Experimental Investigation of the Adsorption Characteristics of Mixed Coal and Variations of Specific Surface Areas before and after CH4 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. WY-98A Gas Adsorption Constant Analyzer
2.3. ASAP-2020 Specific Surface Area Analyzer
3. Experimental Results
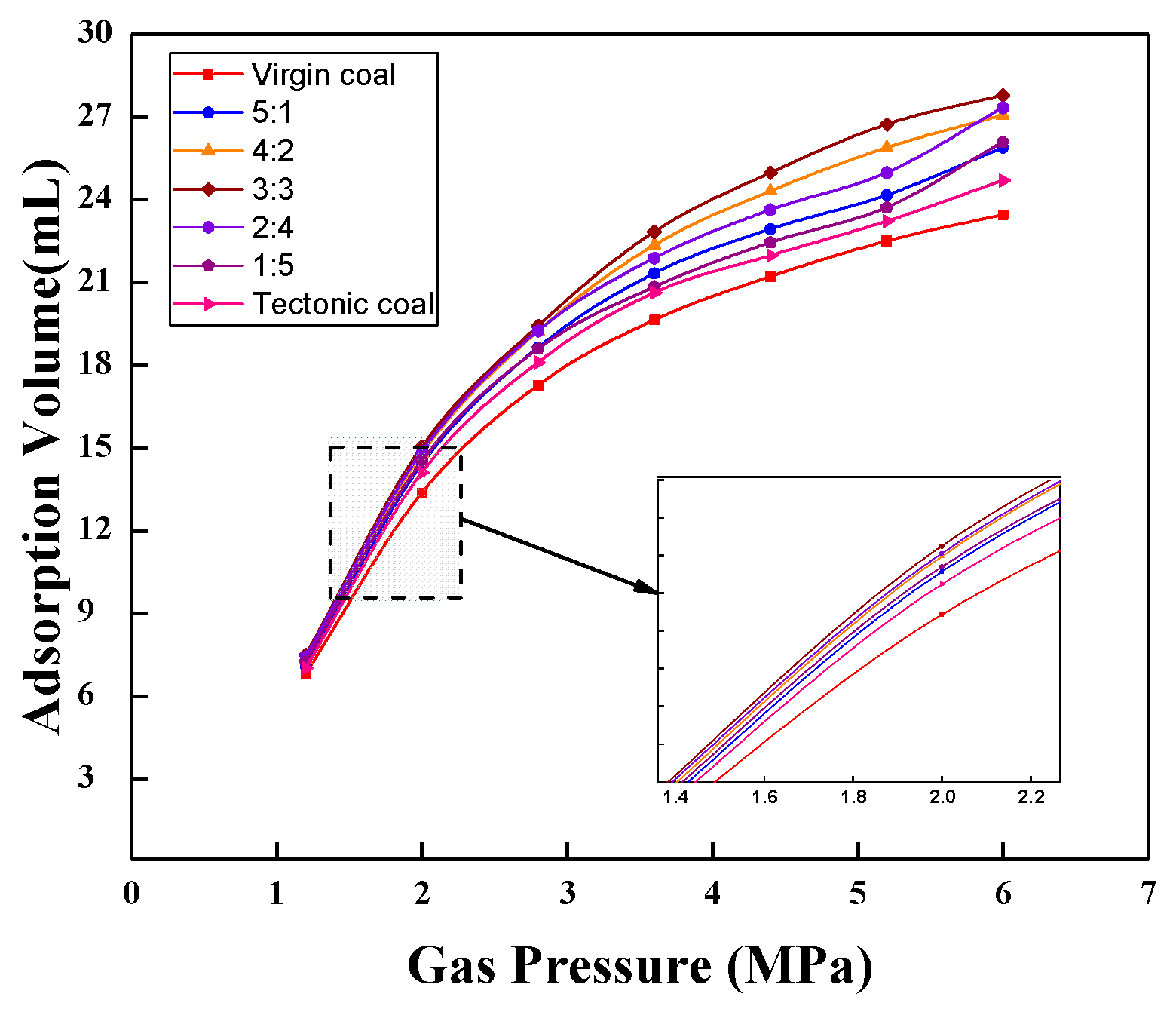
3.1. Isothermal Adsorption Results of Mixed Coal
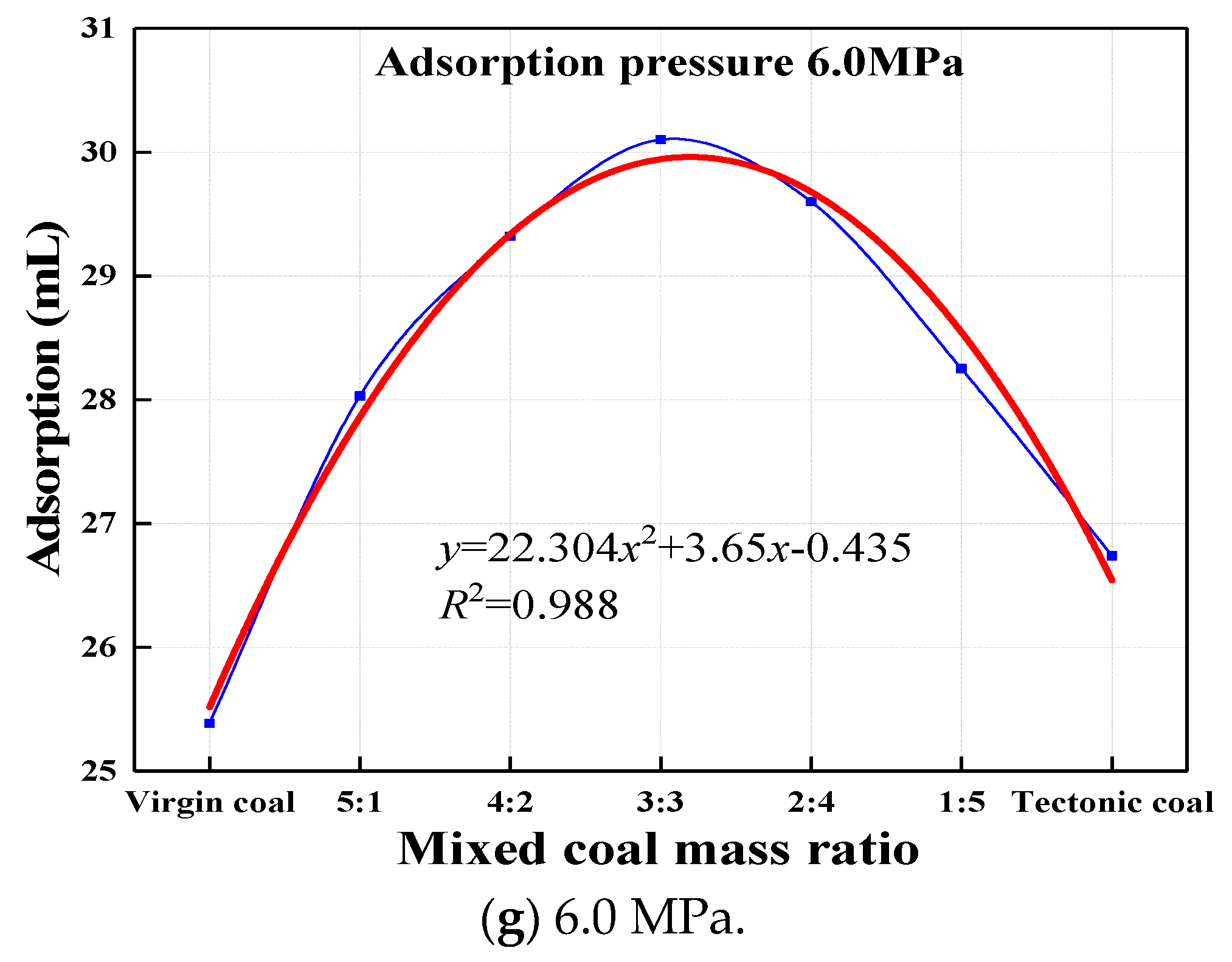
3.2. Adsorption Results of Coal Samples with Different Mass Ratios at Each Pressure Step
3.3. Specific Surface Area Results of Mixed Coal before and after Adsorption
