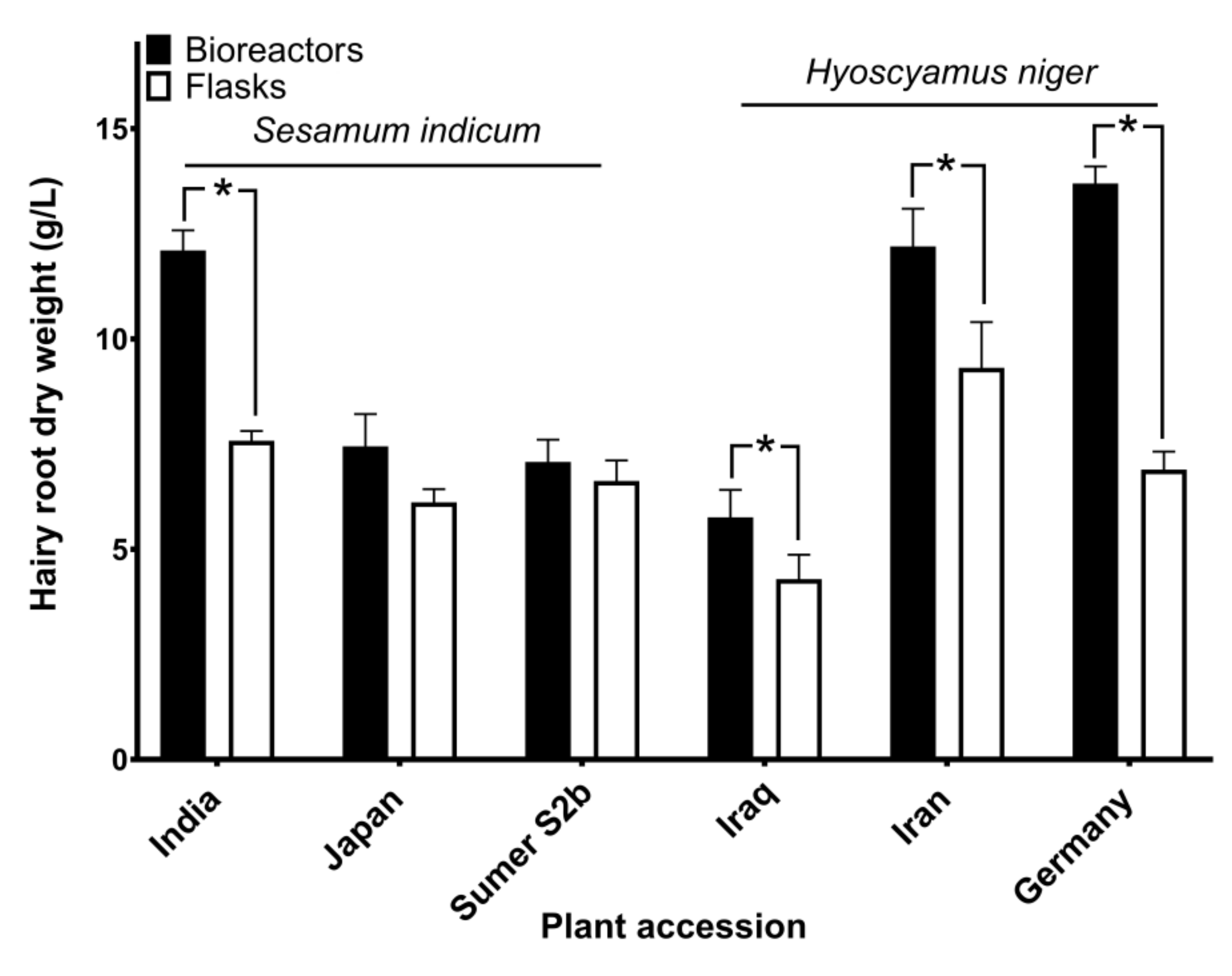
3.7. Nontargeted Analysis of Hairy Root Exudates in Hyoscyamus niger: Comparison to Shaking Flasks
3.8. Metabolic Diversity of Hairy Root Exudates and Genetic Diversity in Hyoscyamus niger
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khorassini, R.; Hettwer, U.; Ratzinger, A.; Steingrobe, B.; Karlovsky, P.; Claassen, N. Citramalic acid and salicylic acid in sugar beet root exudates solubilize soil phosphorus. BMC Plant Biol. 2011, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Tawaraya, K.; Horie, R.; Saito, S.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of root exudates of common bean under phosphorus deficiency. Metabolites 2014, 4, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, C.; Tang, X.; Li, H.; Zhang, F.; Rengel, Z.; Whalley, W.R.; Davies, W.J.; Shen, J. Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol. 2016, 209, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nature Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.; Kirkegaard, J.A.; Passioura, J.B. Rhizosphere biology and crop productivity—a review. Soil Res. 2006, 44, 299–317. [Google Scholar] [CrossRef]
- Miller, S.B.; Heuberger, A.L.; Broeckling, C.D.; Jahn, C.E. Non-targeted metabolomics reveals sorghum rhizosphere-associated exudates are influenced by the belowground interaction of substrate and sorghum genotype. Int. J. Mol. Sci. 2019, 20, 431. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Bont, Z.; Fricke, J.; Brillatz, T.; Aziz, Z.; Gershenzon, J.; Erb, M. A below-ground herbivore shapes root defensive chemistry in natural plant populations. Proc. Biol. Sci. 2016, 283, 20160285. [Google Scholar] [CrossRef] [PubMed]
- Steinkellner, S.; Lendzemo, V.; Langer, I.; Schweiger, P.; Khaosaad, T.; Toussaint, J.P.; Vierheilig, H. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 2007, 12, 1290–1306. [Google Scholar] [CrossRef]
- Lanoue, A.; Burlat, V.; Schurr, U.; Röse, U.S.R. Induced root-secreted phenolic compounds as a belowground plant defense. Plant Signal. Behav. 2010, 5, 1037–1038. [Google Scholar] [CrossRef]
- Arafat, Y.; Wei, X.; Jiang, Y.; Chen, T.; Saqib, H.S.A.; Lin, S.; Lin, W. Spatial distribution patterns of root-associated bacterial communities mediated by root exudates in different aged ratooning tea monoculture systems. Int. J. Mol. Sci. 2017, 18, 1727. [Google Scholar] [CrossRef]
- Hiltpold, I.; Jaffuel, G.; Turlings, T.C.J. The dual effects of root-cap exudates on nematodes: From quiescence in plant-parasitic nematodes to frenzy in entomopathogenic nematodes. J. Exp. Bot. 2015, 66, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.I.; Agostini, E.; Ludwig-Müller, J.; Xu, J. Genetically transformed roots: From plant disease to biotechnological resource. Trends Biotechnol. 2012, 30, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Bulgakov, V.P.; Shkryl, Y.N.; Veremeichik, G.N.; Gorpenchenko, T.Y.; Vereshchagina, Y.V. Recent advances in the understanding of Agrobacterium rhizogenes-derived genes and their effects on stress resistance and plant metabolism. Adv. Biochem. Eng. Biotechnol. 