Strain-Specific Effects of Biochar and Its Water-Soluble Compounds on Bacterial Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Strains and Culture Condition
2.2. Preparation and Physico-Chemical Analysis of Biochar
2.3. Preparation of Water-Soluble Compounds from Biochar
2.4. Effect of Biochar on Bacterial Growth
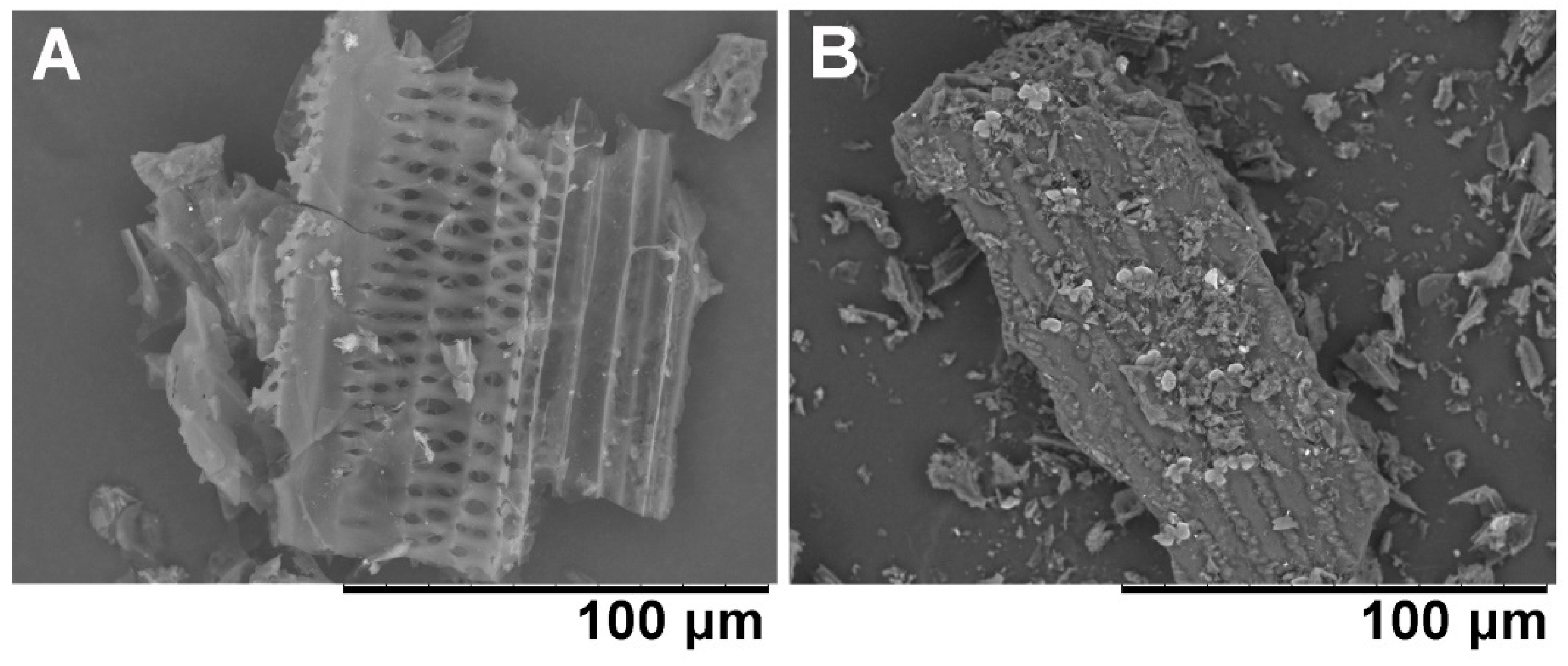
2.5. Adsorption of Bacterial Cells to Biochar
2.6. Effect of Biochar Adsorption on Bacterial Growth Activity
2.7. Influence of Water-Soluble Compounds on Bacterial Growth
2.8. Effect of Biochar on Potassium/Phosphate Solubilizing Strains
2.9. Statistic Analysis
3. Results
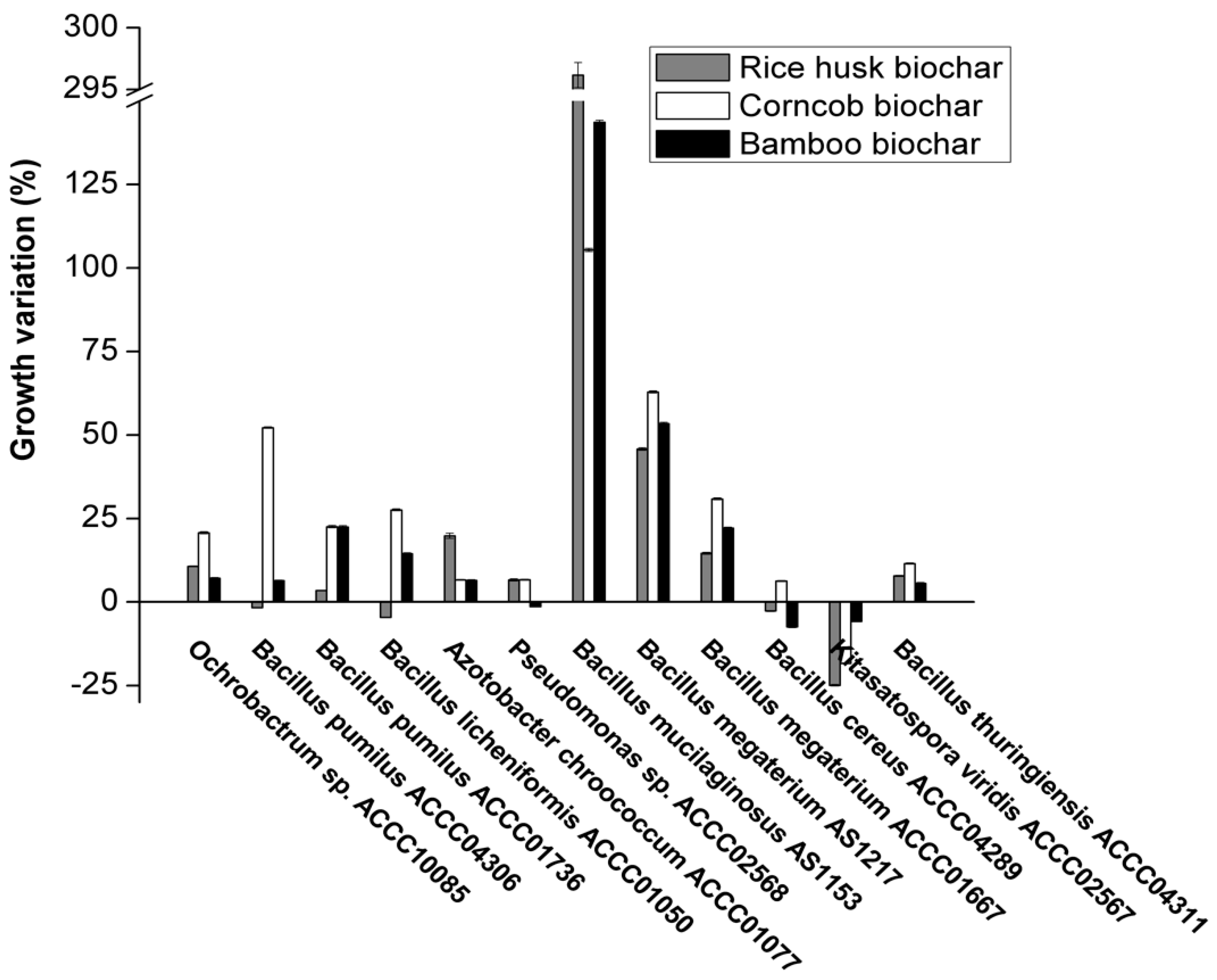
3.1. Impact of Different Biochars on Bacterial Growth
3.2. The Correlation between Adsorption Ability of Biochar and Bacterial Growth
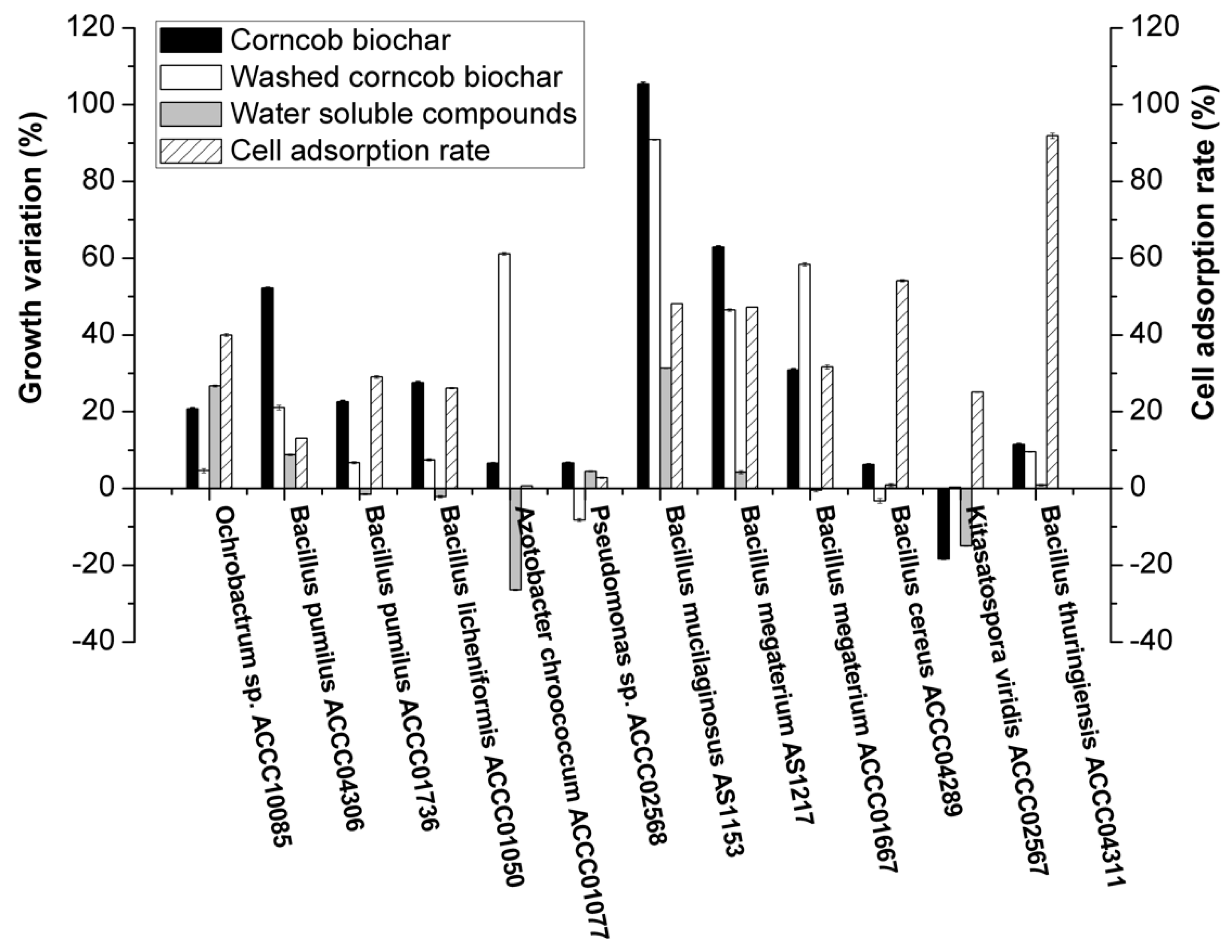
3.3. Effect of Water-Soluble Compounds in Biochar on Bacterial Growth
3.4. Impact of Biochar on Potassium-/Phosphate-Solubilizing Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.; Guo, D.; Jogi, Q.; et al. Recent developments in biochar as an effective tool for agricultural soil management: A review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, N.; Graber, E.R.; Verheijen, F.G.A.; Neve, S.D. Interactions between biochar stability and soil organisms: Review and research needs. Eur. J. Soil Sci. 2013, 64, 379–390. [Google Scholar] [CrossRef]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and applications of biochar for environmental remediation: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Rutigliano, F.A.; Romano, M.; Marzaioli, R.; Baglivo, I.; Baronti, S.; Miglietta, F.; Castaldi, S. Effect of biochar addition on soil microbial community in a wheat crop. Eur. J. Soil Biol. 2014, 60, 9–15. [Google Scholar] [CrossRef]