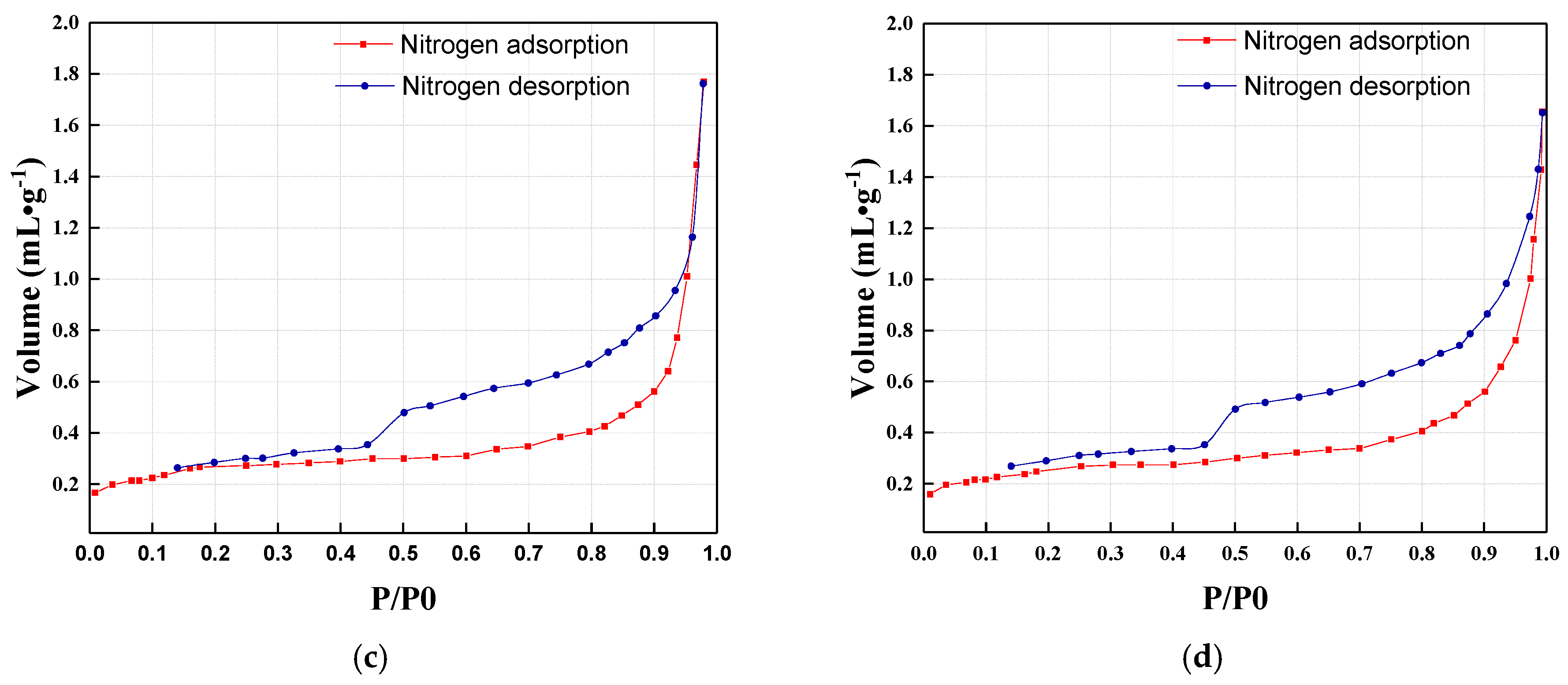
3.4. Nitrogen Adsorption Results of Mixed Coal
4. Discussion
4.1. Effect of Mixed Coal Mass Ratio on Gas Adsorption Constant
4.1.1. Effect of Mixed Coal Mass Ratio on Gas Adsorption Constant a Value
4.1.2. Effect of Mixed Coal Mass Ratio on Gas Adsorption Constant b Value
4.2. Analysis of Specific Surface Area Changes of Mixed Coal before and after Adsorption
4.3. The Relationship between the Specific Surface Area before and after Adsorption and Swelling Deformation and Strain
4.4. Mechanism Analysis of the Effect of Swelling of Mixed Coal on the Amount of Gas Emissions
5. Conclusions
- (1)
- Through the analysis of the test results of the specific surface area of the mixed coal sample, it was concluded that with the increasing proportion of the mass of the tectonic coal in the mixed coal sample, the difference of the proportion of the pore specific surface area before and after the adsorption of the coal sample showed a decreasing trend first and then increased and reached the minimum when the masses of tectonic coal and virgin coal were approximately equal. The change trend of the specific surface area of macro pores was opposite that of the specific surface area of micro pores.