2013, 134, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, W.; Yang, D.; Hou, Z.; Liang, Z. Transcriptional profiles of SmWRKY family genes and their putative roles in the biosynthesis of tanshinone and phenolic acids in Salvia miltiorrhiza. Int. J. Mol. Sci. 2018, 19, 1593. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.Y.; Du, Y.T.; Ma, J.; Min, D.H.; Jin, L.G.; Chen, J.; Chen, M.; Zhou, Y.B.; Ma, Y.Z.; Xu, Z.S.; et al. The WRKY transcription factor GmWRKY12 confers drought and salt tolerance in soybean. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Sańko-Sawczenko, I.; Dmitruk, D.; Łotocka, B.; Różańska, E.; Czarnocka, W. Expression analysis of pin genes in root tips and nodules of Lotus japonicus. Int. J. Mol. Sci. 2019, 20, 235. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Xia, P.; Wang, R.; Jiao, J.; Ru, M.; Liu, J.; Liang, Z. Molecular cloning and characterization of five SmGRAS genes associated with tanshinone biosynthesis in Salvia miltiorrhiza hairy roots. PLoS ONE 2017, 12, e0185322. [Google Scholar] [CrossRef] [PubMed]
- Morriss, S.C.; Studham, M.E.; Tylka, G.L.; MacIntosh, G.C. Validation of a hairy roots system to study soybean-soybean aphid interactions. PLoS ONE 2017, 12, e0174914. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Pavlov, A.I.; Bley, T. Hairy root type plant in vitro systems as sources of bioactive substances. Appl. Microbiol. Biotechnol. 2007, 74, 1175–1185. [Google Scholar] [CrossRef]
- Kochan, E.; Balcerczak, E.; Szymczyk, P.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Szymańska, G. Abscisic acid regulates the 3-hydroxy-3-methylglutaryl CoA reductase gene promoter and ginsenoside production in Panax quinquefolium hairy root cultures. Int. J. Mol. Sci. 2019, 20, 1310. [Google Scholar] [CrossRef]
- Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.S.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) hairy root cultures for the production of anticancer aryltetralin lignans. Int. J. Mol. Sci. 2018, 19, 990. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Srivastava, A.K. Hairy root culture for mass-production of high-value secondary metabolites. Crit. Rev. Biotechnol. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Nakajima, K.; Hashimoto, T. Two tropinone reductases, that catalyze opposite stereospecific reductions in tropane alkaloid biosynthesis, are localized in plant root with different cell-specific patterns. Plant Cell Physiol. 1999, 40, 1099–1107. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ku, K.M.; Becker, T.M.; Juvik, J.A. Chemopreventive glucosinolate accumulation in various broccoli and collard tissues: Microfluidic-based targeted transcriptomics for by-product valorization. PLoS ONE 2017, 12, e0185112. [Google Scholar] [CrossRef]
