Fabrication of a Novel Culture Dish Adapter with a Small Recess Structure for Flow Control in a Closed Environment
Abstract
:Featured Application
Abstract
1. Introduction
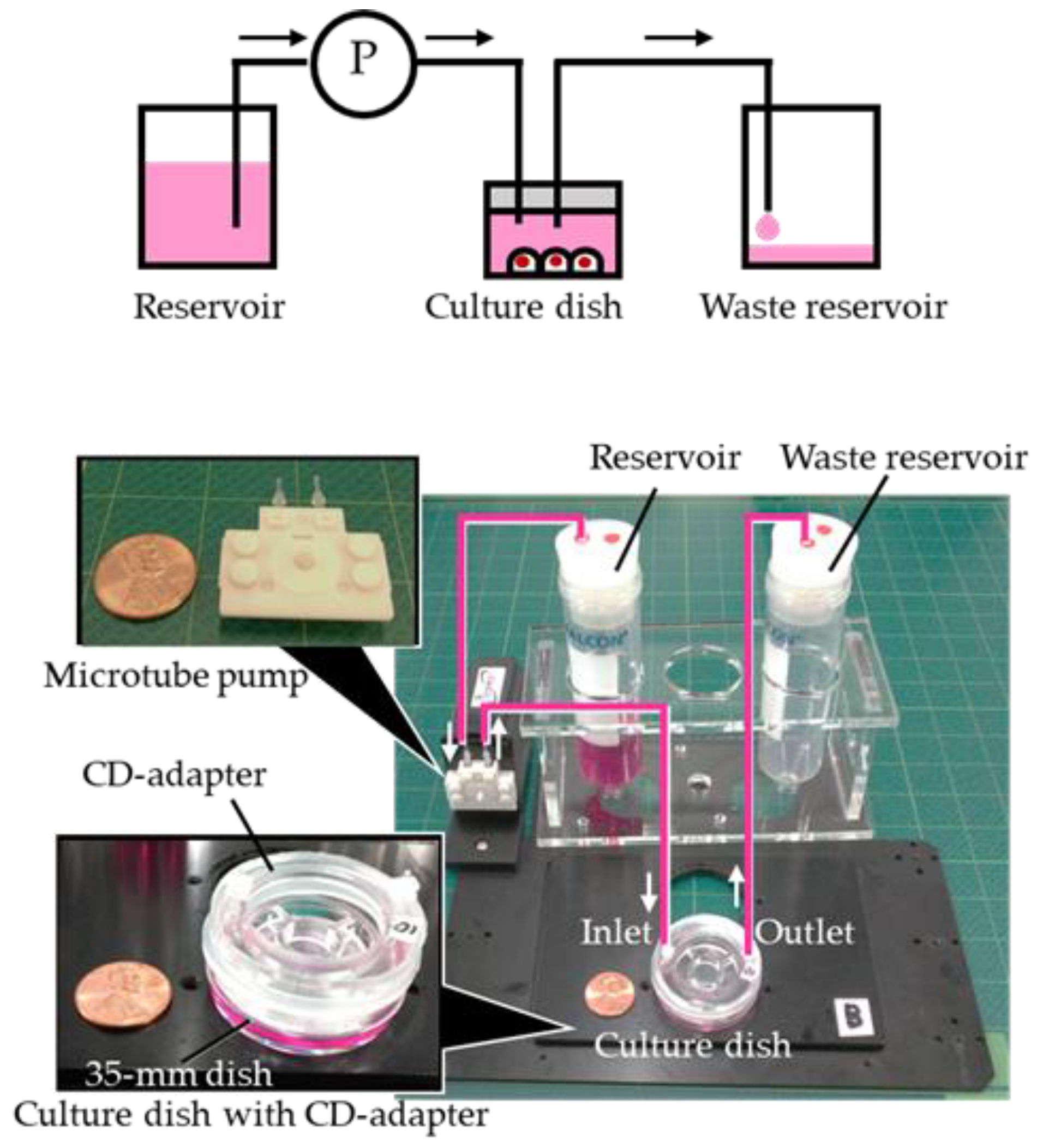
2. Fabrication of the PDMS CD-Adapter
3. Experiment
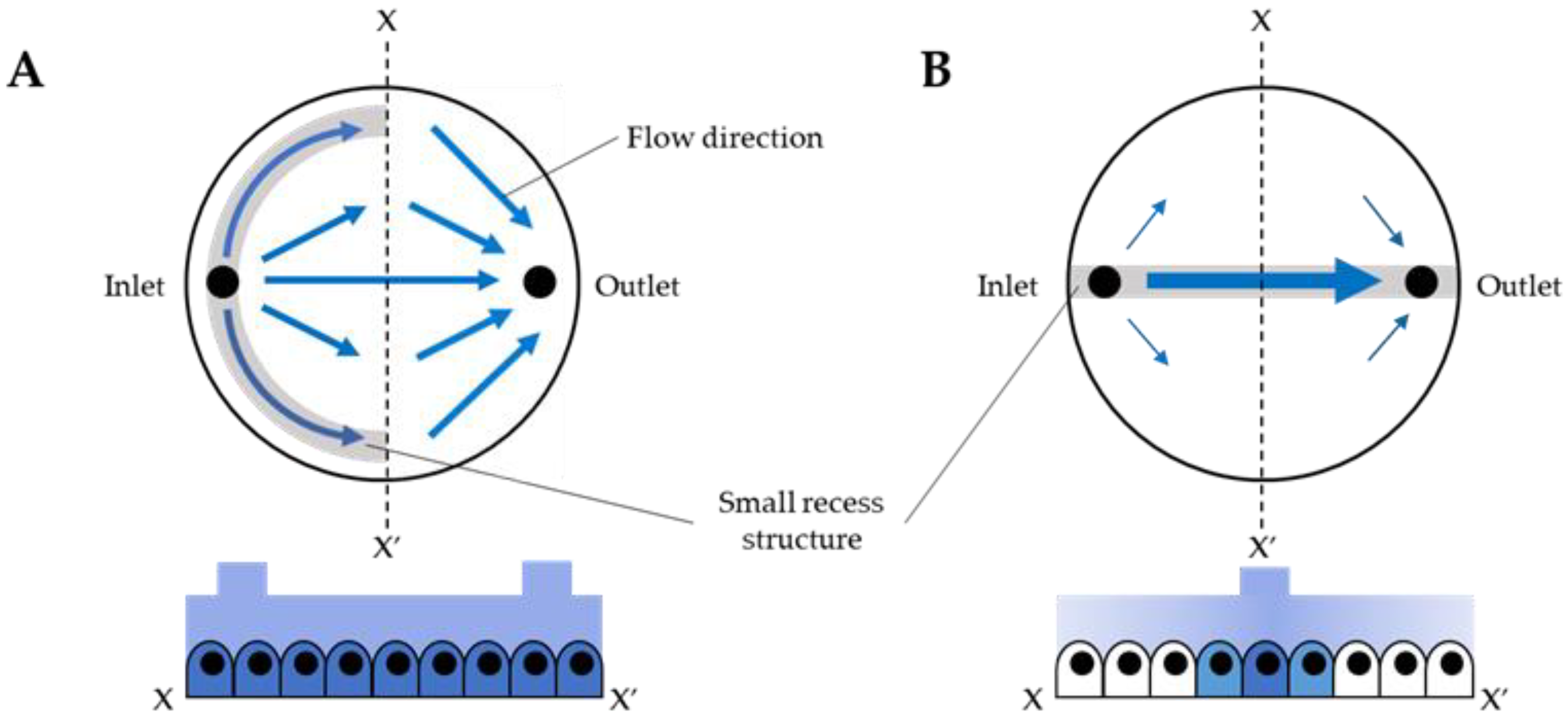
3.1. Fluid Simulation of Flow Control with the CD-Adapter
3.2. Cell Culture Test for Flow Controllability of the Small Recess Structure
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouyang, A.; Ng, R.; Yang, S.T. Long-term culturing of undifferentiated embryonic stem cells in conditioned media and three-dimensional fibrous matrices without extracellular matrix coating. Stem Cells 2007, 25, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.K.; Kim, S.H.; Lee, G.M. Effect of low culture temperature on specific productivity and transcription level of anti-4-1bb antibody in recombinant chinese hamster ovary cells. Biotechnol. Prog. 2003, 19, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.; Alhaque, S.; Szkolnicka, D.; Flint, O.; Hay, D.C. Fluid shear stress modulation of hepatocyte-like cell function. Arch. Toxicol. 2016, 90, 1757–1761. [Google Scholar] [CrossRef] [Green Version]
- Close, D.A.; Camarco, D.P.; Shan, F.; Kochanek, S.J.; Johnston, P.A. The generation of three-dimensional head and neck cancer models for drug discovery in 384-well ultra-low attachment microplates. Methods Mol. Biol. 2018, 1683, 355–369. [Google Scholar] [PubMed]
- Seah, Y.F.S.; Hu, H.; Merten, C.A. Microfluidic single-cell technology in immunology and antibody screening. Mol. Asp. Med. 2018, 59, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.E.; Richard, M. Three-dimensional cell cultures in drug discovery and development. SLAS Discov. Adv. Life Sci. RD 2017, 22, 456–472. [Google Scholar]
- Kano, M.; Suga, H.; Kasai, T.; Ozone, C.; Arima, H. Functional pituitary tissue generation from human embryonic stem cells. Curr. Protoc. Neurosci. 2018, 83, e48. [Google Scholar] [CrossRef] [PubMed]