- (2)
- By analyzing the experimental results, it was concluded that with an increase in the quality of the tectonic coal, the adsorption capacity of the mixed coal gradually increased. Under constant pressure, the amount of adsorbed gas changed with the mass ratio of the mixed coal, showing a downward parabola change characteristic. When the qualities of tectonic coal and virgin coal were approximately equal, the amount of gas adsorption, adsorption constant a value, and gas initial velocity of diffusion reached their maximums, but the adsorption constant b value reached its minimum.
- (3)
- On the basis of the gas adsorption test results of the mixed coal sample, the mathematical model of gas adsorption strain and pore specific surface area changes before and after adsorption in the mixed coal sample and the mathematical model of swelling stress and the variation of pore specific surface area before and after adsorption in the mixed coal sample were created. These models reflect the variation of the expansion strain and stress of the mixed coal sample after gas adsorption, and describe that when the mass ratio of virgin coal to tectonic coal was approximately equal, a large amount of gas is likely to gush out after coal disturbance, providing a theoretical basis for the further study of coal and gas outburst mechanisms.
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C. Distribution Laws of in-Situ Stress in Deep Underground Coal Mines. Procedia Eng. 2011, 26, 909–917. [Google Scholar] [CrossRef]
- Liang, Y. Strategic thinking of simultaneous exploitation of coal and gas in deep mining. J. Chin. Coal Soc. 2016, 41, 1–6. [Google Scholar]
- Jin, K.; Cheng, Y.; Ren, T.; Zhao, W.; Tu, Q.; Dong, J. Experimental investigation on the formation and transport mechanism of outburst coal-gas flow: Implications for the role of gas desorption in the development stage of outburst. Int. J. Coal Geol. 2018, 194, 45–58. [Google Scholar] [CrossRef]
- Si, G.; Sevket, D.; Sergej, J.; Jerneja, L.; Kerstin, A.; Anna, K.; Shi, J.; Simon, Z.; Grzegorz, M.; Adam, L. Seismic monitoring and analysis of excessive gas emissions in heterogeneous coal seams. Int. J. Coal Geol. 2015, 149, 41–54. [Google Scholar] [CrossRef]
- Si, G.; Sergej, J.; Jerneja, L.; Shi, J.; Sevket, D.; Anna, K.; Simon, Z. Monitoring and modelling of gas dynamics in multi-level longwall top coal caving of ultra-thick coal seams, part I: Borehole measurements and a conceptual model for gas emission zones. Int. J. Coal Geol. 2015, 144/145, 98–110. [Google Scholar] [CrossRef]
- Laxminarayana, C.; Crosdale, P.J. Role of coal type and rank on methane sorption characteristics of Bowen Basin, Australia coals. Int. J. Coal Geol. 1999, 40, 309–325. [Google Scholar] [CrossRef]
- Pan, J.; Hou, Q.; Ju, Y.; Bai, H.; Zhao, Y. Coalbed methane sorption related to coal deformation structures at different temperatures and pressures. Fuel 2012, 102, 760–765. [Google Scholar] [CrossRef]
- Levy, J.H.; Day, S.J.; Killingley, J.S. Methane capacities of Bowen Basin coals related to coal properties. Fuel 1997, 76, 713–819. [Google Scholar] [CrossRef]
- Bustin, R.M.; Clarkson, C.R. Geological controls on coalbed methane reservoir capacity and gas content. Int. J. Coal Geol. 1998, 38, 3–26. [Google Scholar] [CrossRef]
