Electrochemical Impedance Characterization of Cell Growth on Reduced Graphene Oxide–Gold Nanoparticles Electrodeposited on Indium Tin Oxide Electrodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of the ITO Electrode-Based Cultureware
2.2. Electrochemical Deposition of rGO–AuNP on the ITO Electrode
2.3. Impedance Measurement of Cells on the rGO–AuNP/ITO Electrode
3. Results and Discussion
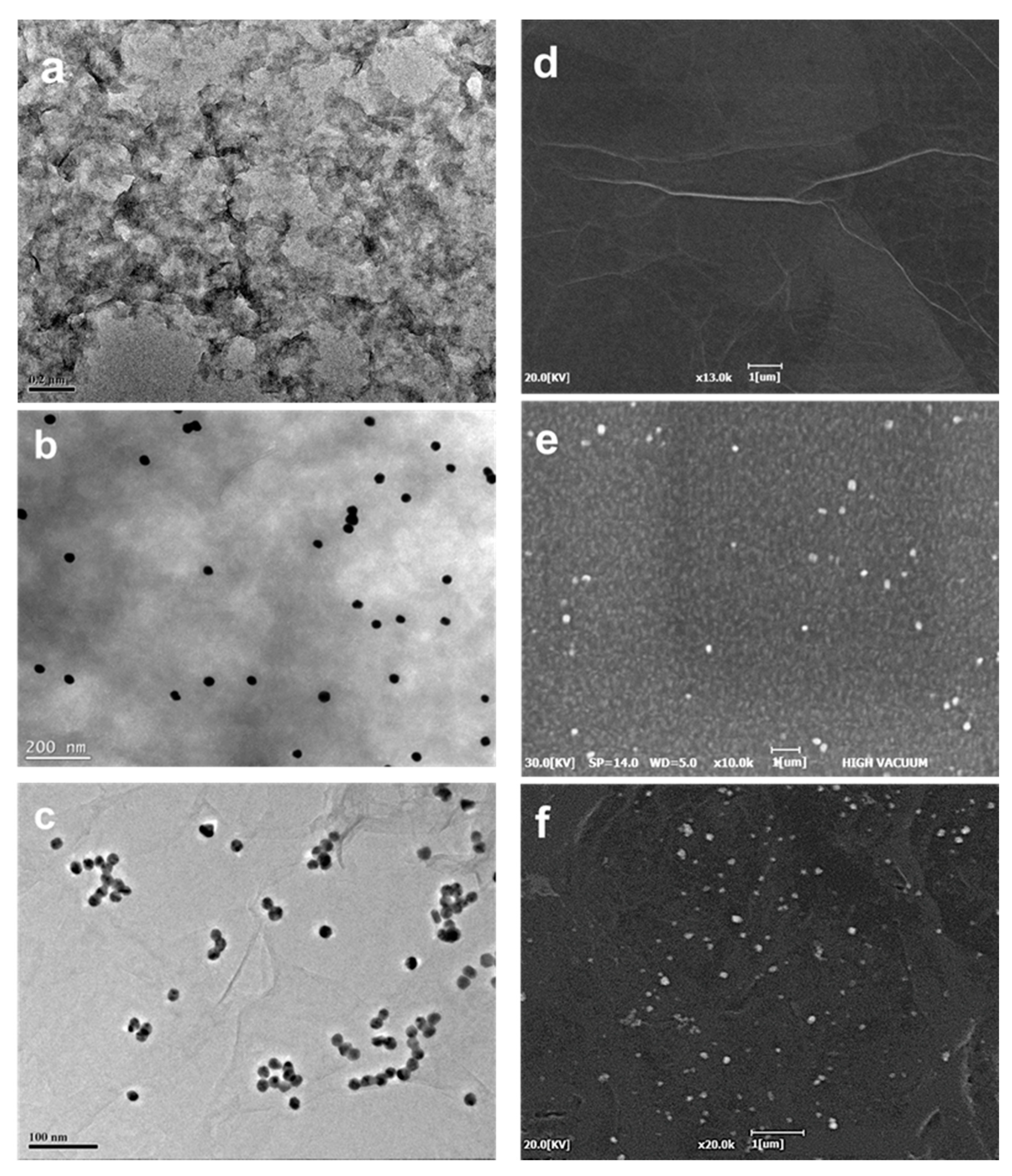
3.1. Characterization of the rGO–AuNP/ITO Electrode
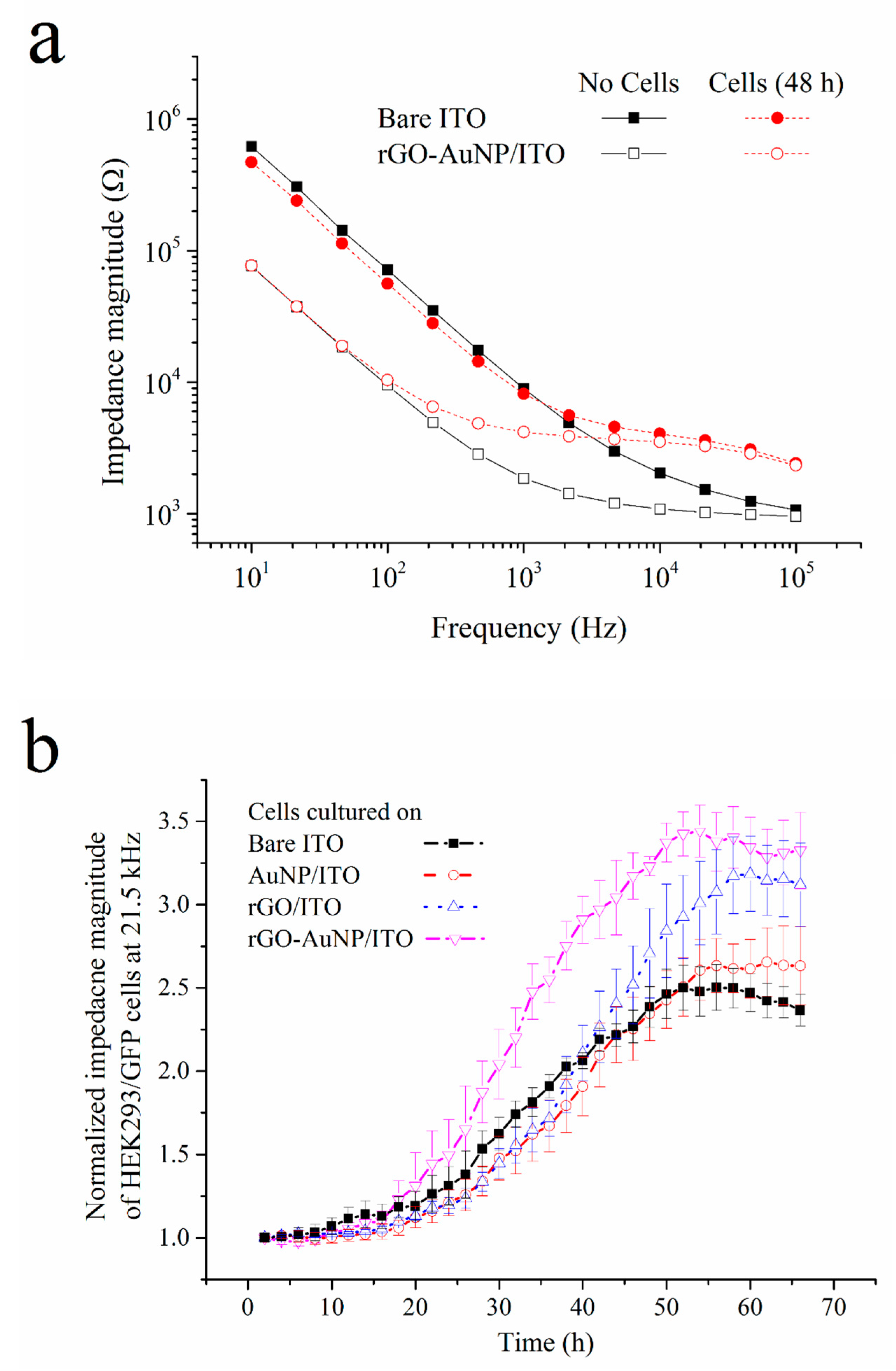
3.2. Impedimetric Measurement of Cell Growth on the rGO–AuNP/ITO Electrode
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Yagati, A.K.; Pyun, J.-C.; Min, J.; Cho, S. Label-free and direct detection of C-reactive protein using reduced graphene oxide-nanoparticle hybrid impedimetric sensor. Bioelectrochemistry 2016, 107, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Vallés, C.; Benito, A.M.; Maser, W.K.; Rius, F.X.; Riu, J. Graphene-based potentiometric biosensor for the immediate detection of living bacteria. Biosens. Bioelectron. 2014, 54, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.D.; Kim, S.K.; Chang, H.; Roh, K.M.; Choi, J.W.; Huang, J. A glucose biosensor based on TiO2-Graphene composite. Biosens. Bioelectron. 2012, 38, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Zhang, J.-J.; Wang, C.; Zhu, J.-J. Graphene-assisted dual amplification strategy for the fabrication of sensitive amperometric immunosensor. Biosens. Bioelectron. 2011, 26, 3627–3632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Liu, Y.; Hu, L.-H.; Jiang, L.-P.; Zhu, J.-J. “Proof-of-principle” concept for ultrasensitive detection of cytokines based on the electrically heated carbon paste electrode. Chem. Commun. 2011, 47, 6551–6553. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Zheng, T.-T.; Cheng, F.-F.; Zhang, J.-R.; Zhu, J.-J. Toward the Early Evaluation of Therapeutic Effects: An Electrochemical Platform for Ultrasensitive Detection of Apoptotic Cells. Anal. Chem. 2011, 83, 7902–7909. [Google Scholar] [CrossRef]
