Multi-Perspective Ultrasound Imaging Technology of the Breast with Cylindrical Motion of Linear Arrays
Abstract
:1. Introduction
2. Experiments and Methods
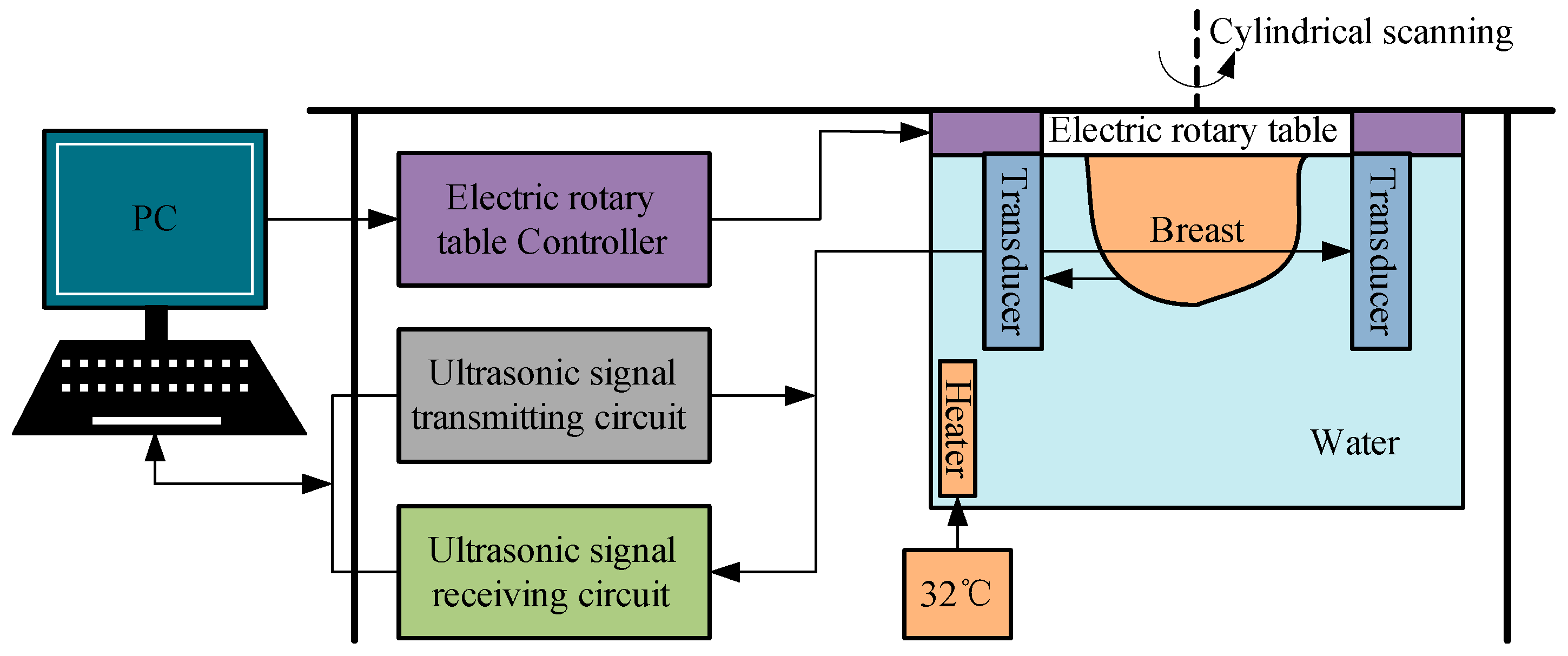
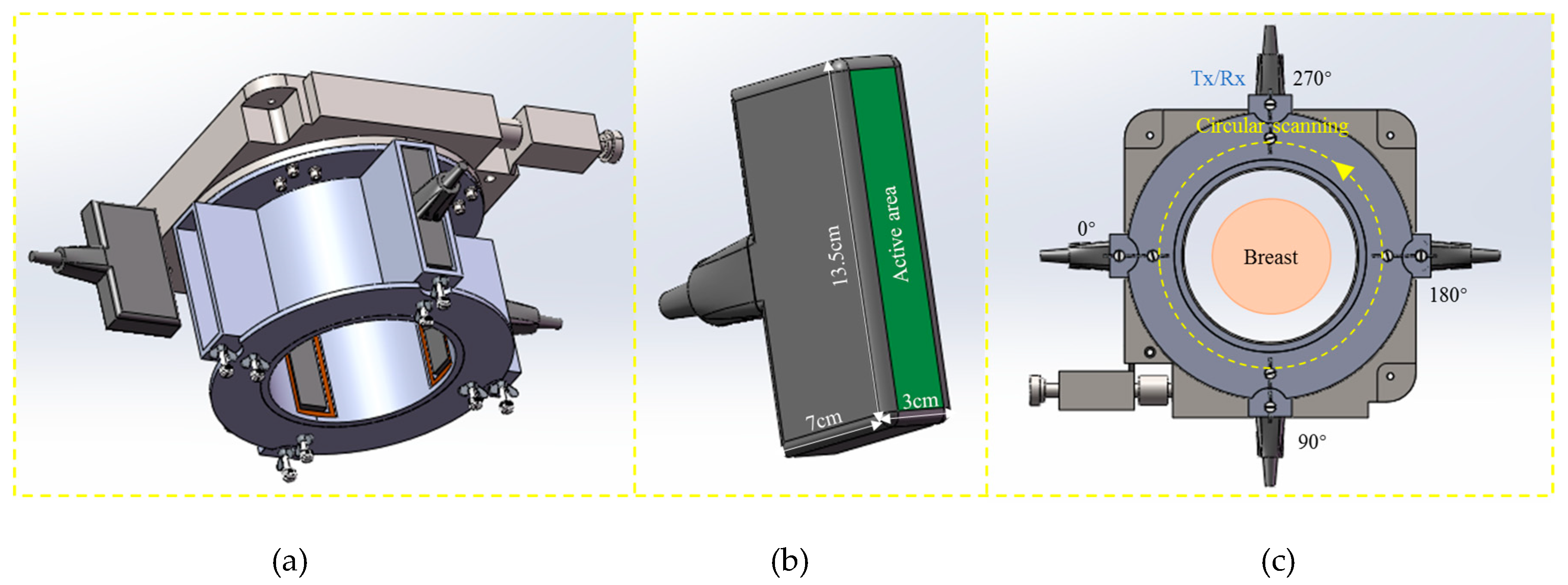
2.1. 3D Imaging System with Cylindrical Motion of Linear Arrays
2.2. Multi-Perspective Ultrasound Imaging
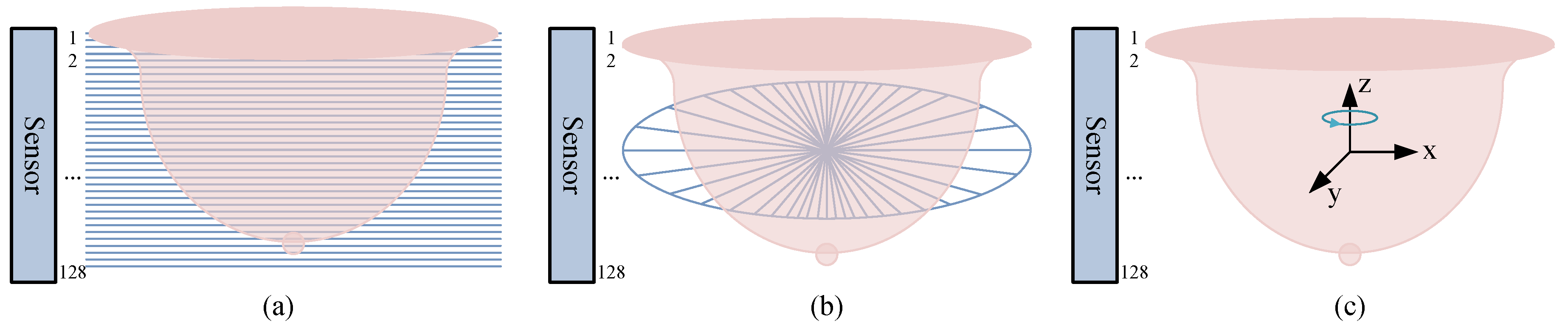
2.2.1. Vertical Slices
2.2.2. Horizontal Slices
2.2.3. 3D Imaging
3. Principles
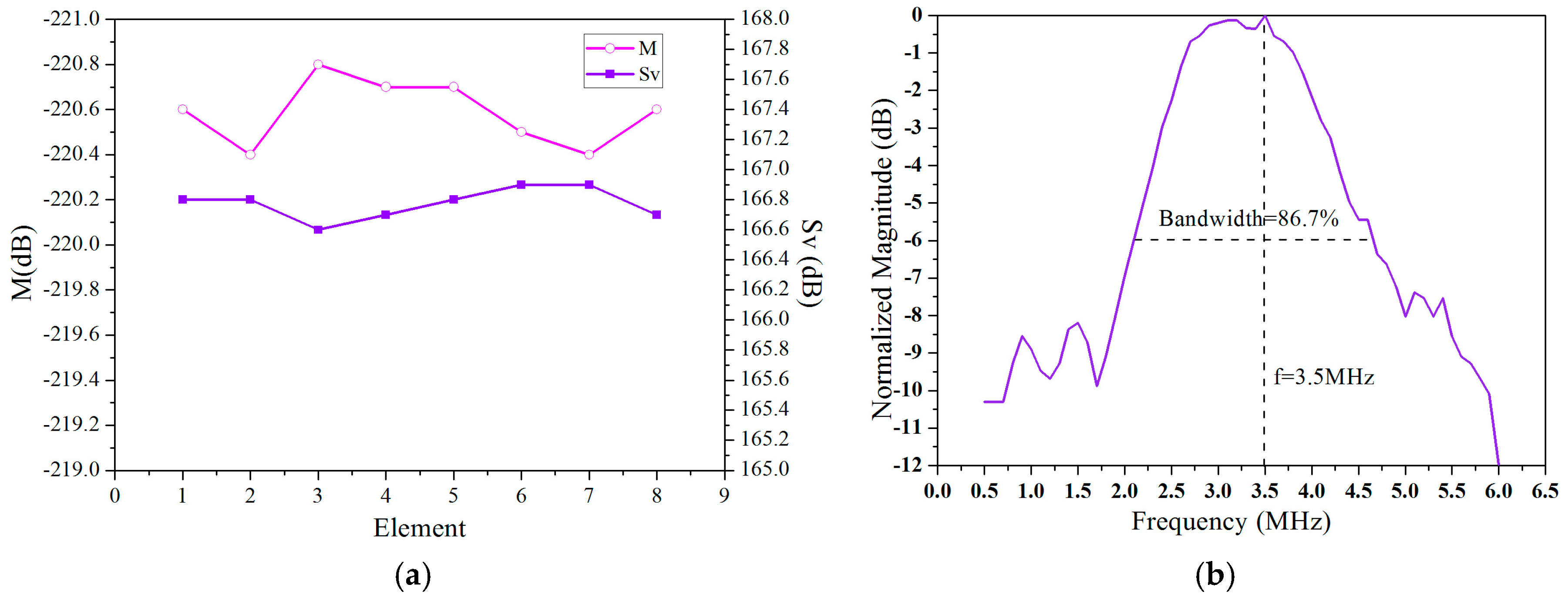
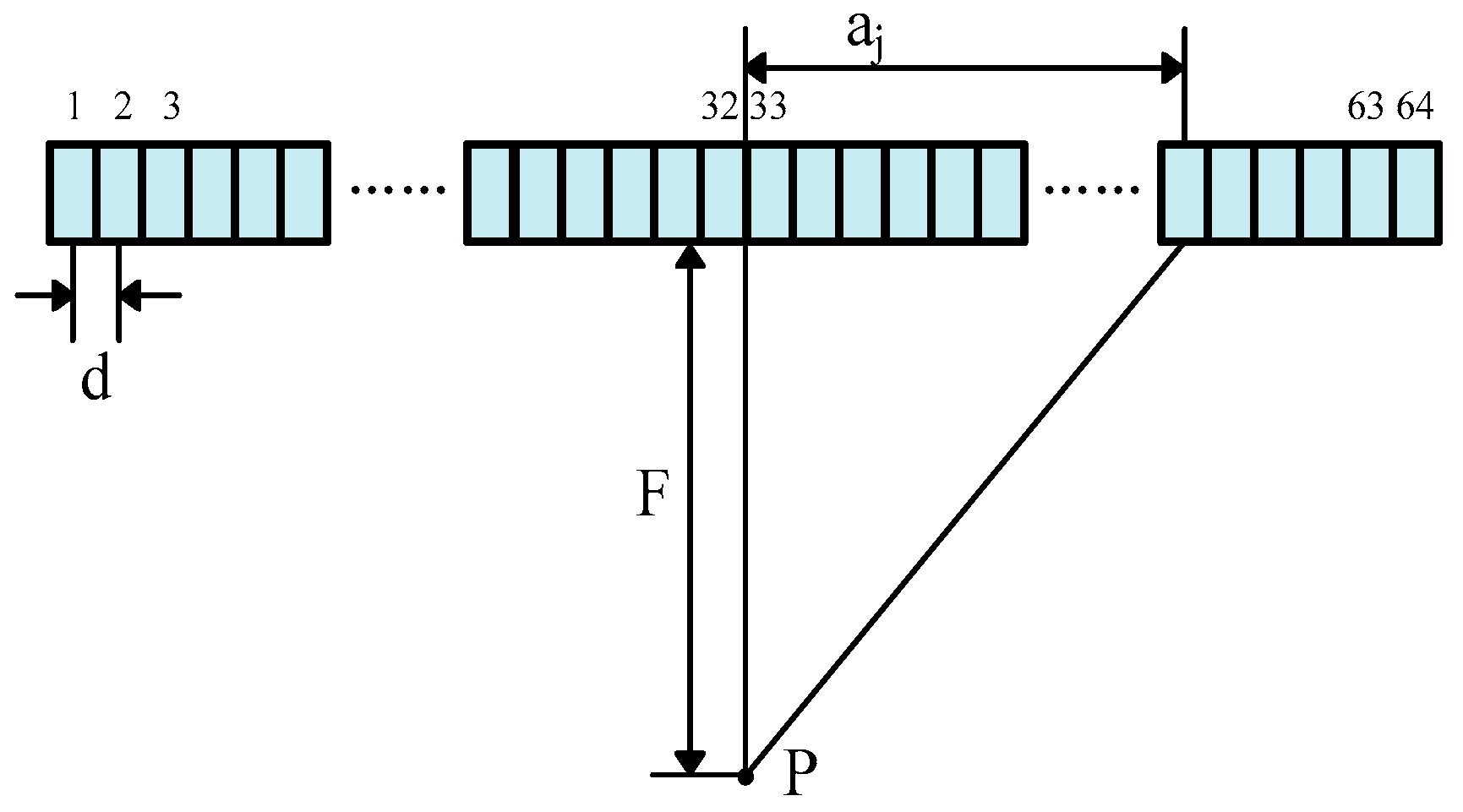
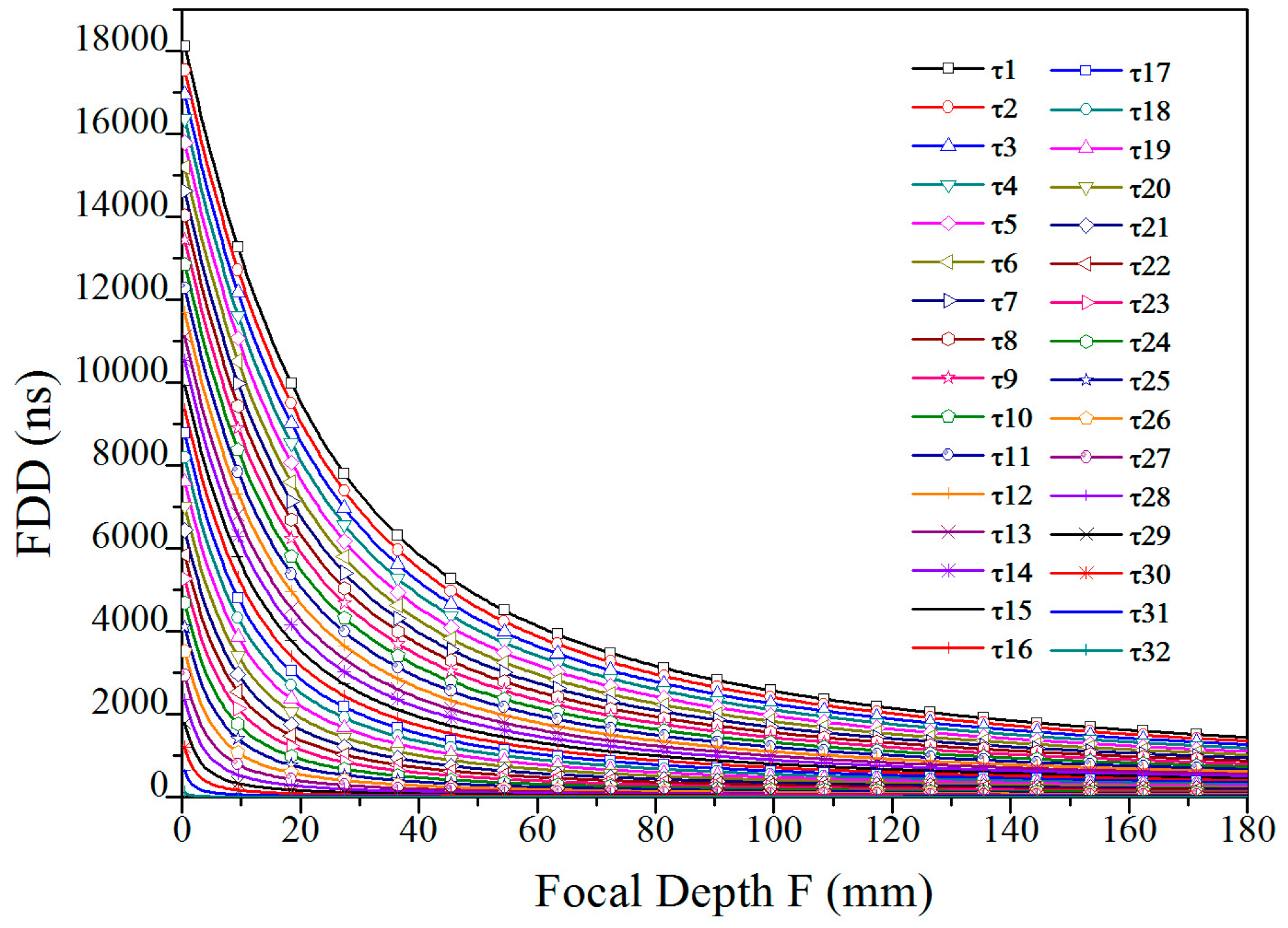
3.1. Focusing Delay Calculation
3.2. Delay Data Analysis
4. Results
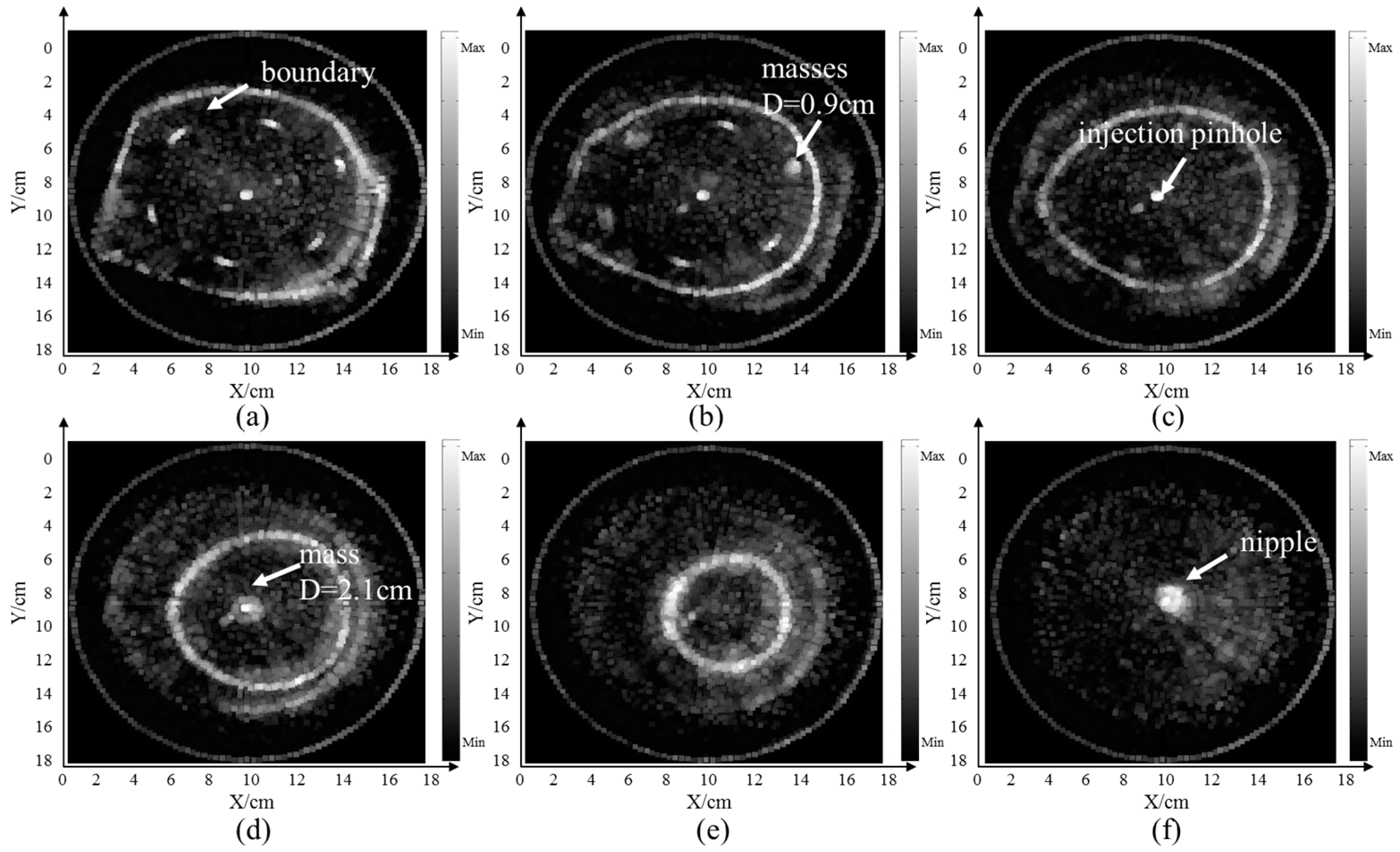
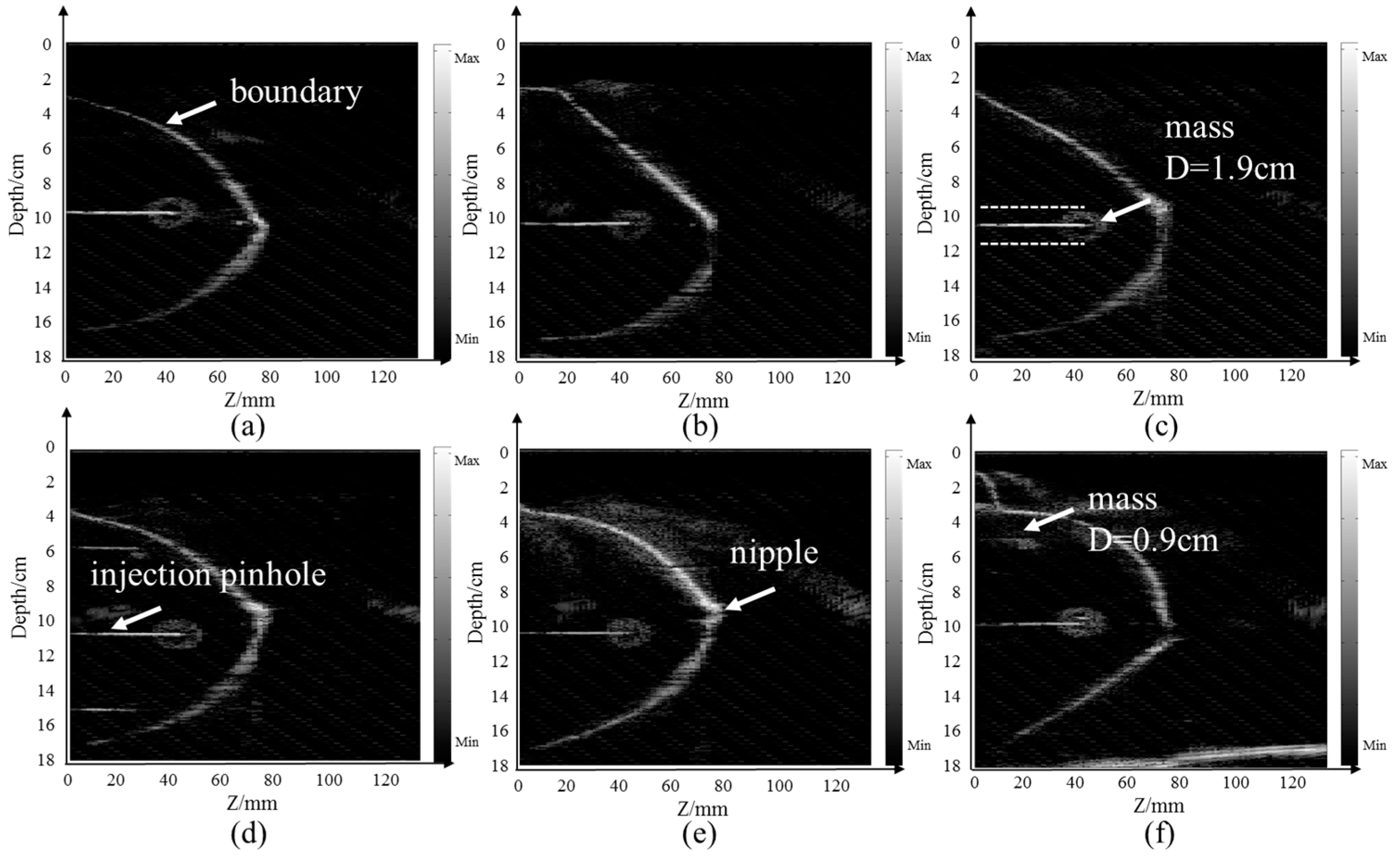
4.1. Breast Model Imaging
5. Discussions and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Grosenick, D.; Rinneberg, H.; Cubeddu, R.; Taroni, P. Review of optical breast imaging and spectroscopy. J. Biomed. Opt. 2016, 21, 91311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stafford, R.J.; Whitman, G.J. Ultrasound Physics and Technology in Breast Imaging. Ultrasound Clin. 2011, 6, 299–312. [Google Scholar] [CrossRef]
