Lithium Distribution in Structured Graphite Anodes Investigated by Laser-Induced Breakdown Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Electrode Material
2.2. Ultrafast Laser Processing
2.3. Electrochemical Analysis
2.4. Laser-Induced Breakdown Spectroscopy (LIBS)
3. Results and Discussion
3.1. Generation of Line Structures and Micro Freestanding Pillars
3.2. Electrochemical Characterization
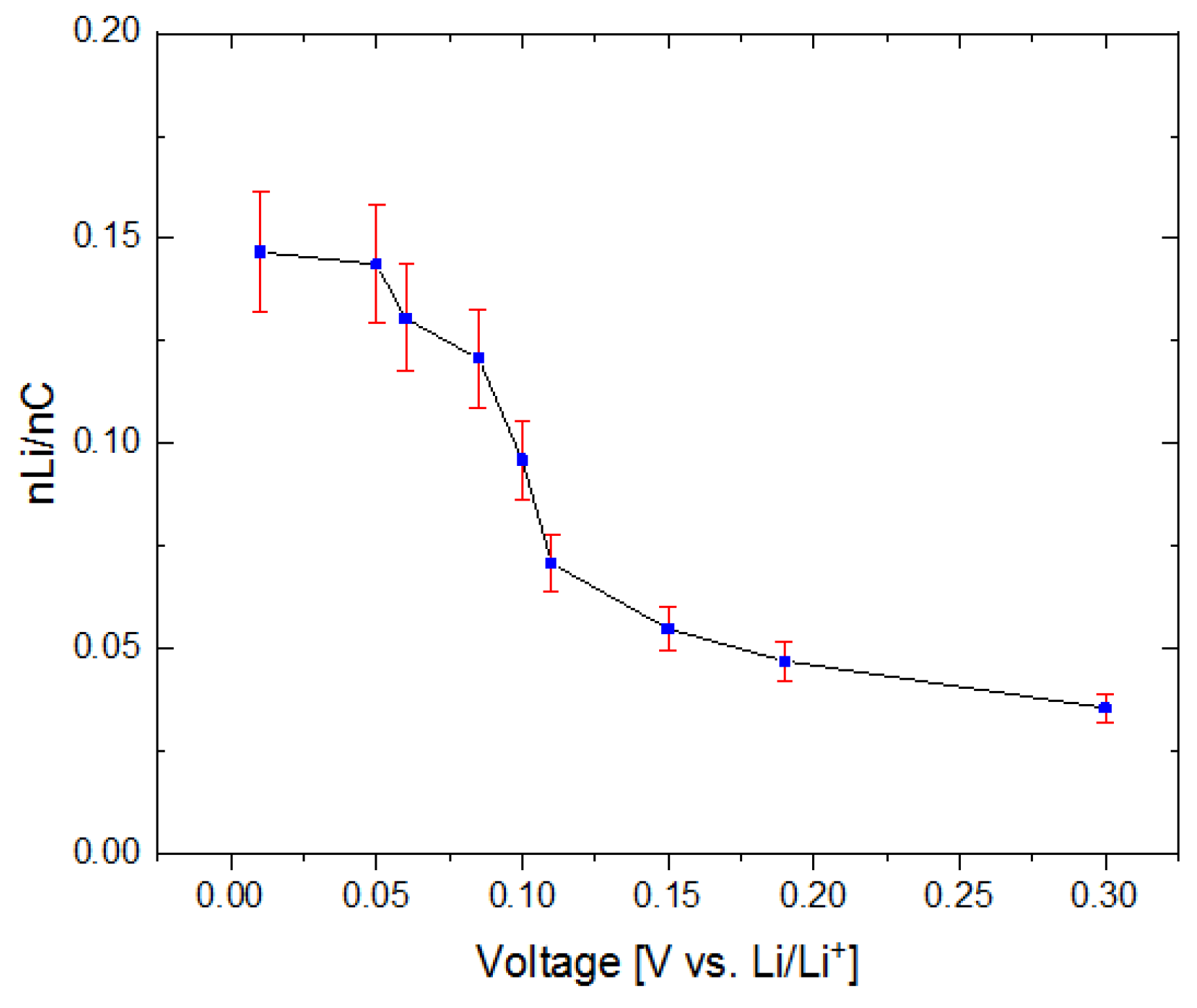
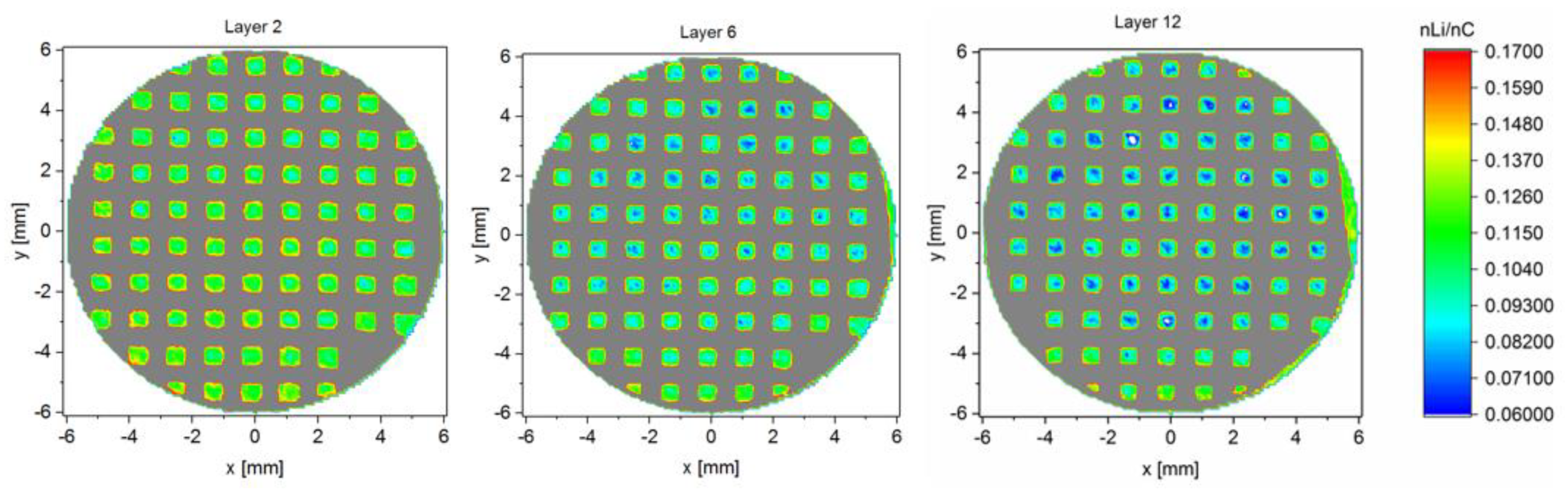
3.3. Analysis of Lithium Distribution
3.3.1. Calibration of LIBS Spectrum
3.3.2. Lithium Distribution within Structured and Unstructured Electrodes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeyen, M. High Power Charging: Schnell, schneller, am schnellsten? eMobil J. Die Fachz. für Smart Mobil. 2018, 30–35. [Google Scholar]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-Dimensional Battery Architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Johns, P.; Owen, J.; Brandell, D.; Edstrom, K.; El Enany, G.; Guery, C.; Golodnitsky, D.; Lacey, M.; Lecoeur, C.; et al. 3D lithium ion batteries—From fundamentals to fabrication. J. Mater. Chem. 2011, 21, 9876. [Google Scholar] [CrossRef]
- Li, J.; Daniel, C.; Wood, D. Materials processing for lithium-ion batteries. J. Power Sources 2011, 196, 2452–2460. [Google Scholar] [CrossRef]
- Kim, H.; Auyeung, R.C.Y.; Piqué, A. Laser-printed thick-film electrodes for solid-state rechargeable Li-ion microbatteries. J. Power Sources 2007, 165, 413–419. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Li, H.; Zhai, T.; Zhou, H. Hierarchical micro/nano porous silicon Li-ion battery anodes. Chem. Commun. (Camb.) 2012, 48, 5079–5081. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Oudenhoven, J.F.M.; Li, D.; Chen, C.; Eichel, R.-A.; Notten, P.H.L. High Power and High Capacity 3D-Structured TiO 2 Electrodes for Lithium-Ion Microbatteries. J. Electrochem. Soc. 2016, 163, A2385–A2389. [Google Scholar] [CrossRef]