- Jouhikainen, K.; Lindgren, L.; Jokelainen, T.; Hiltunen, R.; Teeri, T.H.; Oksman-Caldentey, K.M. Enhancement of scopolamine production in Hyoscyamus muticus L. hairy root cultures by genetic engineering. Planta 1999, 208, 545–551. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Jahn, L.; Lippert, A.; Püschel, J.; Walter, A. Improvement of hairy root cultures and plants by changing biosynthetic pathways leading to pharmaceutical metabolites: Strategies and applications. Biotechnol. Adv. 2014, 32, 1168–1179. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, H.; Lv, Z.; Ji, Q.; Huang, Y.; Liu, J.; Chen, D.; Diao, Y.; Si, J.; Zhang, L. Targeted expression of Vitreoscilla hemoglobin improves the production of tropane alkaloids in Hyoscyamus niger hairy roots. Sci. Rep. 2018, 8, 17969. [Google Scholar] [CrossRef]
- Wei, T.; Gao, Y.; Deng, K.; Zhang, L.; Yang, M.; Liu, X.; Qi, C.; Wang, C.; Song, W.; Zhang, Y.; et al. Enhancement of tanshinone production in Salvia miltiorrhiza hairy root cultures by metabolic engineering. Plant Methods 2019, 15, 53. [Google Scholar] [CrossRef]
- Kirchner, T.W.; Niehaus, M.; Debener, T.; Schenk, M.K.; Herde, M. Efficient generation of mutations mediated by CRISPR/Cas9 in the hairy root transformation system of Brassica carinata. PLoS ONE 2017, 12, e0185429. [Google Scholar] [CrossRef]
- Madeira, L.M.; Szeto, T.H.; Henquet, M.; Raven, N.; Runions, J.; Huddleston, J.; Garrard, I.; Drake, P.M.W.; Ma, J.K.C. High-yield production of a human monoclonal IgG by rhizosecretion in hydroponic tobacco cultures. Plant Biotechnol. J. 2016, 14, 615–624. [Google Scholar] [CrossRef]
- Mai, N.T.P.; Boitel-Conti, M.; Guerineau, F. Arabidopsis thaliana hairy roots for the production of heterologous proteins. Plant Cell Tissue Organ Cult. 2016, 127, 489–496. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Raven, N.; Henquet, M.; Laukkanen, M.L.; Anderlei, T.; Pitkänen, J.P.; Twyman, R.M.; Bosch, D.; Oksman-Caldentey, K.M.; Schillberg, S.; et al. Molecular farming in tobacco hairy roots by triggering the secretion of a pharmaceutical antibody. Biotechnol. Bioeng. 2014, 111, 336–346. [Google Scholar] [CrossRef]
- Van Leeuwen, W.; Ruttink, T.; Borst-Vrenssen, A.W.; van der Plas, L.H.; van der Krol, A.R. Characterization of position-induced spatial and temporal regulation of transgene promoter activity in plants. J. Exp. Bot. 2001, 52, 949–959. [Google Scholar] [CrossRef]
- Laurentin, H.; Ratzinger, A.; Karlovsky, P. Relationship between metabolic and genomic diversity in sesame (Sesamum indicum L.). BMC Genom. 2008, 9, 250. [Google Scholar] [CrossRef]
- Sarrou, E.; Ganopoulos, I.; Xanthopoulou, A.; Masuero, D.; Martens, S.; Madesis, P.; Mavromatis, A.; Chatzopoulou, P. Genetic diversity and metabolic profile of Salvia officinalis populations: Implications for advanced breeding strategies. Planta 2017, 246, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Ohtani, Y.; Aoki, W.; Uno, Y.; Sukekiyo, Y.; Kubokawa, S.; Ueda, M. Detection of betacyanin in red-tube spinach (Spinacia oleracea) and its biofortification by strategic hydroponics. PLoS ONE 2018, 13, e0203656. [Google Scholar] [CrossRef]
