Compression of Biomass Substances—A Study on Springback Effects and Color Formation in Pellet Manufacture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compression, Springback and Mechanical Properties
2.1.1. Test Equipment
2.1.2. Test Procedure
2.1.3. Measurements
2.2. Color Occurrence in Polysaccharides
3. Results
3.1. Compression, Springback and Mechanical Properties
3.2. Color Occurrence in Flexible Polysaccharides
4. Discussion
4.1. Compression, Mechanical Properties and Springback
4.2. Color Occurrence
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stelte, W.; Sanadi, A.R.; Shang, L.; Holm, J.K.; Ahrenfeldt, J.; Henriksen, U.B. Recent developments in biomass pelletization—A Review. BioResources 2012, 7, 4451–4490. [Google Scholar]
- Tumuluru, J.S.; Wright, C.T.; Kenny, K.L.; Hess, J.R. A review on biomass densification technologies for energy application. Ida. Natl. Lab. 2010, 85. [Google Scholar] [CrossRef]
- Nielsen, N.P.K. Importance of Raw Material Properties in Wood Pellet Production—Effects of Differences in Wood Properties for the Energy Requirements of Pelletizing and the Pellet Quality. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2009. [Google Scholar]
- Frodeson, S.; Henriksson, G.; Berghel, J. Pelletizing Pure Biomass Substances to Investigate the Mechanical Properties and Bonding Mechanisms. BioResources 2018, 13, 1202–1222. [Google Scholar] [CrossRef]
- Nielsen, N.P.K.; Gardner, D.J.; Poulsen, T.; Felby, C. Importance of temperature, moisture content, and species for the conversion process of wood residues into fuel pellets. Wood Fiber Sci. 2009, 41, 414–425. [Google Scholar]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Effects of compressive force, particle size and moisture content on mechanical properties of biomass pellets from grasses. Biomass Bioenergy 2006, 30, 648–654. [Google Scholar] [CrossRef]
- Ramírez-Gómez, Á. Research needs on biomass characterization to prevent handling problems and hazards in industry. Part. Sci. Technol. 2016, 34, 432–441. [Google Scholar] [CrossRef]