- Kamaya, A.; Machtaler, S.; Sanjani, S.S.; Nikoozadeh, A.; Sommer, F.G.; Khuri-Yakub, B.T.; Willmann, J.K.; Desser, T.S. New technologies in clinical ultrasound. Semin. Roentgenol. 2013, 48, 214. [Google Scholar] [CrossRef] [PubMed]
- Duric, N.; Littrup, P.; Li, C.; Roy, O.; Schmidt, S.; Cheng, X.; Seamans, J.; Wallen, A.; Bey-Knight, L. Breast imaging with SoftVue: Initial clinical evaluation. Proc. Spie Int. Soc. Opt. Eng. 2014, 9040, 382–385. [Google Scholar]
- Sasieni, P.D.; Shelton, J.; Ormiston-Smith, N.; Thomson, C.S.; Silcocks, P.B. What is the lifetime risk of developing cancer?: The effect of adjusting for multiple primaries. Br. J. Cancer 2011, 105, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Pisano, E. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Eng. J. Med. 2005, 353, 1773–1783. [Google Scholar] [CrossRef] [PubMed]
- Hylton, N. Magnetic Resonance Imaging of the Breast: Opportunities to Improve Breast Cancer Management. J. Clin. Oncol. 2005, 23, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.J.; Lei, W.; Craft, P.; Cawson, J.N.; Morris, I.; Walleser, S.; Griffiths, A.; Parker, S.; Houssami, N. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur. J. Cancer 2007, 43, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.L.; Wang, F. Predicting the Cumulative Risk of False-Positive Mammograms. J. Natl. Cancer Inst. 2000, 92, 1657–1666. [Google Scholar] [CrossRef] [Green Version]
- Holländer, B.; Hendriks, G.A.; Mann, R.M.; Hansen, H.H.; de Korte, C.L. Plane-Wave Compounding in Automated Breast Volume Scanning: A Phantom-Based Study. Ultrasound Med. Biol. 2016, 42, 2493–2503. [Google Scholar] [CrossRef]
- Ruiter, N.V. GPU based 3D SAFT reconstruction including phase aberration. In Proceedings of the Medical Imaging 2014: Ultrasonic Imaging and Tomography, International Society for Optics and Photonics, San Diego, CA, USA, 15–20 February 2014; Volume 10, pp. 252–260. [Google Scholar]
- Wiskin, J.; Borup, D.T.; Johnson, S.A.; Berggren, M. Non-linear inverse scattering: High resolution quantitative breast tissue tomography. J. Acoust. Soc. Am. 2012, 131, 3802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiter, N.V.; Zapf, M.; Hopp, T.; Dapp, R.; Kretzek, E.; Birk, M.; Kohout, B.; Gemmeke, H. 3D ultrasound computer tomography of the breast: A new era? Eur. J. Radiol. 2012, 1, 133–134. [Google Scholar] [CrossRef]