- Lee, W.C.; Lim, C.H.Y.X.; Shi, H.; Tang, L.A.L.; Wang, Y.; Lim, C.T. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef]
- Yagati, A.K.; Min, J.; Cho, S. Electrosynthesis of ERGO-NP Nanocomposite Films for Bioelectrocatalysis of Horseradish Peroxidase towards H2O2. J. Electrochem. Soc. 2014, 161, G133–G140. [Google Scholar] [CrossRef]
- Chauhan, N.; Pundir, C.S. An amperometric acetylcholinesterase sensor based on Fe3O4 nanoparticle/multi-walled carbon nanotube-modified ITO-coated glass plate for the detection of pesticides. Electrochim. Acta 2012, 67, 79–86. [Google Scholar] [CrossRef]
- El-Said, W.A.; Choi, J.W. Electrochemical Biosensor consisted of conducting polymer layer on gold nanodots patterned Indium Tin Oxide electrode for rapid and simultaneous determination of purine bases. Electrochim. Acta 2014, 123, 51–57. [Google Scholar] [CrossRef]
- Golsheikh, A.M.; Huang, N.M.; Lim, H.N.; Zakaria, R.; Yin, C.Y. One-step electrodeposition synthesis of silver-nanoparticle-decorated graphene on indium-tin-oxide for enzymeless hydrogen peroxide detection. Carbon 2013, 62, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Yagati, A.K.; Choi, Y.; Park, J.; Choi, J.-W.; Jun, H.-S.; Cho, S. Silver nanoflower-reduced graphene oxide composite based micro-disk electrode for insulin detection in serum. Biosens. Bioelectron. 2016, 80, 307–314. [Google Scholar] [CrossRef]
- Lin, M.; Pei, H.; Yang, F.; Fan, C.; Zuo, X. Applications of Gold Nanoparticles in the Detection and Identification of Infectious Diseases and Biothreats. Adv. Mater. 2013, 25, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Choi, J.; Wang, W.; Lee, S.; Nam, T.H.; Choi, W.S.; Kim, C.J.; Lee, J.K.; Kim, S.H.; Kang, S.S.; et al. Nullifying tumor efflux by prolonged endolysosome vesicles: Development of low dose anticancer-carbon nanotube drug. ACS Nano 2013, 7, 8484–8497. [Google Scholar] [CrossRef] [PubMed]
- Yadegari, A.; Omidi, M.; Yazdian, F.; Zali, H.; Tayebi, L. An electrochemical cytosensor for ultrasensitive detection of cancer cells using modified graphene–gold nanostructures. RSC Adv. 2017, 7, 2365–2372. [Google Scholar] [CrossRef] [Green Version]