- Tumuluru, J.S.; Wright, C.T.; Hess, J.R.; Kenney, K.L. A review of biomass densification systems to develop uniform feedstock commodities for bioenergy application. Biofuels Bioprod. Biorefining 2011, 5, 683–707. [Google Scholar] [CrossRef]
- Mani, S.; Tabil, L.G.; Sokhansanj, S. Evaluation of compaction equations applied to four mass species. Can. Biosyst. Eng. 2004, 46, 55–61. [Google Scholar]
- O’Dogherty, M.J. A review of the mechanical behaviour of straw when compressed to high densities. J. Agric. Eng. Res. 1989, 44, 241–265. [Google Scholar] [CrossRef]
- Dhamodaran, A.; Afzal, M.T. Compression and springback properties of hardwood and softwood pellets. BioResources 2012, 7, 4362–4376. [Google Scholar]
- Wongsiriamnuay, T.; Tippayawong, N. Effect of densification parameters on the properties of maize residue pellets. Biosyst. Eng. 2015, 139, 111–120. [Google Scholar] [CrossRef]
- Kaliyan, N.; Morey, R.V. Constitutive model for densification of corn stover and switchgrass. Biosyst. Eng. 2009, 104, 47–63. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions, 2nd ed.; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Rydholm, S.A. Pulping Processes; Robert Krieger Publishing Co.: Malabar, Indian, 1985. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Frodeson, S.; Henriksson, G.; Berghel, J. Effects of moisture content during densification of biomass pellets, focusing on polysaccharide substances. Biomass Bioenergy 2019, 122, 322–330. [Google Scholar] [CrossRef]
- Lam, P.S.; Sokhansanj, S.; Bi, X.; Lim, C.J.; Melin, S. Energy input and quality of pellets made from steam-exploded Douglas fir (Pseudotsuga menziesii). Energy Fuels 2011, 25, 1521–1528. [Google Scholar] [CrossRef]
- Lam, P.; Sokhansanj, S.; Bi, X.; Lim, C. Colorimetry applied to steam-treated biomass and pellets made from western Douglas fir (Pseudotsuga menziesii L.). Trans. Asabe 2012, 55, 673–678. [Google Scholar] [CrossRef]
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2008, 4, 370–404. [Google Scholar]
- Boonstra, M.J.; Tjeerdsma, B. Chemical analysis of heat treated softwoods. Holz Als Roh-Und Werkst. 2006, 64, 204. [Google Scholar] [CrossRef]