- Fleischauer, M.D.; Li, J.; Brett, M.J. Columnar Thin Films for Three-Dimensional Microbatteries. Electrochim. Acta 2009, 156, A33. [Google Scholar] [CrossRef]
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573. [Google Scholar] [CrossRef]
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926. [Google Scholar] [CrossRef]
- Proell, J. 3D Structures in Battery Materials. JLMN 2012, 7, 97–104. [Google Scholar] [CrossRef]
- Smyrek, P.; Bergfeldt, T.; Seifert, H.J.; Pfleging, W. Laser-induced breakdown spectroscopy for the quantitative measurement of lithium concentration profiles in structured and unstructured electrodes. J. Mater. Chem. A 2019, 7, 5656–5665. [Google Scholar] [CrossRef] [Green Version]
- Smyrek, P.; Pröll, J.; Seifert, H.J.; Pfleging, W. Laser-Induced Breakdown Spectroscopy of Laser-Structured Li(NiMnCo)O 2 Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A19–A26. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Ion transport restriction in mechanically strained separator membranes. J. Power Sources 2013, 226, 149–155. [Google Scholar] [CrossRef]
- Zheng, Y.; Seifert, H.J.; Shi, H.; Zhang, Y.; Kübel, C.; Pfleging, W. 3D silicon/graphite composite electrodes for high-energy lithium-ion batteries. Electrochim. Acta 2019, 317, 502–508. [Google Scholar] [CrossRef]
- Zheng, Y.; Seifert, H.J.; Smyrek, P.; Pfleging, W. Development of Laser Structured Silicon-based Anodes for Lithium-ion Batteries. In Proceedings of the 2018 IEEE International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale (3M-NANO), Hangzhou, China, 13–17 August 2018; pp. 6–9, ISBN 978-1-5386-6214-4. [Google Scholar]
- Levi, M.D.; Aurbach, D. The mechanism of lithium intercalation in graphite film electrodes in aprotic media. Part 1. High resolution slow scan rate cyclic voltammetric studies and modeling. J. Electroanal. Chem. 1997, 421, 79–88. [Google Scholar] [CrossRef]
