The Use of Chalcedonite as a Biosorption Bed in the Treatment of Groundwater
Abstract
:1. Introduction
2. Materials and Methods
- Water I: single-stage biofiltration on a bed pre-activated with potassium manganese (VII) solution,
- Water II: two-stage biofiltration; I° filtration on a bed pre-activated with potassium manganese (VII), II° filtration on a natural bed,
- Water III: two-stage biofiltration on beds without pre-activation (Figure 1).
- Ammonium nitrogen at a dose of about 2.0–3.0 g N-NH4+/m3,
- Manganese at a dose of about 1.0 g·Mn/m3,
- Iron at a dose of about 1.0 g Fe/m3.
3. Results
4. Discussion of Results
5. Conclusions
- The removal of ammonium nitrogen, iron, and manganese in the biofiltration process on chalcedonite beds is an alternative method to conventional processes used in water treatment plants-filtration on quartz beds. Chalcedonite has greater porosity and a more proper surface than quartz sand. It is a better substrate for immobilization and development of microorganisms involved in the biodegradation of pollutants, which allows for simultaneous removal of NH4+, Mn, and Fe according to different types of mechanisms.
- Among the analyzed systems, the best efficiency of removing ammonium nitrogen, iron, and manganese from groundwater was demonstrated by two-stage biofiltration on chalcedonite beds, with initial chemical activation at the first stage of filtration.
- Chemical activation of the chalcedonite bed had an effect on the efficiency of removal of manganese (II) ions but did not affect the efficiency of iron removal.
- In the pre-activated bed system, a longer biofilm formation time (start-up of the bed) was observed, which resulted in a longer waiting period for obtaining the required concentration of ammonium nitrogen in the treated water.
- Activation of the bed with potassium manganese (VII) reduces the oxygen consumption in the biofiltration process as compared with the non-activated bed. The oxygen deficiency can inhibit the ammonium nitrogen removal process.
- The sorption properties of chalcedonite and the ability to retain water borne microorganisms that cause the biofilm formed on its surface takes over the sorbent functions and retained ammonium ions processing the construction of new bacterial cells. The creation of biofilms and the creation of manganese oxide coatings results in continuous filter operation. Filling the biofilter does not require regeneration.
Author Contributions
Funding
Conflicts of Interest
References
- Sharma, S.K.; Petrusevski, B.; Schippers, J.C. Biological iron removal from groundwater: a review. J. Water Supply Res. Technol. AQUA 2005, 54, 239–247. [Google Scholar] [CrossRef]
- Haddad, M.; Ohkame, T.; Bérubé, P.R.; Barbeau, B. Performance of thin-film composite hollow fiber nanofiltration for the removal of dissolved Mn, Fe and NOM from domestic groundwater supplies. Water Res. 2018, 145, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, D.; Zeng, H.; Zhang, J. Autotrophic nitrogen conversion process and microbial population distribution in biofilter that simultaneously removes Fe, Mn and ammonia from groundwater. Int. Biodeterior. Biodegrad. 2018, 135, 53–61. [Google Scholar] [CrossRef]
- Abu, H.H.; Sheikh, A.S.R.; Kamarudin, S.K.; Tan, K.N. On–off control of aeration time in the simultaneous removal of ammonia and manganese using a biological aerated filter system. Process Saf. Environ. Prot. 2013, 91, 415–422. [Google Scholar] [CrossRef]
- Cheng, Q.; Nengzi, L.; Bao, L.; Huang, Y.; Liu, S.; Cheng, X.; Li, B.; Zhang, J. Distribution and genetic diversity of microbial populations in the pilot-scale biofilter for simultaneous removal of ammonia, iron and manganese from real groundwater. Chemosphere 2017, 182, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Tekerlekopoulou, A.G.; Vayenas, D.V. Ammonia, iron and manganese removal from potable water using trickling filters. Desalination 2007, 210, 225–235. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Chen, Z.; Chen, G.; Zhang, C.; Huang, J.; Nie, X.; Xiong, W.; Zeng, G. Study and health risk assessment of the occurrence of iron and manganese in groundwater at the terminal of the Xiangjiang River. Environ. Sci. Pollut. Res. 2015, 22, 19912–19921. [Google Scholar] [CrossRef]
- Zoni, S.; Albini, E.; Lucchini, R. Neuropsychological testing for the assessment of manganese neurotoxicity: A review and a proposal. Am. J. Ind. Med. 2007, 50, 812–830. [Google Scholar] [CrossRef]
- Tekerlekopoulou, A.G.; Pavlou, S.; Vayenas, D.V. Removal of ammonium, iron and manganese from potable water in biofiltration units: a review: Removal of ammonium, iron and manganese from potable water. J. Chem. Technol. Biotechnol. 2013, 88, 751–773. [Google Scholar] [CrossRef]
- Li, D.; Liu, S. Groundwater Quality Detection. In Water Quality Monitoring and Management; Elsevier: Academic Press: London, UK; San Diego, CA, USA; Cambridge, UK; Oxford, UK, 2018; Volume 1, pp. 269–302. [Google Scholar] [CrossRef]
- Commission Directive (EU) 2015/1787 of 6 October 2015 amending Annexes II and III to Council Directive 98/83/EC on the quality of water intended for human consumption. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2015.260.01.0006.01.ENG (accessed on 14 February 2019).