- Gosavi, R.A.; Salwe, S.; Mukherjee, S.; Dahake, R.; Kothari, S.; Patel, V.; Chowdhary, A.; Deshmukh, R.A. Optimization of ex vivo murine bone marrow derived immature dendritic cells: A comparative analysis of flask culture method and mouse cd11c positive selection kit method. Bone Marrow Res. 2018, 2018, 3495086. [Google Scholar] [CrossRef]
- Kondo, E.; Wada, K.; Hosokawa, K.; Maeda, M. Microfluidic perfusion cell culture system confined in 35 mm culture dish for standard biological laboratories. J. Biosci. Bioeng. 2014, 118, 356–358. [Google Scholar] [CrossRef]
- Yuan, D.; Tan, S.H.; Sluyter, R.; Zhao, Q.; Yan, S.; Nguyen, N.T.; Guo, J.; Zhang, J.; Li, W. On-chip microparticle and cell washing using coflow of viscoelastic fluid and newtonian fluid. Anal. Chem. 2017, 89, 9574–9582. [Google Scholar] [CrossRef]
- Tang, S.Y.; Yi, P.; Soffe, R.; Nahavandi, S.; Shukla, R.; Khoshmanesh, K. Using dielectrophoresis to study the dynamic response of single budding yeast cells to lyticase. Anal. Bioanal. Chem. 2015, 407, 3437–3448. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Fujii, T. A novel assembly technique with semi-automatic alignment for pdms substrates. Lab Chip 2013, 13, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Toh, Y.C.; Voldman, J.; Yu, H. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip 2007, 7, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.C.; Voldman, J. Fluid shear stress primes mouse embryonic stem cells for differentiation in a self-renewing environment via heparan sulfate proteoglycans transduction. FASEB J. 2011, 25, 1208–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdon, T.; Smith, A.; Savatier, P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002, 12, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.N.; Hoey, D.A. Trpv4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef] [PubMed]