- Su, X. Influence of coal rank on coal adsorption capacity. Natl. Gas Ind. 2005, 25, 19–21. [Google Scholar]
- Peng, X.; Cheng, X.; Cao, D. Computer simulations for the adsorption and separation of CO2/CH4/H2/N2 gases by UMCM-1 and UMCM-2 metal organic frameworks. J. Mater. Chem. 2011, 21, 11259–11270. [Google Scholar] [CrossRef]
- Qi, L.; Lin, B.; Zhi, X. Study on the Mechanism of Coal and Gas Dynamic Phenomenon in Majiagou Mine. Chin. Saf. Sci. J. 2006, 12, 30–34. [Google Scholar]
- Weishauptová, Z.; Medek, J.; Kovář, L. Bond forms of methane in porous system of coal II. Fuel 2004, 83, 1759–1764. [Google Scholar] [CrossRef]
- Buss, E. Gravimetric measurement of binary gas adsorption equilibria of methane—Carbon dioxide mixtures on activated carbon. Gas Sep. Purif. 1995, 9, 189–197. [Google Scholar] [CrossRef]
- Talu, O. Needs, status, techniques and problems with binary gas adsorption experiments. Adv. Colloid Interface Sci. 1998, 76, 227–269. [Google Scholar] [CrossRef]
- Cowlard, F.C.; Lewis, J.C. Vitreous carbon—A new form of carbon. J. Mater. Sci. 1967, 2, 507–512. [Google Scholar] [CrossRef]
- Jin, K.; Cheng, Y.; Liu, Q.; Zhao, W.; Wang, L.; Wang, F. Experimental Investigation of Pore Structure Damage in Pulverized Coal: Implications for Methane Adsorption and Diffusion Characteristics. Energy Fuels 2016, 30, 10383–10395. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Manasi, M.M.; Bhatu, K.P. Sorption behavior of coal for implication in coal bed methane an overview. Int. J. Min. Sci. Technol. 2017, 27, 307–314. [Google Scholar]
- Gao, K.; Liu, J.; Deng, D. Physical and Mechanical Characteristics of Tectonic Soft Coal and Their Effects on Coal and Gas Outburst. Chin. Saf. Sci. J. 2013, 23, 129–133. [Google Scholar]
- Okolo, G.N.; Everson, R.C.; Neomagus, H.W.J.P.; Roberts, M.J.; Sakurovs, R. Comparing the porosity and surface areas of coal as measured by gas adsorption, mercury intrusion and SAXS techniques. Fuel 2015, 141, 293–304. [Google Scholar] [CrossRef]
- Akbarzadeh, H.; Chalaturnyk, R.J. Structural changes in coal at elevated temperature pertinent to underground coal gasification: A review. Int. J. Coal Geol. 2014, 131, 126–146. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. Variation in micropore capacity and size distribution with composition in bituminous coal of the Western Canadian Sedimentary Basin. Fuel 1996, 75, 1483–1498. [Google Scholar] [CrossRef]
- Budaeva, A.D.; Zoltoev, E.V. Porous structure and sorption properties of nitrogen-containing activated carbon. Fuel 2010, 89, 2623–2627. [Google Scholar] [CrossRef]
- Takagi, H.; Maruyama, K.; Yoshizawa, N.; Yamada, Y.; Sato, Y. XRD analysis of carbon stacking structure in coal during heat treatment. Fuel 2004, 83, 2427–2433. [Google Scholar] [CrossRef]
- Teng, L.; Tang, T. IR study on surface chemical properties of catalytic grown carbon nanotubes and nanofibers. J. Zhejiang Univ. Sci. A (Appl. Phys. Eng.) 2008, 9, 720–726. [Google Scholar] [CrossRef]