- Puszkarewicz, A.; Kaleta, J. Highly effective conditioning of underground waters with the use of catalytic preparations. Ecol. Technol. 2006, 14, 23–29. [Google Scholar]
- Kaleta, J.; Kida, M.; Koszelnik, P.; Papciak, D.; Puszkarewicz, A.; Tchórzewska-Cieślak, B. The use of activated carbons for removing organic matter from groundwater. Arch. Environ. Prot. 2017, 43, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Jordanowska, J.; Jakubus, M. Evaluation of effectiveness technological process of water purification exemplified on modernized water treatment plant at Otoczna. Civ. Environ. Eng. Rep. 2014, 13, 49–62. [Google Scholar] [CrossRef]
- Štembal, T.; Markić, M.; Ribičić, N.; Briški, F.; Sipos, L. Removal of ammonia, iron and manganese from groundwaters of northern Croatia—pilot plant studies. Process Biochem. 2005, 40, 327–335. [Google Scholar] [CrossRef]
- Vries, D.; Bertelkamp, C.; Schoonenberg Kegel, F.; Hofs, B.; Dusseldorp, J.; Bruins, J.H.; de Vet, W.; van den Akker, B. Iron and manganese removal: Recent advances in modelling treatment efficiency by rapid sand filtration. Water Res. 2017, 109, 35–45. [Google Scholar] [CrossRef]
- Nitzsche, K.S.; Weigold, P.; Lösekann-Behrens, T.; Kappler, A.; Behrens, S. Microbial community composition of a household sand filter used for arsenic, iron, and manganese removal from groundwater in Vietnam. Chemosphere 2015, 138, 47–59. [Google Scholar] [CrossRef]
- de Vet, W.W.; Dinkla, I.J.; Rietveld, L.C.; van Loosdrecht, M.C. Biological iron oxidation by Gallionella spp. in drinking water production under fully aerated conditions. Water Res. 2011, 45, 5389–5398. [Google Scholar] [CrossRef]
- Papciak, D.; Zamorska, J. Single and two-layer nitrifying beds for bio-technologic method of removing ammonium nitrogen from water. Biotechnology 2008, 1, 189–201. [Google Scholar]
- Papciak, D.; Zamorska, J. The biofilters fillers with reference to effectivity of nitrification process. In Scientific Papers of Rzeszow University of Technology; Publishing House of the Rzeszow University of Technology: Rzeszów, Poland, 2003; Volume 35, pp. 147–160. (In Polish) [Google Scholar]
- Papciak, D. Effect of Nitrification-Filter Packing Material on the Time to Reach its Operation Capacity. In Environmental Engineering; Pawłowski, L., Dudzińska, M., Pawłowski, A., Eds.; Taylor & Francis Group: London, UK, 2007; pp. 125–132. [Google Scholar]
- Ruiz, M.; Roset, L.; Demey, H.; Castro, S.; Sastre, A.M.; Pérez, J.J. Equilibrium and dynamic studies for adsorption of boron on calcium alginate gel beads using principal component analysis (PCA) and partial least squares (PLS). Materialwiss. Werkstofftech. 2013, 44, 410–415. [Google Scholar] [CrossRef]
- Jeż-Walkowiak, J.; Dymaczewski, Z.; Szuster-Janiaczyk, A.; Nowicka, A.; Szybowicz, M. Efficiency of Mn removal of different filtration materials for groundwater treatment linking chemical and physical properties. Water 2017, 9, 498. [Google Scholar] [CrossRef]
- Albrektiene, R.; Rimeika, M.; Voisniene, V. The Characterisation of Natural Organic Matter in Ground Water Using Rapid Fractionation. In Proceedings of the Water Pollution 2014, The Algarve, Portugal, 26–28 May 2014; pp. 111–120. [Google Scholar] [CrossRef]
- Albrektienė, R.; Rimeika, M. Efficiency of Removal of Iron, Manganese, Ammonium and Organic Matter from Groundwater. In Proceedings of the Environmental Engineering, Vilnius, Lithuania, 27–28 April 2017. [Google Scholar] [CrossRef]
- Kaleta, J.; Puszkarewicz, A.; Papciak, D. Removal of iron, manganese and nitrogen compounds from underground waters with diverse physical and chemical characteristics. Environ. Prot. Eng. 2007, 33, 5–13. [Google Scholar]
- Papciak, D.; Zamorska, J.; Kaleta, J.; Puszkarewicz, A. Effect of manganese (II) on the time of biofilm formation and on the effectiveness of ammonium nitrogen removal from water in biofiltration process. Pol. J. Environ. Stud. 2009, 2, 43–45. [Google Scholar]
