Thin Coatings of Cerium Oxide Nanoparticles with Anti-Reflective Properties
Abstract
:1. Introduction
2. Thin and Structured Coatings of Densely Packed Nanoparticles
2.1. Convective Self-Assembly
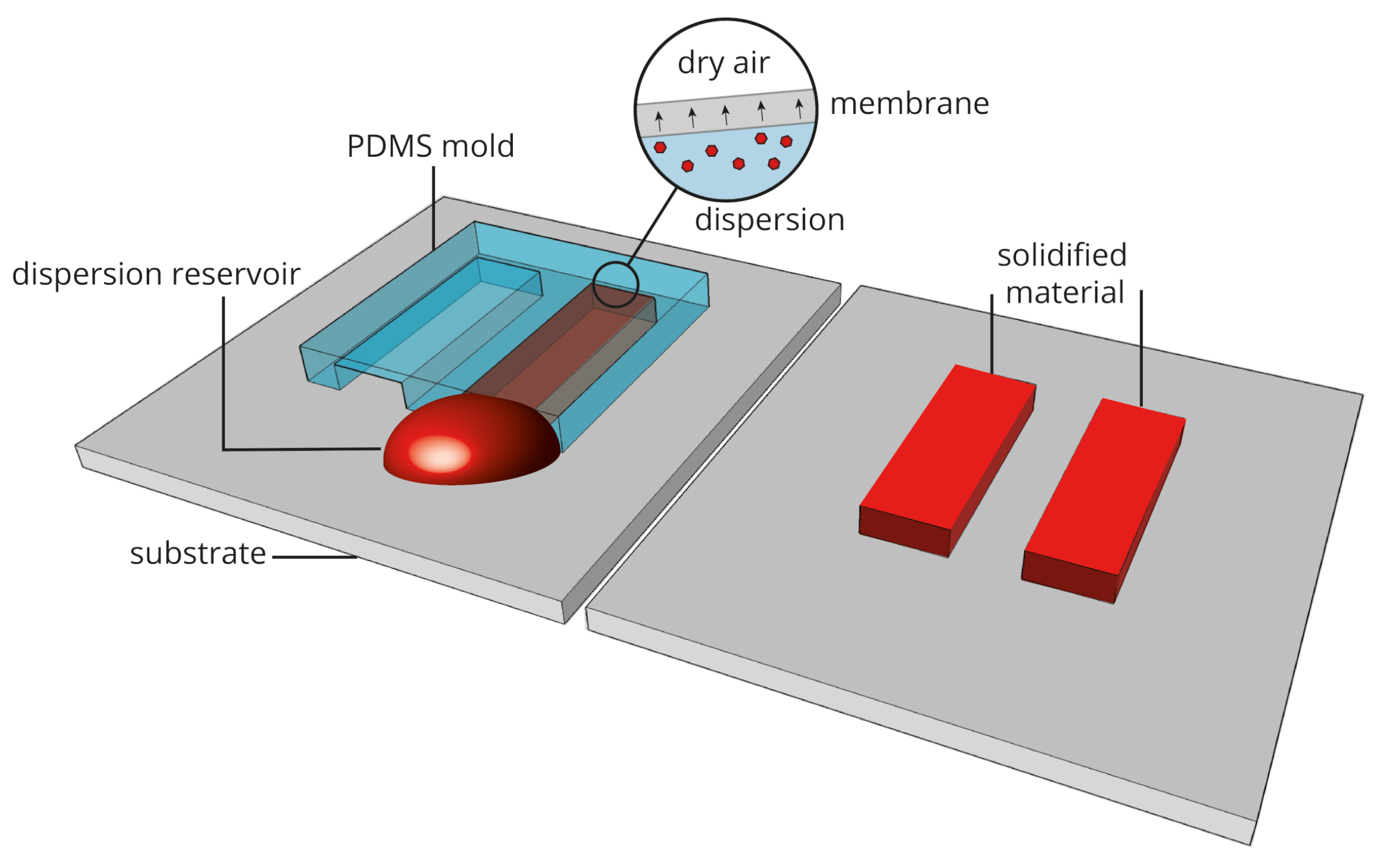
2.2. Microfluidic Pervaporation
3. Optical Features of Nanoporous Coatings
3.1. Refractive Index
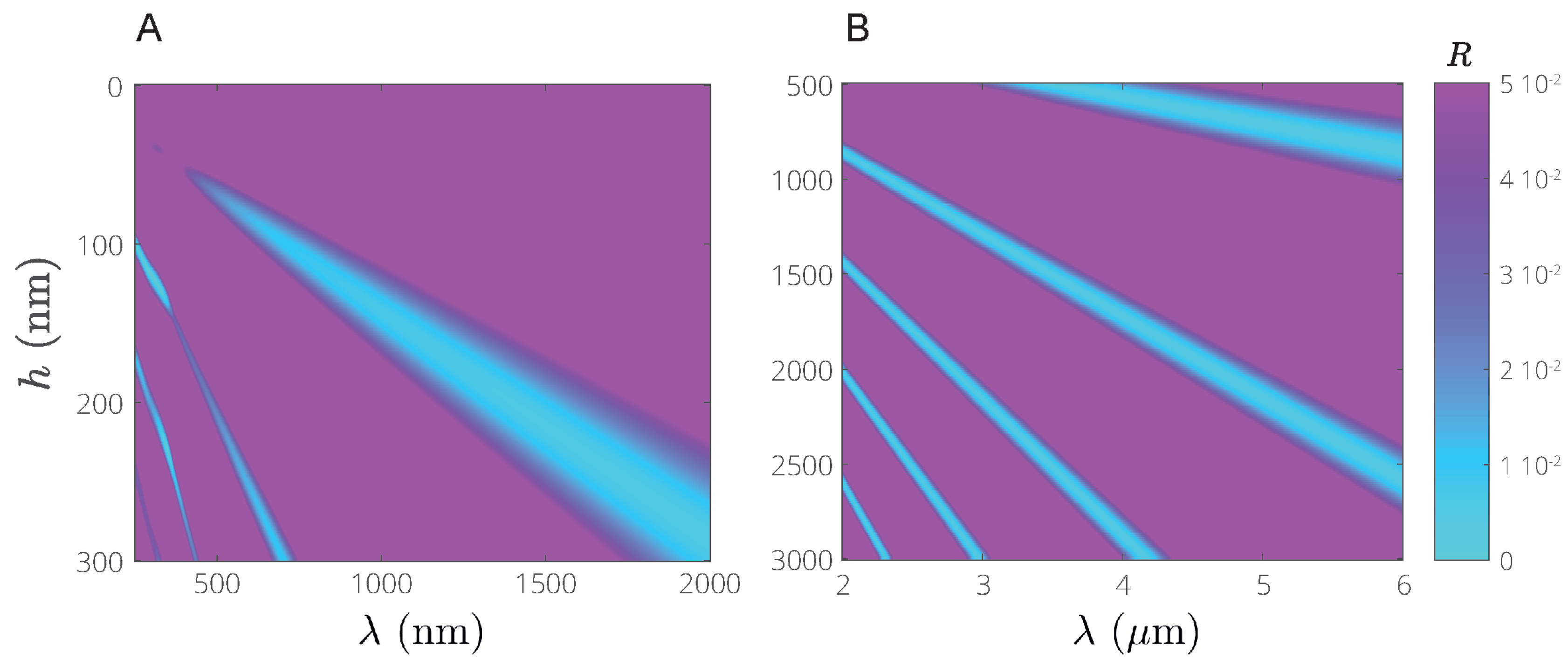
3.2. Reflection Features on Silicon Substrates
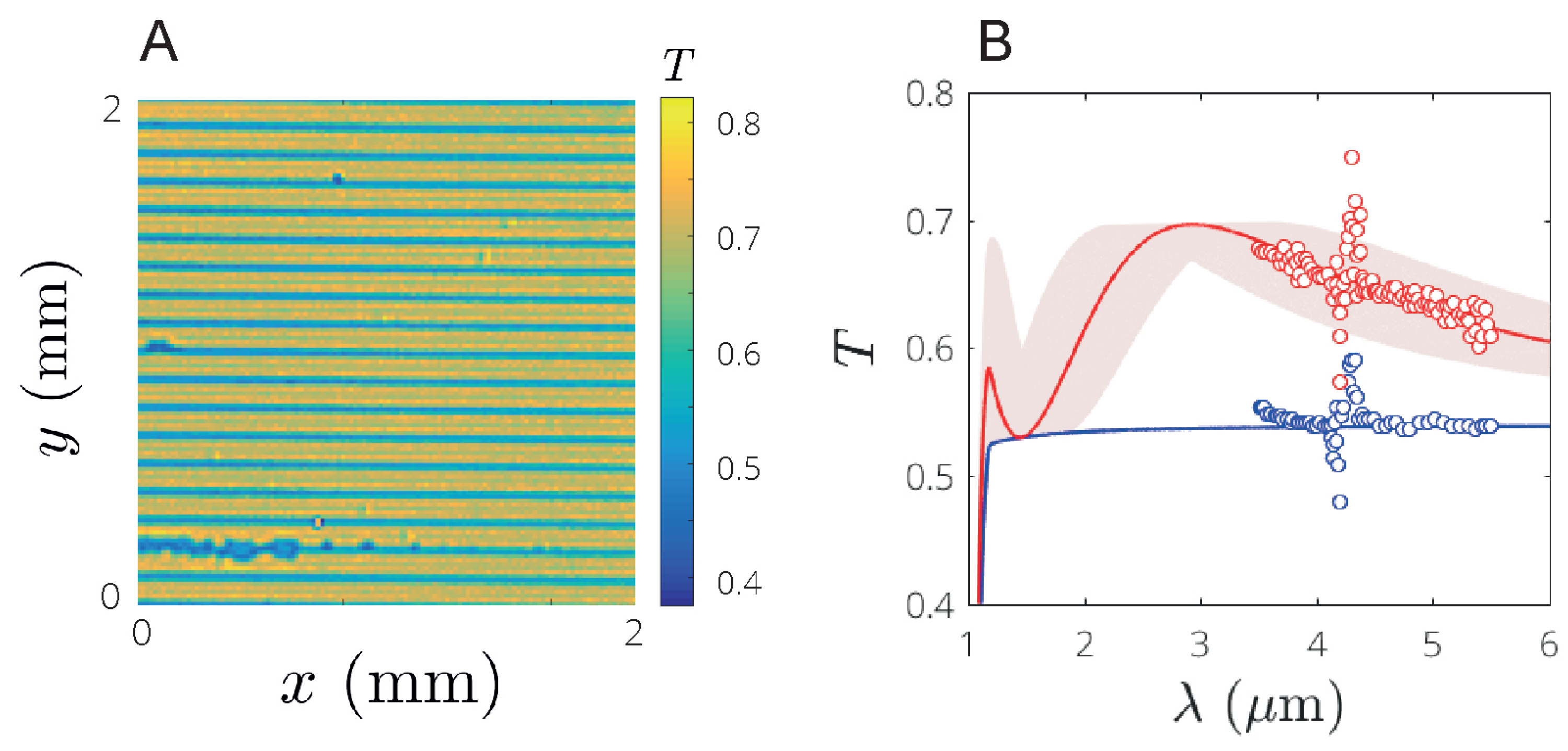
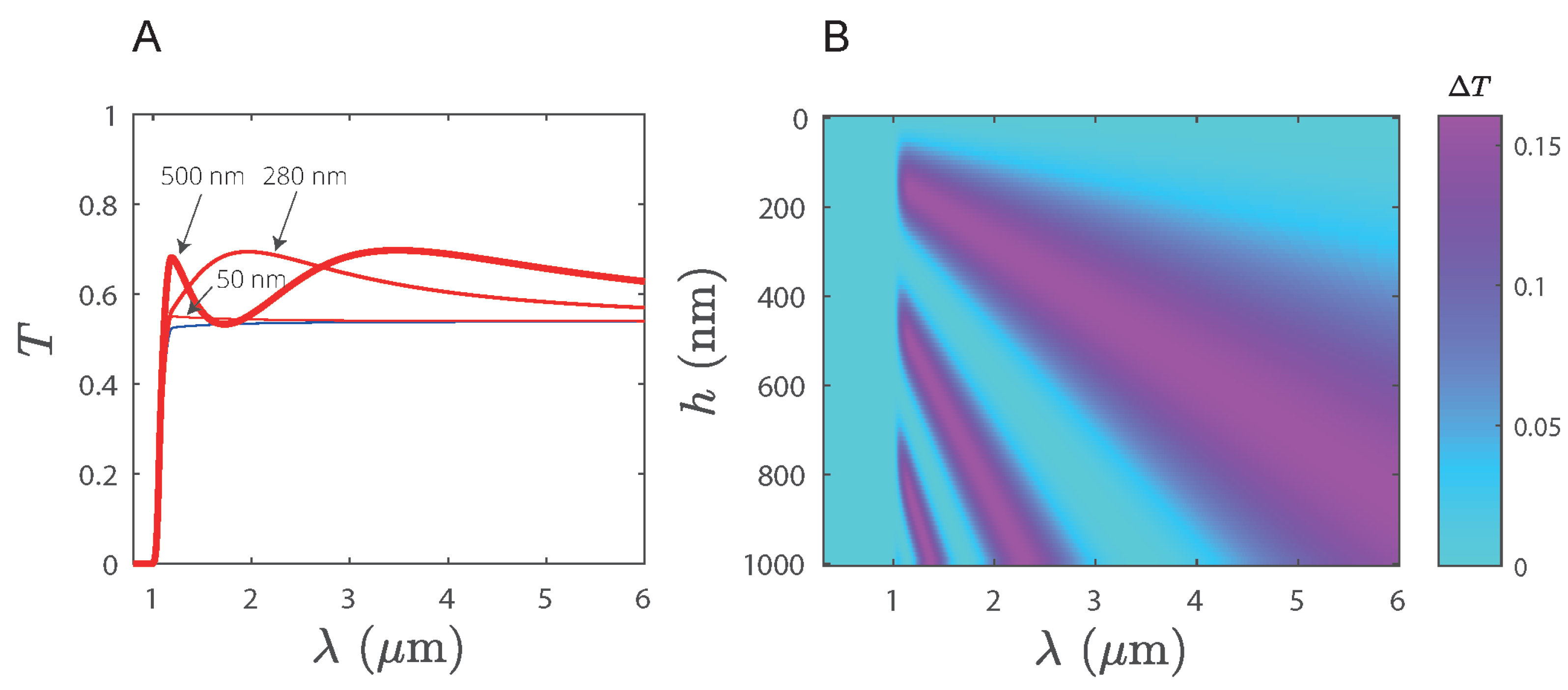
3.3. Enhanced Transmission on Silicon Substrates
4. Materials And Methods
4.1. Dispersions of Nanoparticles
4.2. Assembly Methods
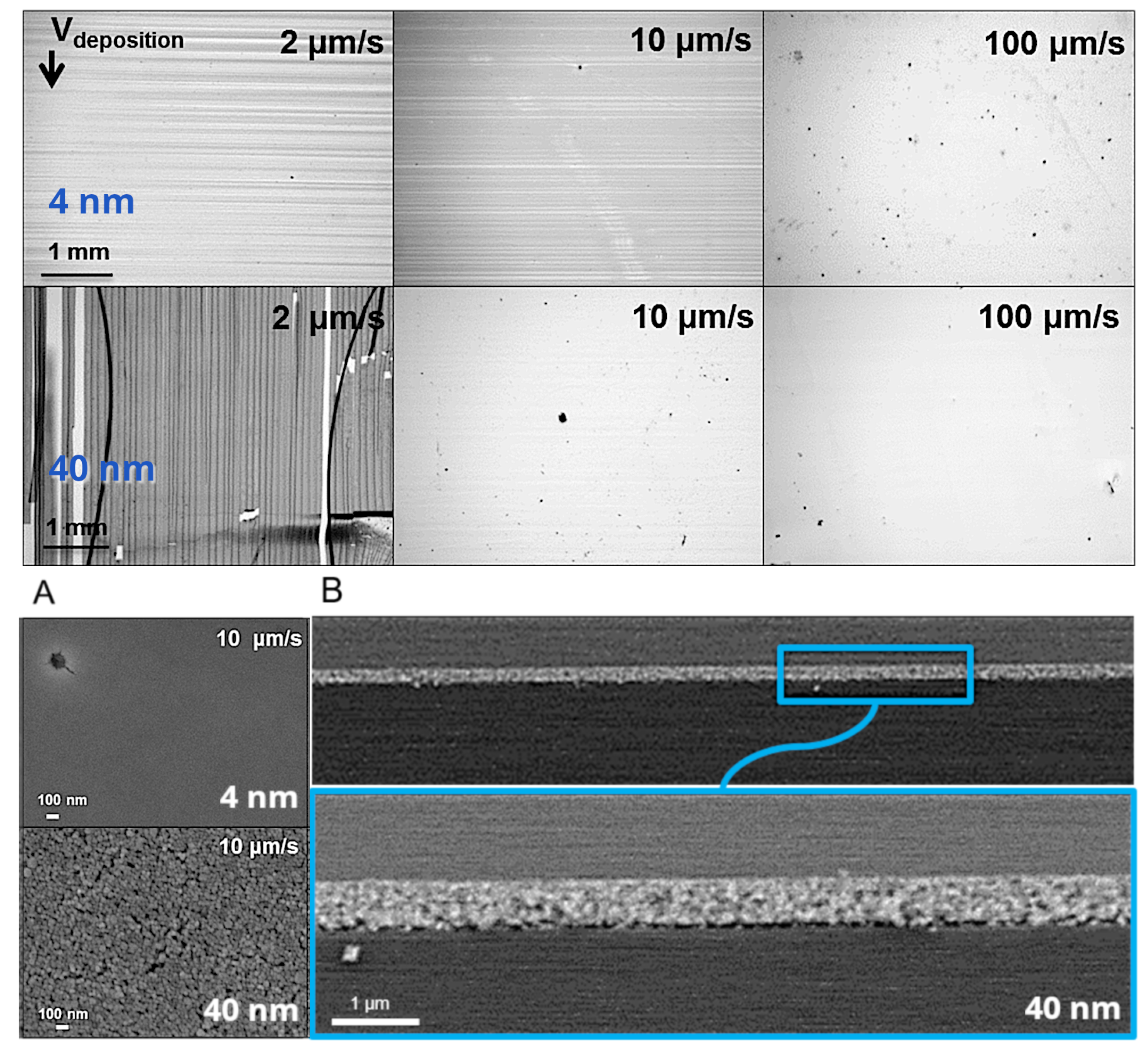
4.2.1. Films Obtained by Convective Self-Assembly (CSA)
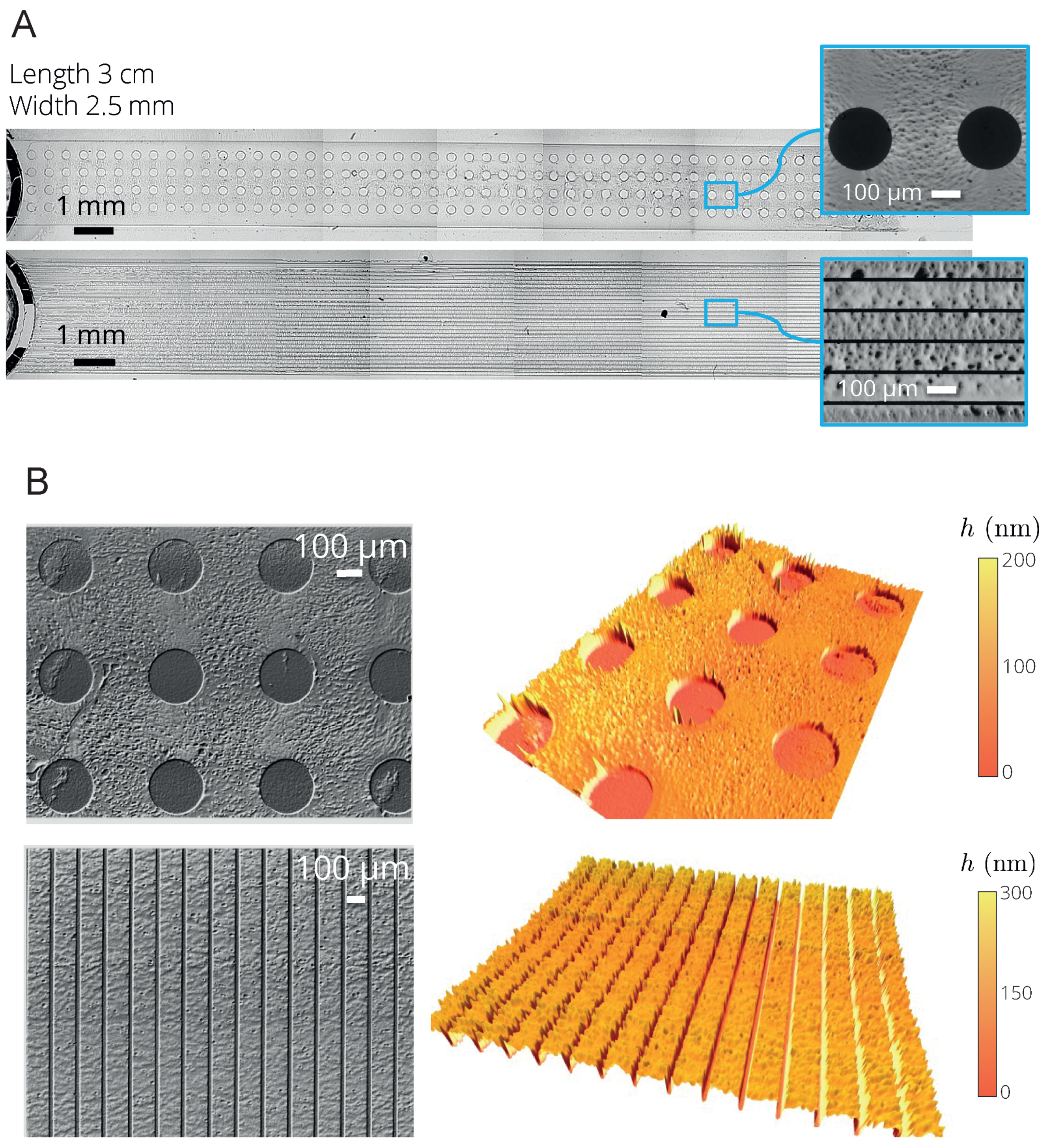
4.2.2. Deposits Engineered with Microfluidic Pervaporation
4.3. Substrates
4.4. Optical Methods
5. Appendix: Optical Performances
5.1. Calculated Reflectance
5.2. Calculated Transmittance