- Kittipongpatana, N.; Hock, R.S.; Porter, J.R. Production of solasodine by hairy root, callus, and cell suspension cultures of Solanum aviculare Forst. Plant Cell Tissue Organ Cult. 1998, 52, 133–143. [Google Scholar] [CrossRef]
- Palavalli, R.R.; Srivastava, S.; Srivastava, A.K. Development of a mathematical model for growth and oxygen transfer in in vitro plant hairy root cultivations. Appl. Biochem. Biotechnol. 2012, 167, 1831–1844. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Eibl, R.; Zhong, J.J. Hosting the plant cells in vitro: Recent trends in bioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 3787–3800. [Google Scholar] [CrossRef]
- Homova, V.; Weber, J.; Schulze, J.; Alipieva, K.; Bley, T.; Georgiev, M. Devil’s claw hairy root culture in flasks and in a 3-L bioreactor: Bioactive metabolite accumulation and flow cytometry. Z. Nat. C J. Biosci. 2010, 65, 472–478. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A.K. Enhanced production of artemisinin by hairy root cultivation of Artemisia annua in a modified stirred tank reactor. Appl. Biochem. Biotechnol. 2014, 174, 2209–2222. [Google Scholar] [CrossRef]
- Medina-Bolívar, F.; Cramer, C. Production of recombinant proteins by hairy roots cultured in plastic sleeve bioreactors. Methods Mol. Biol. 2004, 267, 351–363. [Google Scholar] [CrossRef]
- Kwok, K.H.; Doran, P.M. Kinetic and stoichiometric analysis of hairy roots in a segmented bubble column reactor. Biotechnol. Prog. 1995, 11, 429–435. [Google Scholar] [CrossRef]
- Weathers, P.J.; Giles, K.L. Regeneration of plants using nutrient mist culture. Vitr. Cell. Dev. Biol. 1988, 24, 727–732. [Google Scholar] [CrossRef]
- Tscheschke, B.; Dreimann, J.; von der Ruhr, J.W.; Schmidt, T.; Stahl, F.; Just, L.; Scheper, T. Evaluation of a new mist-chamber bioreactor for biotechnological applications. Biotechnol. Bioeng. 2015, 112, 1155–1164. [Google Scholar] [CrossRef]
- Caspeta, L.; Quintero, R.; Villarreal, M.L. Novel airlift reactor fitting for hairy root cultures: Developmental and performance studies. Biotechnol. Prog. 2005, 21, 735–740. [Google Scholar] [CrossRef]
- Williams, G.R.; Doran, P.M. Hairy root culture in a liquid-dispersed bioreactor: Characterization of spatial heterogeneity. Biotechnol. Prog. 2000, 16, 391–401. [Google Scholar] [CrossRef]
- Higuchi, M.; Kunitomo, N.; Kobayashi, Y.; Kumada, K.; Tanaka, N. Bioreactor using oxygen-enriched micro/nano-bubbles. International Patent Application No. PCT/JP2015/071597, 2 February 2017. [Google Scholar]
- Khuntia, S.; Majumder, S.K.; Ghosh, P. Microbubble-aided water and wastewater purification: A review. Rev. Chem. Eng. 2012, 28, 191–221. [Google Scholar] [CrossRef]