- Srinivas, K.; Pandey, K.K. Effect of Heat Treatment on Color Changes, Dimensional Stability, and Mechanical Properties of Wood. J. Wood Chem. Technol. 2012, 32, 304–316. [Google Scholar] [CrossRef]
- Sehlstedt-Persson, M. Colour responses to heat-treatment of extractives and sap from pine and spruce. In Proceedings of the International IUFRO Wood Drying Conference, Brasov, Romania, 25–29 August 2003; pp. 459–464. [Google Scholar]
- McDonald, A.; Fernandez, M.; Kreber, B. Chemical and UV-VIS spectroscopic study on kiln brown stain formation in Radiata pine. In Proceedings of the 9th International Symposium of Wood and Pulping Chemistry, Montreal, QC, Canada, 9–12 June 1997; pp. 1–5. [Google Scholar]
- Sundqvist, B.; Morén, T. The influence of wood polymers and extractives on wood colour induced by hydrothermal treatment. Holz Als Roh-Und Werkst. 2002, 60, 375–376. [Google Scholar] [CrossRef]
- Bekhta, P.; Niemz, P. Effect of High Temperature on the Change in Color, Dimensional Stability and Mechanical Properties of Spruce Wood. Holzforschung 2003, 57, 539. [Google Scholar] [CrossRef]
- Mitsui, K.; Inagaki, T.; Tsuchikawa, S. Monitoring of Hydroxyl Groups in Wood during Heat Treatment Using NIR Spectroscopy. Biomacromolecules 2008, 9, 286–288. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R. The Initial Phase of Sodium Sulfite Pulping of Softwood: A Comparison of Different Pulping Options; Karlstad University: Karlstad, Sweden, 2016. [Google Scholar]
- Salmén, L.; Olsson, A.-M. Interactions between hemicelluloses, lignin and cellulose: Structure-Properties relationships. J. Pulp Pap. Sci. 1998, 24, 99–103. [Google Scholar]
- Liggett, R.W.; Deitz, V.R. Color and turbidity of sugar products. In Advances in Carbohydrate Chemistry; Elsevier: Amsterdam, The Netherlands, 1954; Volume 9, pp. 247–284. [Google Scholar]
- Kroh, L.W. Caramelisation in food and beverages. Food Chem. 1994, 51, 373–379. [Google Scholar] [CrossRef]
- De Bruijn, J.M.; Kieboom, A.P.G. Reactions of monosaccharides in aqueous alkaline solutions. [A review]. Sugar Technol. Rev. 1986, 13. [Google Scholar]