- Gomez, J.D.; Denef, K.; Stewart, C.E.; Zheng, J.; Cotrufo, M.F. Biochar addition rate influences soil microbial abundance and activity in temperate soils. Eur. J. Soil Sci. 2014, 65, 28–39. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thi, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota: A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influence plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ‘charosphere’—Does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, L.; Borenshtein, M.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Lu, H.; Lashari, M.S.; Liu, X.; Ji, H.; Li, L.; Zheng, J.; Kibue, G.W.; Joseph, S.; Pan, G. Changes in soil microbial community structure and enzyme activity with amendment of biochar-manure compost and pyroligneous solution in a saline soil from central China. Eur. J. Soil Biol. 2015, 70, 67–76. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Chan, K.Y.; Zwieten, L.V.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Bradstreet, R.B. Kjeldahl method for organic nitrogen. Anal. Chem. 1954, 26, 185–187. [Google Scholar] [CrossRef]
- Muhammad, N.; Dai, Z.; Xiao, K.; Meng, J.; Brookes, P.C.; Liu, X.; Wang, H.; Wu, J.; Xu, J. Changes in microbial community structure due to biochars generated from different feedstock and their relationships with soil chemical properties. Geoderma 2014, 226, 270–278. [Google Scholar] [CrossRef]
- Mosmaan, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Yang, F.; Meng, J.; Chen, W.; Li, X. Influence of biochar application on potassium-solubilizing Bacillus mucilaginosus as potential biofertilizer. Prep. Biochem. Biotechnol. 2016, 47, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Rowe, J.J.; Grimaldi, F.S. Molybdenum blue reaction and determination of phosphorus in waters containing arsenic, silicon and germanium. Anal. Chem. 1955, 27, 258–262. [Google Scholar] [CrossRef]