- Sanghavi, B.J.; Wolfbeis, O.S.; Hirsch, T.; Swami, N.S. Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim. Acta 2015, 182, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, P.; Deng, X.; Wang, F.; Chen, Z. Fabrication of electrochemical NO sensor based on nanostructured film and its application in drug screening. Biosens. Bioelectron. 2013, 50, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Jo, H.; Her, J.; Lee, H.Y.; Ban, C. Ultrasensitive electrochemical detection of engrailed-2 based on homeodomain-specific DNA probe recognition for the diagnosis of prostate cancer. Biosens. Bioelectron. 2015, 66, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Min, J.; Cho, S. Indium tin oxide based chip for optical and electrochemical characterization of protein–cell interaction. Jpn. J. Appl. Phys. 2015, 54, 06FN03. [Google Scholar] [CrossRef]
- Park, I.; Nguyen, T.; Park, J.; Yoo, A.Y.; Park, J.K.; Cho, S. Impedance Characterization of Chitosan Cytotoxicity to MCF-7 Breast Cancer Cells Using a Multidisc Indium Tin Oxide Microelectrode Array. J. Electrochem. Soc. 2018, 165, B55–B59. [Google Scholar] [CrossRef]
- Park, I.; Hong, Y.; Jun, Y.-H.; Lee, G.-Y.; Jun, H.-S.; Pyun, J.-C.; Choi, J.-W.; Cho, S. Electrical Impedance Monitoring of C2C12 Myoblast Differentiation on an Indium Tin Oxide Electrode. Sensors 2016, 16, 2068. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, R.C.; Silva, E.R.; Antonia, L.H.D.; Ferrerira, F.F.; Alves, W.A. A Nonenzymatic Biosensor Based on Gold Electrodes Modified with Peptide Self-Assemblies for Detecting Ammonia and Urea Oxidation. Langmuir 2014, 30, 11464–11473. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, B.; Luo, S.C.; Lin, H.A.; Nakao, A.; Yamashita, Y.; Yu, H. Controlled Protein Absorption and Cell Adhesion on Polymer-Brush-Grafted Poly(3,4-ethylenedioxythiophene) Films. Appl. Mater. Interfaces 2013, 5, 4536–4543. [Google Scholar] [CrossRef]