- Kroh, L.W.; Fiedler, T.; Wagner, J. α-Dicarbonyl Compounds—Key Intermediates for the Formation of Carbohydrate-based Melanoidins. Ann. N. Y. Acad. Sci. 2008, 1126, 210–215. [Google Scholar] [CrossRef]
- Kamuf, W.; Nixon, A.; Parker, O.; Barnum, G.C.; Willamson, D.D. Overview of caramel colors. Cereal Foods World 2003, 48, 64–69. [Google Scholar]
- Luna, M.P.; Aguilera, J.M. Kinetics of colour development of molten glucose, fructose and sucrose at high temperatures. Food Biophys. 2014, 9, 61–68. [Google Scholar] [CrossRef]
- Kocadağlı, T.; Gökmen, V. Multiresponse kinetic modelling of Maillard reaction and caramelisation in a heated glucose/wheat flour system. Food Chem. 2016, 211, 892–902. [Google Scholar] [CrossRef]
- Belkacemi, K.; Abatzoglou, N.; Overend, R.P.; Chornet, E. Phenomenological kinetics of complex systems: Mechanistic considerations in the solubilization of hemicelluloses following aqueous/steam treatments. Ind. Eng. Chem. Res. 1991, 30, 2416–2425. [Google Scholar] [CrossRef]
- Teleman, A.; Siika-aho, M.; Sorsa, H.; Buchert, J.; Perttula, M.; Hausalo, T.; Tenkanen, M. 4-O-Methyl-β-idopyranosyluronic acid linked to xylan from kraft pulp: Isolation procedure and characterisation by NMR spectroscopy. Carbohydr. Res. 1996, 293, 1–13. [Google Scholar] [CrossRef]
- Pettersen, R.C. The chemical composition of wood. Chem. Solid Wood 1984, 207, 57–126. [Google Scholar]
- Claude, J.; Ubbink, J. Thermal degradation of carbohydrate polymers in amorphous states: A physical study including colorimetry. Food Chem. 2006, 96, 402–410. [Google Scholar] [CrossRef]
- Whittaker, C.; Shield, I. Factors affecting wood, energy grass and straw pellet durability—A review. Renew. Sustain. Energy Rev. 2017, 71, 1–11. [Google Scholar] [CrossRef]
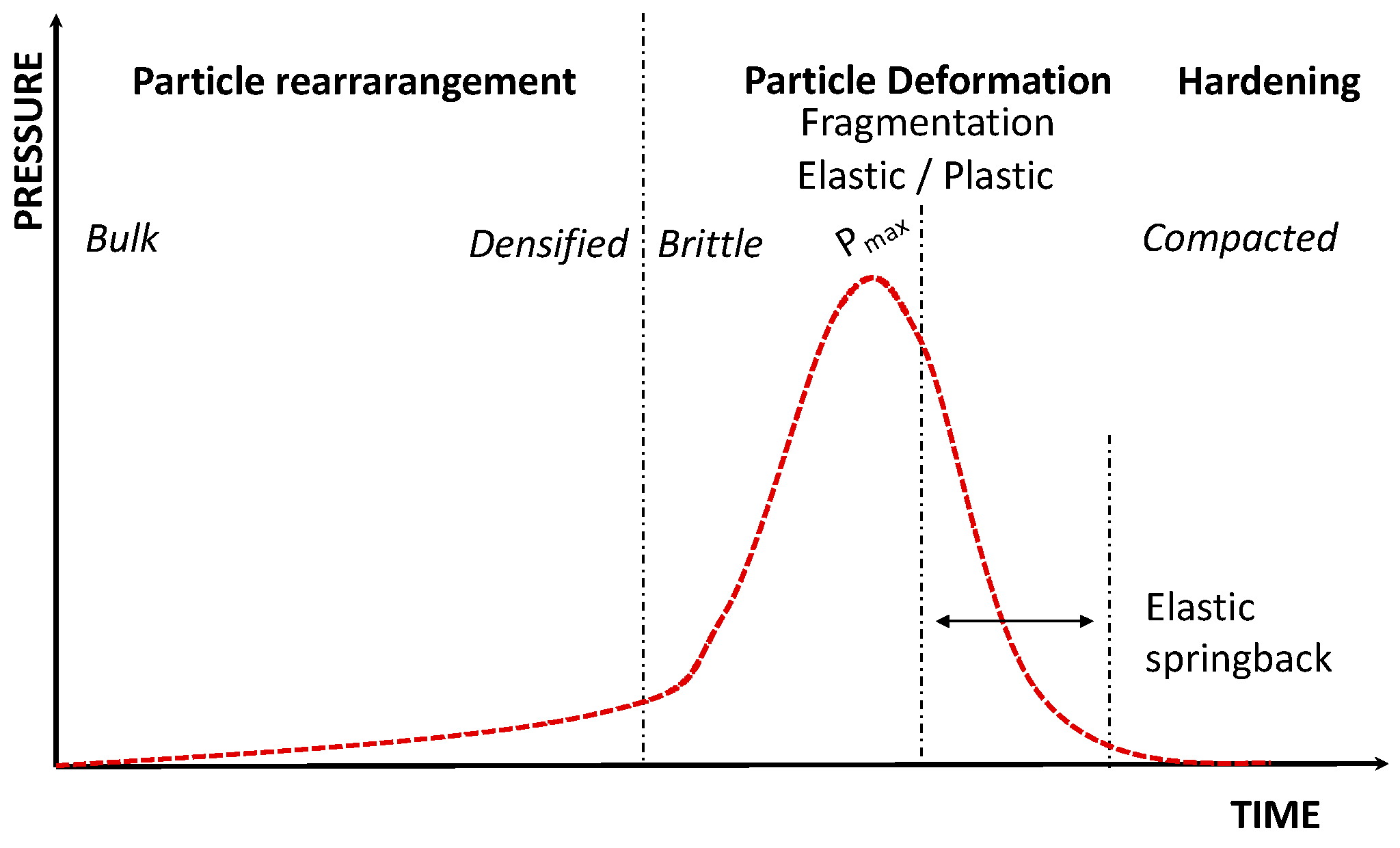

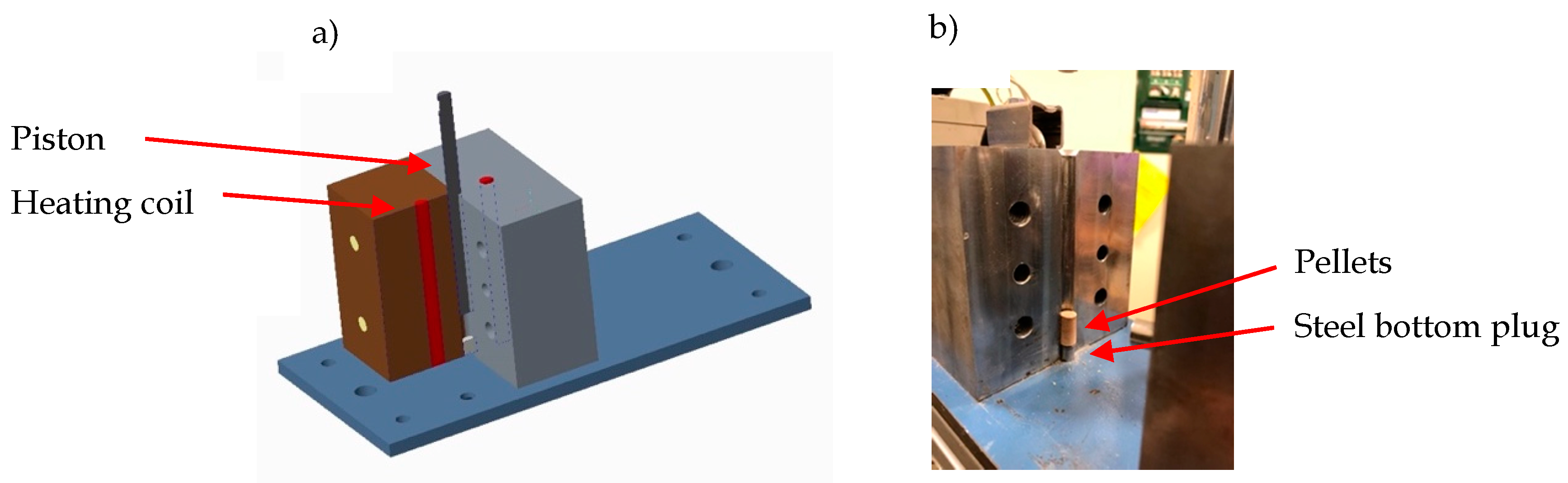



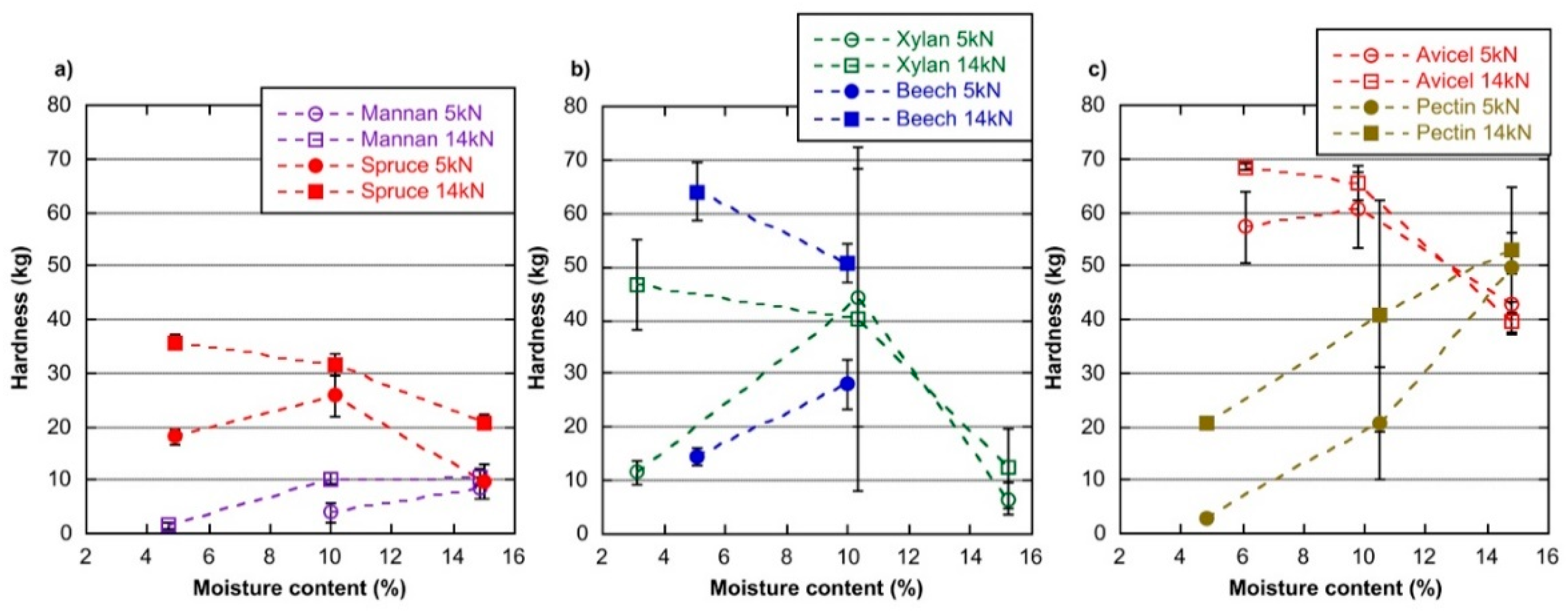


| Test | Name | Origin and Comment |
|---|---|---|
| T1 | Avicel | Sigma-Aldrich (Darmstadt, Germany), Avicel®PH-101 product number 11365; short-chain highly ordered cellulose from hydrolyzed hardwood chemical pulp |