- Sun, D.; Hale, L.; Crowley, D. Nutrient supplementation of pinewood biochar for use as a bacterial inoculum carrier. Biol. Fertil. Soils 2016, 52, 515–522. [Google Scholar] [CrossRef]
- Thies, J.E.; Rillig, M. Characteristics of biochar: Biological properties. In Biochar for Environmental Management; Lehmann, J., Joseph, S., Eds.; Science and Technology Earthscan: London, UK, 2009; pp. 85–105. [Google Scholar]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M.A.S. Impact of biochar amendment on fertility of a southeastern coastal plain soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Sun, D.; Meng, J.; Liang, H.; Yang, E.; Huang, Y.; Chen, W.; Jiang, L.; Lan, Y.; Zhang, W.; Gao, J. Effect of volatile organic compounds absorbed to fresh biochar on survival of Bacillus mucilaginosus and structure of soil microbial communities. J. Soils Sediments 2015, 15, 271–281. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; Neve, S.D. Biochar amendment to soils with contrasting organic matter level: Effects on N mineralization and biological soil properties. GCB Bioenergy 2013, 7, 135–144. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source of contamination–phytotoxic effects on cress seed (Lepidium sativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Rombolà, A.G.; Marisi, G.; Torri, C.; Fabbri, D.; Buscaroli, A.; Ghidotti, M. Relationships between chemical characteristics and phytotoxicity of biochar from poultry litter pyrolysis. J. Agric. Food Chem. 2015, 63, 6660–6667. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Spokas, K.A.; Baker, J.M.; Reicosky, D.C. Ethylene: Potential key for biochar amendment impacts. Plant Soil 2010, 33, 443–452. [Google Scholar] [CrossRef]