- Kafi, M.A.; El-Said, W.A.; Kim, T.H.; Choi, J.W. Cell adhesion, spreading, and proliferation on surface functionalized with RGD nanopillar arrays. Biomaterials 2012, 33, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.S.; Dao, L.T.M.; Pyun, J.C.; Cho, S. Effect of cell senescence on the impedance measurement of adipose tissue-derived stem cells. Enzym. Microb. Technol. 2013, 53, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kumar, V.; Ali, M.A.; Solanki, P.R.; Srivastava, A.; Sumana, G.; Saxena, P.S.; Joshi, A.G.; Malhotra, B.D. Electrophoretically deposited reduced graphene oxide platform for food toxin detection. Nanoscale 2013, 5, 3043–3051. [Google Scholar] [CrossRef]
- Narváez, E.M.; Merkoçi, A. Graphene Oxide as an Optical Biosensing Platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and Ultrafast Molecular Sieving Through Graphene Oxide Membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Rathod, D.; Warren, S.; Keane, K.; Egan, D.A.; Dempsey, E. Evaluation of a modified carbon micromesh electrodeas a new substrate for electrochemical immunosensing. Anal. Methods 2011, 3, 799–805. [Google Scholar] [CrossRef]
- Guin, S.K.; Pillai, J.S.; Ambolikar, A.S.; Saha, A.; Aggarwal, S.K. Template-free electrosynthesis of gold nanoparticles of controlled size dispersion for the determination of lead at ultratrace levels. RSC Adv. 2013, 3, 17977–17988. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnadayyala, S.R.; Park, J.; Choi, Y.; Han, J.-H.; Yagati, A.K.; Cho, S. Electrochemical Impedance Characterization of Cell Growth on Reduced Graphene Oxide–Gold Nanoparticles Electrodeposited on Indium Tin Oxide Electrodes. Appl. Sci. 2019, 9, 326. https://doi.org/10.3390/app9020326
Chinnadayyala SR, Park J, Choi Y, Han J-H, Yagati AK, Cho S. Electrochemical Impedance Characterization of Cell Growth on Reduced Graphene Oxide–Gold Nanoparticles Electrodeposited on Indium Tin Oxide Electrodes. Applied Sciences. 2019; 9(2):326. https://doi.org/10.3390/app9020326
Chicago/Turabian StyleChinnadayyala, Somasekhar R., Jinsoo Park, Yonghyun Choi, Jae-Hee Han, Ajay Kumar Yagati, and Sungbo Cho. 2019. "Electrochemical Impedance Characterization of Cell Growth on Reduced Graphene Oxide–Gold Nanoparticles Electrodeposited on Indium Tin Oxide Electrodes" Applied Sciences 9, no. 2: 326. https://doi.org/10.3390/app9020326