- Terada, T.; Yamanaka, K.; Suzuki, A.; Tsubota, Y.; Wu, W.; Kawabata, K.I. Highly precise acoustic calibration method of ring-shaped ultrasound transducer array for plane-wave-based ultrasound tomography. Jpn. J. Appl. Phys. 2017, 56, 07JF07. [Google Scholar] [CrossRef]
- Oeri, M.; Bost, W.; Tretbar, S.; Fournelle, M. Calibrated Linear Array-Driven Photoacoustic/Ultrasound Tomography. Ultrasound Med. Biol. 2016, 42, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Stiller, F.; Lerch, R.; Ermert, H. An ultrasound tomography system with polyvinyl alcohol (PVA) moldings for coupling: In vivo results for 3-D pulse-echo imaging of the female breast. Ultrason. Ferroelectr. Freq. Control IEEE Trans. 2015, 62, 266–279. [Google Scholar] [CrossRef]
- Zhang, X.; Fincke, J.; Kuzmin, A.; Lempitsky, V.; Anthony, B. A single element 3D ultrasound tomography system. In Proceedings of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; pp. 5541–5544. [Google Scholar]
- Tasinkevych, J.; Trots, I. Circular Radon Transform Inversion Technique in Synthetic Aperture Ultrasound Imaging: An Ultrasound Phantom Evaluation. Arch. Acoust. 2014, 39, 569–582. [Google Scholar] [CrossRef]
- Nebeker, J.; Nelson, T.R. Imaging of sound speed using reflection ultrasound tomography. J Ultrasound Med 2012, 31, 1389–1404. [Google Scholar] [CrossRef]
- Sandhu, G.Y.; Li, C.; Roy, O.; Schmidt, S.; Duric, N. Frequency Domain Ultrasound Waveform Tomography: Breast Imaging Using a Ring Transducer. Phys. Med. Biol. 2016, 60, 5381–5398. [Google Scholar] [CrossRef]
- Sanpanich, A.; Hamamoto, K.; Pintavirooj, C. An investigation on attenuation UCT with wave paths enhancement for breast ultrasound. Ieej Trans. Electrical Electronic Eng. 2012, 7, S105–S113. [Google Scholar] [CrossRef]
- Rouyer, J.; Mensah, S.; Franceschini, E.; Lasaygues, P.; Lefebvre, J.P. Conformal ultrasound imaging system for anatomical breast inspection. IEEE. Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1457–1469. [Google Scholar] [CrossRef]