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| IR | Infra-Red |
| UV | Ultra-Violet |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
References
- Das, S.; Dowding, J.M.; Klump, K.E.; McGinnis, J.F.; Self, W.; Seal, S. Cerium oxide nanoparticles: Applications and prospects in nanomedicine. Nanomedicine 2013, 8, 1483–1508. [Google Scholar] [CrossRef]
- Younis, A.; Chu, D.; Li, S. Cerium Oxide Nanostructures and their Applications. In Functionalized Nanomaterials; InTech: London, UK, 2016. [Google Scholar] [Green Version]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Prevo, B.G.; Hwang, Y.; Velev, O.D. Convective Assembly of Antireflective Silica Coatings with Controlled Thickness and Refractive Index. Chem. Mater. 2005, 17, 3642–3651. [Google Scholar] [CrossRef]
- Prevo, B.G.; Hon, E.W.; Velev, O.D. Assembly and characterization of colloid-based antireflective coatings on multicrystalline silicon solar cells. J. Mater. Chem. 2007, 17, 791–799. [Google Scholar] [CrossRef]
- Dimitrov, A.S.; Nagayama, K. Continuous Convective Assembling of Fine Particles into Two-Dimensional Arrays on Solid Surfaces. Langmuir 1996, 12, 1303–1311. [Google Scholar] [CrossRef]
- Doumenc, F.; Salmon, J.B.; Guerrier, B. Modeling Flow Coating of Colloidal Dispersions in the Evaporative Regime: Prediction of Deposit Thickness. Langmuir 2016, 32, 13657–13668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loussert, C.; Doumenc, F.; Salmon, J.B.; Nikolayev, V.S.; Guerrier, B. Role of Vapor Mass Transfer in Flow Coating of Colloidal Dispersions in the Evaporative Regime. Langmuir 2017, 33, 14078–14086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, K.; Gilchrist, J.F. Estimation of drying length during particle assembly by convective deposition. J. Colloid Interface Sci. 2017, 496, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, D.; Xia, Y.; Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 2010, 5, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, B.; Jafek, A.; Lambert, C.; Goenner, B.; Moghimifam, H.; Nze, U.; Kamarapu, S. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef]
- Leng, J.; Lonetti, B.; Tabeling, P.; Joanicot, M.; Ajdari, A. Microevaporators for Kinetic Exploration of Phase Diagrams. Phys. Rev. Lett. 2006, 96, 084503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angly, J.; Iazzolino, A.; Salmon, J.B.; Leng, J.; Chandran, S.P.; Ponsinet, V.; Désert, A.; Le Beulze, A.; Mornet, S.; Tréguer-Delapierre, M.; et al. Microfluidic-Induced Growth and Shape-Up of Three-Dimensional Extended Arrays of Densely Packed Nanoparticles. ACS Nano 2013, 7, 6465–6477. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Xia, Y.; Whitesides, G.M. Micromolding in Capillaries: Applications in Materials Science. J. Am. Chem. Soc. 1996, 118, 5722–5731. [Google Scholar] [CrossRef]
- Le Berre, M.; Chen, Y.; Baigl, D. From Convective Assembly to Landau Levich Deposition of Multilayered Phospholipid Films of Controlled Thickness. Langmuir 2009, 25, 2554–2557. [Google Scholar] [CrossRef] [PubMed]
- Kumnorkaew, P.; Gilchrist, J.F. Effect of Nanoparticle Concentration on the Convective Deposition of Binary Suspensions. Langmuir 2009, 25, 6070–6075. [Google Scholar] [CrossRef]
- Grosso, D. How to exploit the full potential of the dip-coating process to better control film formation. J. Mater. Chem. 2011, 21, 17033. [Google Scholar] [CrossRef]
- Farcau, C.; Sangeetha, N.M.; Moreira, H.; Viallet, B.; Grisolia, J.; Ciuculescu-Pradines, D.; Ressier, L. High-Sensitivity Strain Gauge Based on a Single Wire of Gold Nanoparticles Fabricated by Stop-and-Go Convective Self-Assembly. ACS Nano 2011, 5, 7137–7143. [Google Scholar] [CrossRef]
- Bodiguel, H.; Doumenc, F.; Guerrier, B. Stick-Slip Patterning at Low Capillary Numbers for an Evaporating Colloidal Suspension. Langmuir 2010, 26, 10758–10763. [Google Scholar] [CrossRef]