- Muirhead, D.K.; Parnell, J.; Taylor, C.; Bowden, S.A. A kinetic model for the thermal evolution of sedimentary and meteoritic organic carbon using Raman spectroscopy. J. Anal. Appl. Pyrolysis 2012, 96, 153–161. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, H.; Wang, J.; Cen, K. Effect of temperature on the sintering behavior of Zhundong coal ash in oxy-fuel combustion atmosphere. Fuel 2015, 150, 526–537. [Google Scholar] [CrossRef]
- Lee, G.-J.; Pyun, S.-I.; Rhee, C.-K. Characterisation of geometric and structural properties of pore surfaces of reactivated microporous carbons based upon image analysis and gas adsorption. Microporous Mesoporous Mater. 2006, 93, 217–225. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Elsworth, D.; Jiang, Y.; Jie, Z. Pore Structure Characterization of Coal by Synchrotron Small-Angle X-ray Scattering and Transmission Electron Microscopy. Energy Fuels 2014, 28, 3704–3711. [Google Scholar] [CrossRef]
- Dean, C.; Horn, E. A hard, high-carbon, lignomor with conchoidal fracture: Cunnite, from mature myrtle beech (Nothofagus cunninghamii (Hook. f.) Oerst.). Geoderma 2019, 333, 178–187. [Google Scholar] [CrossRef]
- Gamson, P.; Beamish, B.; Johnson, D. Coal microstructure and secondary mineralization: Their effect on methane recovery. Geol. Soc. Lond. Spec. Publ. 1996, 109, 165–179. [Google Scholar] [CrossRef]
- Pohl, W.L. Economic Geology Principles and Practice: Metals, Minerals, Coal and Hydrocarbons—Introduction to Formation and Sustainable Exploitation of Mineral Deposits. Econ. Geol. 2013, 108, 1517–1518. [Google Scholar]
- Li, Y.; Lu, G.; Rudolph, V. Compressibility and fractal dimension of fine coal particles in relation to pore structure characterisation using mercury porosimetry. Part. Part. Syst. Charact. 1999, 16, 25–31. [Google Scholar] [CrossRef]
- Fishman, N.S.; Hackley, P.C.; Lowers, H.A.; Hill, R.J.; Egenhoff, S.O.; Eberl, D.D.; Blum, A.E. The nature of porosity in organic-rich mudstones of the Upper Jurassic Kimmeridge Clay Formation, North Sea, offshore United Kingdom. Int. J. Coal Geol. 2012, 103, 32–50. [Google Scholar] [CrossRef]
- Vanherendael, B.; Oberti, C.; Brosens, I. Microanatomy of human amniotic membranes—Light microscopic, transmission, and scanning electron-microscopic study. Aust. Plant Pathol. Soc. Newsl. 1978, 3, 62. [Google Scholar]
- Ortiz, M. A constitutive theory for the inelastic behavior of concrete. Mech. Mater. 1985, 4, 67–93. [Google Scholar] [CrossRef]
- Park, S.; Heo, H.A.; Lee, K.B.; Kim, H.G.; Pyo, S.W. Improved Bone Regeneration with Multiporous PLGA Scaffold and BMP-2-Transduced Human Adipose-Derived Stem Cells by Cell-Permeable Peptide. Implant Dent. 2017, 26, 4. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. Binary gas adsorption/desorption isotherms: Effect of moisture and coal composition upon carbon dioxide selectivity over methane. Int. J. Coal Geol. 2000, 42, 241–271. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Wang, G.; Booth, P.; Nemcik, J. Experimental investigation of N2 injection to enhance gas drainage in CO2-rich low permeable seam. Fuel 2018, 215, 665–674. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Wang, G.; Booth, P.; Nemcik, J. Experimental study of the adsorption-induced coal matrix swelling and its impact on ECBM. J. Earth Sci. 2017, 28, 917–925. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Wang, G.; Nemcik, J. Simulation investigation of N2-injection enhanced gas drainage: Model development and identification of critical parameters. J. Natl. Gas Sci. Eng. 2018, 55, 30–41. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2 -sorption data by DR-, BET- and t-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