| T2 | Locust bean gum mannan | Sigma-Aldrich, product number G0753; galactomannan polysaccharide from locust bean seeds with large similarities to the hemicellulose glucomannan |
| T3 | Eucalypt xylan | The hemicellulose xylan from a hardwood, delivered by KTH Royal Institute of Technology |
| T4 | Apple pectin | Sigma-Aldrich, product number 93854; pectin from apples, probably rather similar to wood pectin |
| T5 | Spruce wood | Norway spruce (Picea abies) representative reference from softwood |
| T6 | Beech wood | European beech (Fagus sylvatica) representative reference from hardwood |
| Test | Eucalypt xylan | CMC |
|---|---|---|
| 1 | Pure original powder | Pure original powder |
| 2 | 5 kN 5% MC | 5 kN 5% MC |
| 3 | 5 kN 10% MC | 5 kN 10% MC |
| 4 | 5 kN 15% MC | 5 kN 15% MC |
| 5 | 14 kN 5% MC | 14 kN 5% MC |
| 6 | 14 kN 10% MC | 14 kN 10% MC |
| 7 | 14 kN 15 % MC | 14 kN 15% MC |
| 8 | Heated 5% MC | Heated 5% MC |
| 9 | Heated 15% MC | Heated 15% MC |
| Inlet MC | Pellet | |||||||
|---|---|---|---|---|---|---|---|---|
| Wcomp | Weight | Diameter (mm) | Length (mm) | |||||
| Test | (%) | (J/g) | (g) | Green | Cured | Comp | Green | Cured |
| T15kN5% | 6.10 | 31.35 ± 1.88 | 0.970 ± 0.023 | 8.26 ± 0.0075 | 8.23 ± 0.0075 | 13.56 ± 0.43 | 13.87 ± 0.40 | 13.78 ± 0.41 |
| T15kN10% | 9.80 | 25.12 ± 1.66 | 1.001 ± 0.008 | 8.27 ± 0.034 | 8.26 ± 0.034 | 12.57 ± 0.11 | 13.44 ± 0.11 | 13.40 ± 0.09 |
| T15kN15% | 14.80 | 21.5 ± 2.88 | 1.001 ±0.010 | 8.27 ± 0.0082 | 8.23 ± 0.015 | 12.73 ± 0.20 | 13.44 ± 0.23 | 13.34 ± 0.23 |
| T25kN5% | 4.70 | 23.36 ± 6.56 | 0.789 ± 0.322 | 8.34 ± 0.070 | - | 14.75 ± 1.08 | 17.09 ± 0.68 | - |
| T25kN10% | 10.0 | 17.30 ± 0.62 | 1.015 ± 0.037 | 8.28 ± 0.047 | 8.28 ± 0.0098 | 13.35 ± 0.59 | 15.58 ± 0.65 | 15.23 ± 0.64 |
| T25kN15% | 14.90 | 9.77 ± 1.14 | 0.994 ± 0.031 | 8.23 ± 0.049 | 8.22 ± 0.029 | 12.48 ± 0.33 | 16.15 ± 0.47 | 16.25 ± 0.67 |
| T35kN5% | 3.10 | 16.46 ± 0.84 | 0.986 ± 0.059 | 8.28 ± 0.022 | 8.26 ± 0.021 | 14.45 ± 1.01 | 15.21 ± 0.99 | 15.14 ± 1.02 |