- Marks, E.A.; Mattana, S.; Alcaniz, J.M.; Domene, X. Biochars provoke diverse soil mesofauna reproductive responses in laboratory bioassays. Eur. J. Soil Biol. 2014, 60, 104–111. [Google Scholar] [CrossRef]
- Kołtowski, M.; Oleszczuk, P. Toxicity of biochars after polycyclic aromatic hydrocarbons removal by thermal treatment. Ecol. Eng. 2015, 75, 79–85. [Google Scholar] [CrossRef]

| Property | Rice Husk Biochar | Corncob Biochar | Bamboo Biochar |
|---|---|---|---|
| pH | 10.01 | 9.64 | 9.27 |
| TN (g·kg−1) | 6.68 | 12.69 | 9.17 |
| TOC (g·kg−1) | 460.50 | 541.00 | 859.80 |
| Volatile matter (%) | 16.45 | 21.94 | 13.90 |
| Ash content (%) | 42.75 | 18.85 | 0.66 |
| Surface area (m2·g−1) | 92.52 | 58.38 | 0.11 |
| Average pore size (nm) | 2.72 | 3.34 | — |
| Characteristic | Bacillus Mucilaginosus AS1153 | Bacillus Megaterium AS1217 | Ochrobactrum sp. ACCC10085 | |||
|---|---|---|---|---|---|---|
| −biochar | +biochar | −biochar | +biochar | −biochar | +biochar | |
| Potassium content (μg·mL−1) | 9.51 ± 0.43 | 67.27 ± 0.44 | — | — | — | — |
| Phosphate content (μg·mL−1) | — | — | 28.37 ± 0.65 | 98.98 ± 1.13 | 86.32 ± 0.89 | 107.11 ± 0.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Zhou, Y.; Liu, W.; Tang, W.; Meng, J.; Chen, W.; Li, X. Strain-Specific Effects of Biochar and Its Water-Soluble Compounds on Bacterial Growth. Appl. Sci. 2019, 9, 3209. https://doi.org/10.3390/app9163209
Yang F, Zhou Y, Liu W, Tang W, Meng J, Chen W, Li X. Strain-Specific Effects of Biochar and Its Water-Soluble Compounds on Bacterial Growth. Applied Sciences. 2019; 9(16):3209. https://doi.org/10.3390/app9163209
Chicago/Turabian StyleYang, Fan, Yue Zhou, Weiming Liu, Wenzhu Tang, Jun Meng, Wenfu Chen, and Xianzhen Li. 2019. "Strain-Specific Effects of Biochar and Its Water-Soluble Compounds on Bacterial Growth" Applied Sciences 9, no. 16: 3209. https://doi.org/10.3390/app9163209