- Ju, W.; Shen, L.; Guo, D. Nitrogen Adsorption Method and Mercury Injection Method Determination the Pore Structure of Alumina Carrier. Guangdong Chem. Ind. 2009, 36, 213–228. [Google Scholar]
- Perera, M.; Ranjith, P.G.; Choi, S.; Airey, D.; Weniger, P. Estimation of gas adsorption capacity in coal: A review and an analytical study. Int. J. Coal Prep. Util. 2012, 32, 25–55. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, S.; Lin, Y. Isothermal adsorption and desorption experiment of coal and experimental results accuracy fitting. J. Chin. Coal Soc. 2011, 36, 477–480. [Google Scholar]
- Schpfer, M.; Abe, S.; Childs, C.; Walsh, J. The impact of porosity and crack density on the elasticity, strength and friction of cohesive granular materials: Insights from dem modelling. Int. J. Rock Mech. Min. Sci. 2009, 46, 250–261. [Google Scholar] [CrossRef]
- Yang, Y.; Aplin, A.C. Definition and practical application of mudstone porosity-effective stress relationships. Pet. Geosci. 2004, 10, 153–162. [Google Scholar] [CrossRef]
- Zhang, J. Effective stress, porosity, velocity and abnormal pore pressure prediction accounting for compaction disequilibrium and unloading. Mar. Pet. Geol. 2013, 45, 2–11. [Google Scholar] [CrossRef]
- Huang, J.; Xu, G.; Hu, G.; Kizil, M.; Chen, Z. A coupled electromagnetic irradiation, heat and mass transfer model for microwave heating and its numerical simulation on coal. Fuel Process. Technol. 2018, 177, 237–245. [Google Scholar] [CrossRef]
- Yang, K.; Lu, X.; Lin, Y.; Neimark, A.V. Deformation of Coal Induced by Methane Adsorption at Geological Conditions. Energy Fuels 2016, 24, 5955–5964. [Google Scholar] [CrossRef]
- Zhang, Z. Adsorption characteristics of coal and distribution of pores in coal. Saf. Coal Mines 2006, 37, 1–3. [Google Scholar]
- Wang, J.R.; Deng, C.B.; Deng, H.Z. Study on the microcosmic mechanism for coal-gas outburst. J. Chin. Coal Soc. 2008, 33, 131–135. [Google Scholar]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Liu, H.; Li, S.; Lin, H.; Jia, Y.; Yan, M.; Yuan, M.; Lin, J. Experimental Investigation of the Adsorption Characteristics of Mixed Coal and Variations of Specific Surface Areas before and after CH4 Adsorption. Appl. Sci. 2019, 9, 524. https://doi.org/10.3390/app9030524
Zhao P, Liu H, Li S, Lin H, Jia Y, Yan M, Yuan M, Lin J. Experimental Investigation of the Adsorption Characteristics of Mixed Coal and Variations of Specific Surface Areas before and after CH4 Adsorption. Applied Sciences. 2019; 9(3):524. https://doi.org/10.3390/app9030524
Chicago/Turabian StyleZhao, Pengxiang, Hui Liu, Shugang Li, Haifei Lin, Yongyong Jia, Min Yan, Meiqi Yuan, and Jia Lin. 2019. "Experimental Investigation of the Adsorption Characteristics of Mixed Coal and Variations of Specific Surface Areas before and after CH4 Adsorption" Applied Sciences 9, no. 3: 524. https://doi.org/10.3390/app9030524