- Müller, M. Neue Wege zur Quantifizierung mit der Laserinduzierten Plasmaspektroskopie (LIBS). Dissertation, Bundesanstalt für Materialforschung und -prüfung (BAM), Berlin, Germany, 2010. [Google Scholar]
- Kramida, A.; Ralchenko, Y.; Reader, J.; NIST ASD Team. NIST Atomic Spectra Database, NIST Standard Reference Database 78; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2019. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Gor, G.Y.; Cannarella, J.; Prévost, J.H.; Arnold, C.B. A Model for the Behavior of Battery Separators in Compression at Different Strain/Charge Rates. J. Electrochem. Soc. 2014, 161, F3065–F3071. [Google Scholar] [CrossRef] [Green Version]





| Cell State for LIBS Measurements | Step 1 Formation | Step 2 | Step 3 | Step 4 | Step 5 before Cell Disassembly |
|---|---|---|---|---|---|
| Lithiated state at 0.01 V (C/20) | Charging/Discharging*: C/20 Cycles: 3 | - | - | - | Discharging: C/20, CV at 0.01 V for 10 min |
| Lithiated state at 0.01 V (1C) | Charging/Discharging*: 1C Cycles: 3 | Charging/Discharging*: C/5 Cycles: 1 | Charging/Discharging*: 1C Cycles: 1 | Discharging: 1C, CV at 0.01 V for 10 min | |
| Delithiated state at 1 V (1C) | Charging/Discharging*: 1C Cycles: 2 | CV for 10 min after last charging to 1 V |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Pfäffl, L.; Seifert, H.J.; Pfleging, W. Lithium Distribution in Structured Graphite Anodes Investigated by Laser-Induced Breakdown Spectroscopy. Appl. Sci. 2019, 9, 4218. https://doi.org/10.3390/app9204218
Zheng Y, Pfäffl L, Seifert HJ, Pfleging W. Lithium Distribution in Structured Graphite Anodes Investigated by Laser-Induced Breakdown Spectroscopy. Applied Sciences. 2019; 9(20):4218. https://doi.org/10.3390/app9204218
Chicago/Turabian StyleZheng, Yijing, Lisa Pfäffl, Hans Jürgen Seifert, and Wilhelm Pfleging. 2019. "Lithium Distribution in Structured Graphite Anodes Investigated by Laser-Induced Breakdown Spectroscopy" Applied Sciences 9, no. 20: 4218. https://doi.org/10.3390/app9204218