- Mehling, M.; Tay, S. Microfluidic cell culture. Curr. Opin. Biotechnol. 2014, 25, 95–102. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yang, S. A microfluidic-based lid device for conventional cell culture dishes to automatically control oxygen level. Biotechniques 2018, 64, 231–234. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.S.; Cho, H.S.; Kim, J.A.; Lee, K.H. The flow rate of the elastomeric balloon infusor is influenced by the internal pressure of the infusor. J. Korean Med. Sci. 2001, 16, 702–706. [Google Scholar] [CrossRef]
- DasGupta, S.S.; Jeffrey, A.; Kim, I.Y.; Wayner, P.C., Jr. Use of the augmented young-laplace equation to model equilibrium and evaporating extended menisci. J. Colloid Interface Sci. 1993, 157, 332–342. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, J.; Yan, S.; Peng, G.; Zhao, Q.; Alici, G.; Du, H.; Li, W. Investigation of particle lateral migration in sample-sheath flow of viscoelastic fluid and newtonian fluid. Electrophoresis 2016, 37, 2147–2155. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, R.; Adachi, S.; Okonogi, A.; Anzai, Y.; Kamiyama, T.; Katano, K.; Hoshi, N.; Natsume, T.; Mogi, K. Fabrication of a Novel Culture Dish Adapter with a Small Recess Structure for Flow Control in a Closed Environment. Appl. Sci. 2019, 9, 269. https://doi.org/10.3390/app9020269
Yasuda R, Adachi S, Okonogi A, Anzai Y, Kamiyama T, Katano K, Hoshi N, Natsume T, Mogi K. Fabrication of a Novel Culture Dish Adapter with a Small Recess Structure for Flow Control in a Closed Environment. Applied Sciences. 2019; 9(2):269. https://doi.org/10.3390/app9020269
Chicago/Turabian StyleYasuda, Reiko, Shungo Adachi, Atsuhito Okonogi, Youhei Anzai, Tadataka Kamiyama, Keiji Katano, Nobuhiko Hoshi, Tohru Natsume, and Katsuo Mogi. 2019. "Fabrication of a Novel Culture Dish Adapter with a Small Recess Structure for Flow Control in a Closed Environment" Applied Sciences 9, no. 2: 269. https://doi.org/10.3390/app9020269