- Smith, M.I.; Sharp, J.S. Effects of Substrate Constraint on Crack Pattern Formation in Thin Films of Colloidal Polystyrene Particles. Langmuir 2011, 27, 8009–8017. [Google Scholar] [CrossRef]
- Farr, R.S.; Groot, R.D. Close packing density of polydisperse hard spheres. J. Chem. Phys. 2009, 131, 244104. [Google Scholar] [CrossRef] [Green Version]
- Whitesides, G.M. Self-Assembly at All Scales. Science 2002, 295, 2418–2421. [Google Scholar] [CrossRef] [Green Version]
- Salmon, J.B.; Leng, J. Application of microevaporators to dynamic exploration of the phase diagram. J. Appl. Phys. 2010, 107, 084905. [Google Scholar] [CrossRef] [Green Version]
- Laval, C.; Poulin, P.; Salmon, J.B. Investigation of the dynamics of growth of polymer materials obtained by combined pervaporation and micro-moulding. Soft Matter 2016, 12, 1810–1819. [Google Scholar] [CrossRef]
- Bouchaudy, A.; Salmon, J.B. Drying-induced stresses before solidification in colloidal dispersions: In situ measurements. Soft Matter 2019, 15, 2768–2781. [Google Scholar] [CrossRef]
- Tompkins, H.G.; Irene, E.A. (Eds.) Handbook of Ellipsometry; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Oh, T.S.; Tokpanov, Y.S.; Hao, Y.; Jung, W.; Haile, S.M. Determination of optical and microstructural parameters of ceria films. J. Appl. Phys. 2012, 112, 103535. [Google Scholar] [CrossRef] [Green Version]
- Vangelista, S.; Piagge, R.; Ek, S.; Sarnet, T.; Ghidini, G.; Martella, C.; Lamperti, A. Structural, chemical and optical properties of cerium dioxide film prepared by atomic layer deposition on TiN and Si substrates. Thin Solid Films 2017, 636, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Yvon, H.J. New Amorphous Diserpsion Formula; Technical Report; Horiba Jobin Yvon: France, 2012; Available online: http://www.horiba.com/fileadmin/uploads/Scientific/Downloads/OpticalSchool_CN/TN/ellipsometer/New_Amorphous_Dispersion_Formula.pdf (accessed on 15 June 2019).