- Al-Mashhadani, M.K.H.; Wilkinson, S.J.; Zimmerman, W.B. Airlift bioreactor for biological applications with microbubble mediated transport processes. Chem. Eng. Sci. 2015, 137, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Mochida, K.; Furuta, T.; Ebana, K.; Shinozaki, K.; Kikuchi, J. Correlation exploration of metabolic and genomic diversity in rice. BMC Genom. 2009, 10, 568. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Rengel, Z. Genetic control of root exudation. Plant Soil 2002, 245, 59–70. [Google Scholar] [CrossRef]
- Klessig, D.F.; Malamy, J. The salicylic acid signal in plants. Plant Mol. Biol. 1994, 26, 1439–1458. [Google Scholar] [CrossRef]
- Ratzinger, A.; Riediger, N.; von Tiedemann, A.; Karlovsky, P. Salicylic acid and salicylic acid glucoside in xylem sap of Brassica napus infected with Verticillium longisporum. J. Plant Res. 2009, 122, 571–579. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Glavina del Rio, T.; Jones, C.D.; Tringe, S.G.; et al. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [Green Version]
- Sirrenberg, A.; Nutz, S.; Ratzinger, A.; Karlovsky, P. Replant disease of roses—new research approaches. Gartenpraxis 2009, 9, 16–20. [Google Scholar]
- Zedníková, M.; Orvalho, S.; Fialová, M.; Ruzicka, M.C. Measurement of volumetric mass transfer coefficient in bubble columns. ChemEngineering 2018, 2, 19. [Google Scholar] [CrossRef]
- Vervliet, G.; Holsters, M.; Teuchy, H.; Van Montagu, M.; Schell, J. Characterization of different plaque-forming and defective temperate phages in Agrobacterium. J. Gen. Virol. 1975, 26, 33–48. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hand, D.W. Effects of atmospheric humidity on greenhouse crops. Acta Hortic. 1988, 229, 143–158. [Google Scholar] [CrossRef]
- Cámara-Zapata, J.M.; Sánchez-Molina, J.A.; Rodríguez, F.; López, J.C. Evaluation of a dehumidifier in a mild weather greenhouse. Appl. Therm. Eng. 2019, 146, 92–103. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Kang, S.M.; Jung, H.Y.; Kang, Y.M.; Yun, D.J.; Bahk, J.D.; Yang, J.; Choi, M.S. Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of PMT and H6H in adventitious root cultures of Scopolia parviflora. Plant Sci. 2004, 166, 745–751. [Google Scholar] [CrossRef]
- Harfi, B.; Khelifi, L.; Khelifi-Slaoui, M.; Assaf-Ducrocq, C.; Gontier, E. Tropane alkaloids GC/MS analysis and low dose elicitors’ effects on hyoscyamine biosynthetic pathway in hairy roots of Algerian Datura species. Sci. Rep. 2018, 8, 17951. [Google Scholar] [CrossRef]
- Adnani, N.; Michel, C.R.; Bugni, T.S. Universal quantification of structurally diverse natural products using an evaporative light scattering detector. J. Nat. Prod. 2012, 75, 802–806. [Google Scholar] [CrossRef]
- Houshyani, B.; Kabouw, P.; Muth, D.; de Vos, R.C.H.; Bino, R.J.; Bouwmeester, H.J. Characterization of the natural variation in Arabidopsis thaliana metabolome by the analysis of metabolic distance. Metabolomics 2012, 8, 131–145. [Google Scholar] [CrossRef]