| T35kN10% | 10.30 | 25.21 ± 1.28 | 0.986 ± 0.034 | 8.28 ± 0.014 | 8.24 ± 0.012 | 11.62 ± 0.52 | 12.22 ± 0.48 | 12.16 ± 0.50 |
| T35kN15% | 15.20 | 15.40 ± 0.98 | 0.970 ± 0.032 | 8.29 ± 0.0084 | 8.25 ± 0.0075 | 11.58 ± 0.43 | 12.17 ± 0.42 | 12.13 ± 0.43 |
| T45kN5% | 4.80 | 22.93 ± 1.97 | 0.912 ± 0.039 | 8.35 ± 0.017 | 8.39 ± 0.023 | 16.28 ± 0.37 | 17.53 ± 1.68 | 16.25 ± 0.67 |
| T45kN10% | 10.50 | 19.92 ± 1.29 | 1.029 ± 0.043 | 8.28 ± 0.027 | 8.22 ± 0.023 | 15.10 ± 0.86 | 16.45 ± 0.82 | 16.27 ± 0.84 |
| T45kN15% | 14.80 | 15.06 ± 2.51 | 1.015 ± 0.037 | 8.22 ± 0.019 | 8.19 ± 0.012 | 12.96 ± 0.82 | 13.97 ± 0.79 | 13.91 ± 0.81 |
| T55kN5% | 4.90 | 24.80 ± 0.71 | 1.006 ± 0.019 | 8.34 ± 0.027 | 8.32 ± 0.023 | 14.57 ± 0.19 | 17.03 ± 0.23 | 16.93 ± 0.21 |
| T55kN10% | 10.10 | 20.25 ± 1.81 | 0.981 ± 0.018 | 8.40 ± 0.20 | 8.31 ± 0.017 | 13.27 ± 0.34 | 16.11 ± 0.31 | 15.92 ± 0.29 |
| T55kN15% | 15.0 | 16.69 ± 0.94 | 0.977 ± 0.015 | 8.46 ± 0.057 | 8.44 ± 0.063 | 13.16 ± 0.26 | 18.66 ± 0.71 | 18.43 ± 0.67 |
| T65kN5% | 5.10 | 29.19 ± 2.76 | 1.020 ± 0.025 | 8.29 ± 0.021 | 8.30 ± 0.021 | 16.31 ± 0.48 | 18.61 ± 0.69 | 18.67 ± 0.60 |
| T65kN10% | 10.0 | 15.65 ± 2.81 | 1.002 ± 0.012 | 8.34 ± 0.025 | 8.40 ± 0.039 | 14.20 ± 0.18 | 18.60 ± 0.33 | 18.34 ± 0.41 |
| T114kN5% | 6.10 | 55.70 ± 3.86 | 1.017 ± 0.025 | 8.28 ± 0.017 | 8.26 ± 0.016 | 12.96 ± 0.33 | 12.83 ± 0.35 | 12.75 ± 0.34 |
| T114N10% | 9.80 | 40.91 ± 1.97 | 0.989 ± 0.029 | 8.30 ± 0.053 | 8.30 ± 0.038 | 12.02 ± 0.38 | 12.61 ± 0.50 | 12.5 ± 0.48 |
| T114kN15% | 14.80 | 47.53 ± 5.67 | 0.900 ± 0.024 | 8.32 ± 0.063 | 8.33 ± 0.025 | 11.26 ± 0.51 | 11.55 ± 0.26 | 11.61 ± 0.27 |
| T214kN5% | 4.70 | 45.82 ± 1.93 | 1.000 ± 0.023 | 8.44 ± 0.037 | 8.39 ± 0.027 | 13.08 ± 0.33 | 14.76 ± 0.35 | 13.22 ± 0.78 |
| T214kN10% | 10.0 | 37.98 ± 0.58 | 1.035 ± 0.054 | 8.32 ± 0.031 | 8.30 ± 0.037 | 12.58 ± 0.66 | 15.03 ± 0.80 | 14.85 ± 0.82 |
| T214kN15% | 14.90 | 22.6 ± 1.03 | 0.981 ± 0.039 | 8.25 ± 0.015 | 8.19 ± 0.023 | 12.25 ± 0.56 | 16.05 ± 0.61 | 16.00 ± 0.71 |
| T314kN5% | 3.10 | 45.18 ± 1.90 | 0.960 ± 0.080 | 8.29 ± 0.018 | 8.29 ± 0.016 | 11.19 ± 1.10 | 11.70 ± 1.02 | 11.67 ± 1.04 |