- Birk, M.; Dapp, R.; Ruiter, N.V.; Becker, J. GPU-based iterative transmission reconstruction in 3D ultrasound computer tomography. J. Parallel Distrib. Comput. 2014, 74, 1730–1743. [Google Scholar] [CrossRef]
- Jaeger, M.; Held, G.; Peeters, S.; Preisser, S.; Grünig, M.; Frenz, M. Computed ultrasound tomography in echo mode for imaging speed of sound using pulse-echo sonography: Proof of principle. Ultrasound Med. Biol. 2015, 41, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, G.Y.; West, E.; Li, C.; Roy, O.; Duric, N. 3D frequency-domain ultrasound waveform tomography breast imaging. In Proceedings of the Medical Imaging 2017: Ultrasonic Imaging and Tomography. International Society for Optics and Photonics, Orlando, FL, USA, 11–16 February 2017; p. 1013909. [Google Scholar]
- Yu, S.; Wu, S.; Zhuang, L.; Wei, X.; Sak, M.; Neb, D.; Hu, J.; Xie, Y. Efficient Segmentation of a Breast in B-Mode Ultrasound Tomography Using Three-Dimensional GrabCut (GC3D). Sensors 2017, 17, 1827. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Choi, J.Y.; Chung, Y.E.; Bang, S.; Kim, M.J.; Park, M.S.; Kim, K.W. Comparison of MRI and endoscopic ultrasound in the characterization of pancreatic cystic lesions. Ajr