- MacLeod, H.A. Antireflection Coatings. In Thin-Film Optical Filters, Fourth Edition; CRC Press: Boca Raton, FL, USA, 2010; pp. 105–184. [Google Scholar]
- Kats, M.A.; Blanchard, R.; Genevet, P.; Capasso, F. Nanometre optical coatings based on strong interference effects in highly absorbing media. Nat. Mater. 2013, 12, 20–24. [Google Scholar] [CrossRef]
- RefractiveIndex.INFO. Optical Constants of Si (Silicon) after Aspnes and Studna, 1983 (n, k for λ = 0.21–0.83 μm). 2019. Available online: https://refractiveindex.info/?shelf=main&book=Si&page=Aspnes (accessed on 4 May 2019).
- Romano, M.; Ndiaye, C.; Duphil, A.; Sommier, A.; Morikawa, J.; Mascetti, J.; Batsale, J.; Servant, L.; Pradere, C. Fast infrared imaging spectroscopy technique (FIIST). Infrared Phys. Technol. 2015, 68, 152–158. [Google Scholar] [CrossRef]
- Kirchner, S.; Narinsamy, S.; Sommier, A.; Romano, M.; Ryu, M.; Morikawa, J.; Leng, J.; Batsale, J.C.; Pradère, C. Calibration Procedure for Attenuation Coefficient Measurements in Highly Opaque Media Using Infrared Focal Plane Array (IRFPA) Spectroscopy. Appl. Spectrosc. 2018, 72, 177–187. [Google Scholar] [CrossRef]
- Dimitrov, A.S.; Nagayama, K. Steady-state unidirectional convective assembling of fine particles into two-dimensional arrays. Chem. Phys. Lett. 1995, 243, 462–468. [Google Scholar] [CrossRef]
- Verneuil, E.; Buguin, A.; Silberzan, P. Permeation-induced flows: Consequences for silicone-based microfluidics. Europhys. Lett. (EPL) 2004, 68, 412–418. [Google Scholar] [CrossRef]
- Randall, G.C.; Doyle, P.S. Permeation-driven flow in poly(dimethylsiloxane) microfluidic devices. Proc. Natl. Acad. Sci. USA 2005, 102, 10813–10818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, A.; Iazzolino, A.; Ehrhardt, K.; Salmon, J.B.; Aradian, A.; Kravets, V.; Grigorenko, A.N.; Leng, J.; Le Beulze, A.; Tréguer-Delapierre, M.; et al. Bulk optical metamaterials assembled by microfluidic evaporation. Opt. Mater. Express 2013, 3, 1792. [Google Scholar] [CrossRef]
- Gómez-Graña, S.; Fernández-López, C.; Polavarapu, L.; Salmon, J.B.; Leng, J.; Pastoriza-Santos, I.; Pérez-Juste, J. Gold Nanooctahedra with Tunable Size and Microfluidic-Induced 3D Assembly for Highly Uniform SERS-Active Supercrystals. Chem. Mater. 2015, 27, 8310–8317. [Google Scholar] [CrossRef]
- Gomez-Graña, S.; Le Beulze, A.; Treguer-Delapierre, M.; Mornet, S.; Duguet, E.; Grana, E.; Cloutet, E.; Hadziioannou, G.; Leng, J.; Salmon, J.B.; et al. Hierarchical self-assembly of a bulk metamaterial enables isotropic magnetic permeability at optical frequencies. Mater. Horiz. 2016, 3, 596–601. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romasanta, L.J.; D’Alençon, L.; Kirchner, S.; Pradère, C.; Leng, J. Thin Coatings of Cerium Oxide Nanoparticles with Anti-Reflective Properties. Appl. Sci. 2019, 9, 3886. https://doi.org/10.3390/app9183886
Romasanta LJ, D’Alençon L, Kirchner S, Pradère C, Leng J. Thin Coatings of Cerium Oxide Nanoparticles with Anti-Reflective Properties. Applied Sciences. 2019; 9(18):3886. https://doi.org/10.3390/app9183886
Chicago/Turabian StyleRomasanta, Laura J., Lauriane D’Alençon, Sara Kirchner, Christophe Pradère, and Jacques Leng. 2019. "Thin Coatings of Cerium Oxide Nanoparticles with Anti-Reflective Properties" Applied Sciences 9, no. 18: 3886. https://doi.org/10.3390/app9183886