- Lamine, M.; Rahali, F.Z.; Hamdaoui, G.; Selmi, S.; Mliki, A.; Gargouri, M. Associating chemical analysis to molecular markers for the valorization of Citrus aurantium leaves: A useful starting point for marker-assisted selection. Euphytica 2017, 213, 44. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Gadzikowska, M. Tropane alkaloids as medicinally useful natural products and their synthetic derivatives as new drugs. Pharmacol. Rep. 2008, 60, 439–463. [Google Scholar]
- Hashimoto, T.; Yukimune, Y.; Yamada, Y. Tropane alkaloid production in Hyoscyamus root cultures. J. Plant Physiol. 1986, 124, 61–75. [Google Scholar] [CrossRef]





| Label | Origin | Year | Source |
|---|---|---|---|
| Iraq | Iraq, Daray Mar | 2014 | Collected by ZJK |
| Iran | Iran, Isfahan | 2015 | Pakan Bazr * |
| Germany | Germany, Göttingen | 2016 | Botanical garden |
| Label | Origin | Year * | Accession |
|---|---|---|---|
| India | India, Hyderabad | 2004 | Acc. 92–2918 ** |
| Japan | Japan | 2004 | Acc. 92–3030 ** |
| Sumer S2b | Iraq, Erbil | 2014 | Sumer S2b *** |
| Accession | RT * | Iran | Germany | Iraq | |||
|---|---|---|---|---|---|---|---|
| Metabolite | Bioreactor | Flasks | Bioreactor | Flasks | Bioreactor | Flasks | |
| Compound 1 | 3.6 | N.d. | 224 ± 191 ** | N.d. | N.d. | N.d. | N.d. |
| Compound 2 | 4.3 | 41 ± 4 | N.d. | 41 ± 4 | N.d. | N.d. | 100 ± 125 |
| Compound 3 | 6.1 | 128 ± 18 | 20 ± 2 | 24 ± 1 | 32 ± 2 | 30 ± 4 | 41 ± 2 |
| Compound 4 | 6.8 | 12,500 ± 600 | 2300 ± 00 | 5700 ± 490 | 5100 ± 200 | 7000 ± 300 | 5900 ± 200 |
| Compound 5 | 7.7 | 29 ± 5 | N.d. | N.d. | N.d. | N.d. | N.d. |
| Compound 9 | 9.5 | 40 ± 8 | N.d. | N.d. | N.d. | 35 ± 9 | N.d. |
| Compound 10 | 9.7 | 75 ± 29 | 750 ± 350 | 1300 ± 650 | 485 ± 175 | N.d. | 1750 ± 490 |
| Compound 11 | 9.9 | 42 ± 29 | N.d. | N.d. | N.d. | N.d. | N.d. |
| Compound 12 | 10.5 | N.d. | N.d. | 32 ± 2 | 52 ± 13 | N.d. | 120 ± 30 |
| Compound 13 | 11.1 | N.d. | N.d. | 51 ± 18 | 110 ± 41 | N.d. | 67 ± 2 |
| Compound 14 | 11.3 | N.d. | N.d. | 21 ± 5 | N.d. | N.d. | N.d. |
| Compound 17 | 14.8 | N.d. | 87 ± 8 | N.d. | N.d. | N.d. | N.d. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kareem, Z.J.; Su, L.; Rathgeb, A.; Sirrenberg, A.; Hadacek, F.; Rashid, A.H.A.H.; Karlovsky, P. Small-Scale Bioreactor for Sterile Hydroponics and Hairy Roots: Metabolic Diversity and Salicylic Acid Exudation by Hairy Roots of Hyoscyamus niger. Appl. Sci. 2019, 9, 3044. https://doi.org/10.3390/app9153044
Kareem ZJ, Su L, Rathgeb A, Sirrenberg A, Hadacek F, Rashid AHAH, Karlovsky P. Small-Scale Bioreactor for Sterile Hydroponics and Hairy Roots: Metabolic Diversity and Salicylic Acid Exudation by Hairy Roots of Hyoscyamus niger. Applied Sciences. 2019; 9(15):3044. https://doi.org/10.3390/app9153044
Chicago/Turabian StyleKareem, Zana Jamal, Ling Su, Anna Rathgeb, Anke Sirrenberg, Franz Hadacek, Ahmad Hama Ameen H. Rashid, and Petr Karlovsky. 2019. "Small-Scale Bioreactor for Sterile Hydroponics and Hairy Roots: Metabolic Diversity and Salicylic Acid Exudation by Hairy Roots of Hyoscyamus niger" Applied Sciences 9, no. 15: 3044. https://doi.org/10.3390/app9153044