Am. J. Roentgenol 2010, 195, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.; Fanti, Z.; Cosío, F.A. 3D freehand ultrasound for medical assistance in diagnosis and treatment of breast cancer: Preliminary results. In Proceedings of the IX International Seminar on Medical Information Processing & Analysis, Mexico City, Mexico, 11–14 November 2013. [Google Scholar]
- Kretzek, E.; Zapf, M.; Birk, M.; Gemmeke, H.; Ruiter, N.V. GPU based acceleration of 3D USCT image reconstruction with efficient integration into MATLAB. In Proceedings of the Medical Imaging: Ultrasonic Imaging, Tomography, & Therapy, Lake Buena Vista (Orlando Area), FL, USA, 9–14 February 2013. [Google Scholar]
- Liu, C.; Xue, C.; Zhang, B.; Zhang, G.; He, C. The Application of an Ultrasound Tomography Algorithm in a Novel Ring 3D Ultrasound Imaging System. Sensors 2018, 18, 1332. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Tao, C.; Liu, X. Dynamic focusing of acoustic wave utilizing a randomly scattering lens and a single fixed transducer. J. Appl. Phys. 2017, 121, 1606. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, J.; Luo, H.; Li, F.; Sun, G.; Cui, S. The research of compression and generation of high-precision dynamic focusing delay data for ultrasound beamformer. Clust. Comput. 2017, 20, 1–11. [Google Scholar] [CrossRef]
- U-Wai, L.; Gang-Wei, F.; Pai-Chi, L. Lossless data compression for improving the performance of a GPU-based beamformer. Ultrason. Imaging 2015, 37, 135–151. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhang, B.; Xue, C.; Zhang, W.; Zhang, G.; Cheng, Y. Multi-Perspective Ultrasound Imaging Technology of the Breast with Cylindrical Motion of Linear Arrays. Appl. Sci. 2019, 9, 419. https://doi.org/10.3390/app9030419
Liu C, Zhang B, Xue C, Zhang W, Zhang G, Cheng Y. Multi-Perspective Ultrasound Imaging Technology of the Breast with Cylindrical Motion of Linear Arrays. Applied Sciences. 2019; 9(3):419. https://doi.org/10.3390/app9030419
Chicago/Turabian StyleLiu, Chang, Binzhen Zhang, Chenyang Xue, Wendong Zhang, Guojun Zhang, and Yijun Cheng. 2019. "Multi-Perspective Ultrasound Imaging Technology of the Breast with Cylindrical Motion of Linear Arrays" Applied Sciences 9, no. 3: 419. https://doi.org/10.3390/app9030419