| T314kN10% | 10.30 | 44.54 ± 2.32 | 0.930 ± 0.009 | 8.33 ± 0.015 | 8.31 ± 0.017 | 10.90 ± 0.10 | 11.36 ± 0.07 | 11.34 ± 0.08 |
| T314kN15% | 15.20 | 29.01 ± 1.74 | 0.820 ± 0.101 | 8.31 ± 0.072 | 8.29 ± 0.065 | 10.03 ± 1.11 | 11.69 ± 0.27 | 11.56 ± 0.39 |
| T414kN5% | 4.80 | 57.38 ± 11.59 | 1.009 ± 0.083 | 8.38 ± 0.0071 | 8.28 ± 0.057 | 14.49 ± 2.24 | 15.66 ± 2.57 | 14.60 ± 1.09 |
| T414kN10% | 10.50 | 38.84 ± 7.33 | 1.003 ± 0.044 | 8.27 ± 0.047 | 8.26 ± 0.043 | 12.23 ± 0.54 | 13.89 ± 0.72 | 13.82 ± 0.72 |
| T414kN15% | 14.80 | 37.16 ± 4.83 | 0.974 ± 0.016 | 8.22 ± 0.017 | 8.21 ± 0.019 | 11.64 ± 0.21 | 12.78 ± 0.18 | 12.77 ± 0.20 |
| T514kN5% | 4.90 | 47.65 ± 6.37 | 1.002 ± 0.016 | 8.32 ± 0.014 | 8.31 ± 0.0075 | 12.92 ± 0.22 | 15.04 ± 0.25 | 14.89 ± 0.28 |
| T514kN10% | 10.10 | 38.27 ± 1.53 | 1.007 ± 0.014 | 8.33 ± 0.017 | 8.33 ± 0.018 | 12.95 ± 0.20 | 15.83 ± 0.20 | 15.64 ± 0.20 |
| T514kN15% | 15.0 | 33.35 ± 1.80 | 0.972 ± 0.024 | 8.46 ± 0.043 | 8.43 ± 0.035 | 12.70 ± 0.32 | 17.64 ± 0.71 | 17.25 ± 0.70 |
| T614kN5% | 5.10 | 58.01 ± 1.77 | 1.004 ± 0.020 | 8.29 ± 0.022 | 8.35 ± 0.025 | 13.56 ± 0.43 | 13.87 ± 0.40 | 13.78 ± 0.41 |
| T614kN10% | 10.0 | 37.72 ± 2.59 | 1.000 ± 0.017 | 8.35 ± 0.032 | 8.34 ± 0.018 | 12.57 ± 0.11 | 13.44 ± 0.11 | 13.40 ± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frodeson, S.; Lindén, P.; Henriksson, G.; Berghel, J. Compression of Biomass Substances—A Study on Springback Effects and Color Formation in Pellet Manufacture. Appl. Sci. 2019, 9, 4302. https://doi.org/10.3390/app9204302
Frodeson S, Lindén P, Henriksson G, Berghel J. Compression of Biomass Substances—A Study on Springback Effects and Color Formation in Pellet Manufacture. Applied Sciences. 2019; 9(20):4302. https://doi.org/10.3390/app9204302
Chicago/Turabian StyleFrodeson, Stefan, Pär Lindén, Gunnar Henriksson, and Jonas Berghel. 2019. "Compression of Biomass Substances—A Study on Springback Effects and Color Formation in Pellet Manufacture" Applied Sciences 9, no. 20: 4302. https://doi.org/10.3390/app9204302