- Sozański, M.M.; Jeż-Walkowiak, J.; Weber, Ł. Methods of research on technological suitability of filtration materials for iron and manganese removal from groundwater based on tests with chalcedonit bed. Instal 2011, 10, 61–65. [Google Scholar]
- Kaleta, J.; Puszkarewicz, A.; Papciak, D. Natural and modified minerals in remediation of groundwaters. Economy Min. Resour. 2009, 25, 51–63. [Google Scholar]
- Demey, H.; Lapo, B.; Ruiz, M.; Fortuny, A.; Marchand, M.; Sastre, A.M. Neodymium recovery by chitosan/iron(III)hydroxide [ChiFer(III)] sorbent material: Batch and column systems. Polymers 2018, 10, 204. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zikoudi, A.; Hug, S.J. Arsenic removal from groundwaters containing iron, ammonium, manganese and phosphate: a case study from a treatment unit in northern Greece. Desalination 2008, 224, 330–339. [Google Scholar] [CrossRef]
- Michel, M.M. Characteristics of chalcedonite from Teofilów deposit for possible use in technology of water and wastewater treatment. Economy Min. Resour. 2011, 27, 49–67. [Google Scholar]
- Michel, M.M.; Kiedryńska, L.; Tyszko, E. Manganese removal from groundwater on modified chalcedonite and Purolite MZ-10 beds. Environ. Prot. 2008, 30, 15–20. [Google Scholar]
- Kasza, B.; Naziemiec, Z.; Pabis, J. Utilization chalcedownite waste from the Inowłódz mine. In Proceedings of the Conference Materials on Economy of Mineral Raw Materials from Mining and Energy, Przegorzały, Poland, 1996; Volume 1, pp. 1–6. [Google Scholar] [CrossRef]
- Tchórzewska, D.; Pabis, J.; Kosk, I.; Nieć, M. New applications of chalcedonite as a sorbent in the water treatment process. Geol. Rev. 2001, 49, 303–306. [Google Scholar]
- Clack MTM® is Used for Reducing Iron, Manganese and Hydrogen Sulfide from Water Supplies. Available online: http://www.clackcorp.com/downloads/ion_exchange_resin_and_filter_media/mtm_2353.pdf (accessed on 14 February 2019).
- Clack MTM® Safety Data Sheet. Available online: http://www.clackcorp.com/downloads/msds/Active%20Online%20Filter%20Medias/MTM-EU.pdf (accessed on 14 February 2019).
- Greensand Plus—Technical Data. Available online: https://www.inversand.com/wp-content/uploads/2018/02/Greensand-Technical-Data.pdf (accessed on 14 February 2019).
- Greensand PlusTM—Safety Data Sheet. Available online: http://www.clackcorp.com/downloads/msds/Active%20Online%20Filter%20Medias/A8042%20GreensandPlus%20SDS%208-20-2015.pdf (accessed on 14 February 2019).
- Catalyst G1. Available online: http://gc2000.pl/wp-content/uploads/docs/G1.pdf (accessed on 14 February 2019).
- Defeman Deposit. Available online: http://gc2000.pl/wp-content/uploads/docs/DEFEMAN.pdf (accessed on 14 February 2019).
- Multiman 3M—Filtration Media. Available online: http://www.dynamikfiltr.pl/df_files/files/pl_74_pl_9_ZLOZE_FILTRACYJNE_MULTIMAN_3M.pdf (accessed on 14 February 2019).
- Pyrolox®—Filtration Media. Available online: http://www.clackcorp.com/downloads/ion_exchange_resin_and_filter_media/pyrolox_2356.pdf (accessed on 14 February 2019).
- 2018 Pure Water Commercial Catalog. Available online: https://www.watts.com/dfsmedia/0533dbba17714b1ab581ab07a4cbb521/29526-source/options/download/c-wq-purewatercommercial (accessed on 14 February 2019).
- Filtration Media. Available online: https://www.swtwater.com/catalog/pdf/1093%20-%20MetalEase.pdf (accessed on 14 February 2019).
- Cai, Y.; Li, D.; Liang, Y.; Zeng, H.; Zhang, J. Operational parameters required for the start-up process of a biofilter to remove Fe, Mn, and NH3-N from low-temperature groundwater. Desalin. Water Treat. 2016, 57, 3588–3596. [Google Scholar] [CrossRef]
- Pruss, A. Contribution of biofilm thickness on sand filter grains to oxygen uptake during ammonia nitrogen removal. Environ. Prot. 2007, 29, 5–39. [Google Scholar]
- Das, A.P.; Sukla, L.B.; Pradhan, N.; Nayak, S. Manganese biomining: A review. Bioresour. Technol. 2011, 102, 381–7387. [Google Scholar] [CrossRef]
- Papciak, D.; Kaleta, J.; Puszkarewicz, A. Removal of ammonia nitrogen from groundwater on chalcedony deposits in two-stage biofiltration process. Annu. Set Environ. Prot. 2013, 15, 1352–1366. [Google Scholar]
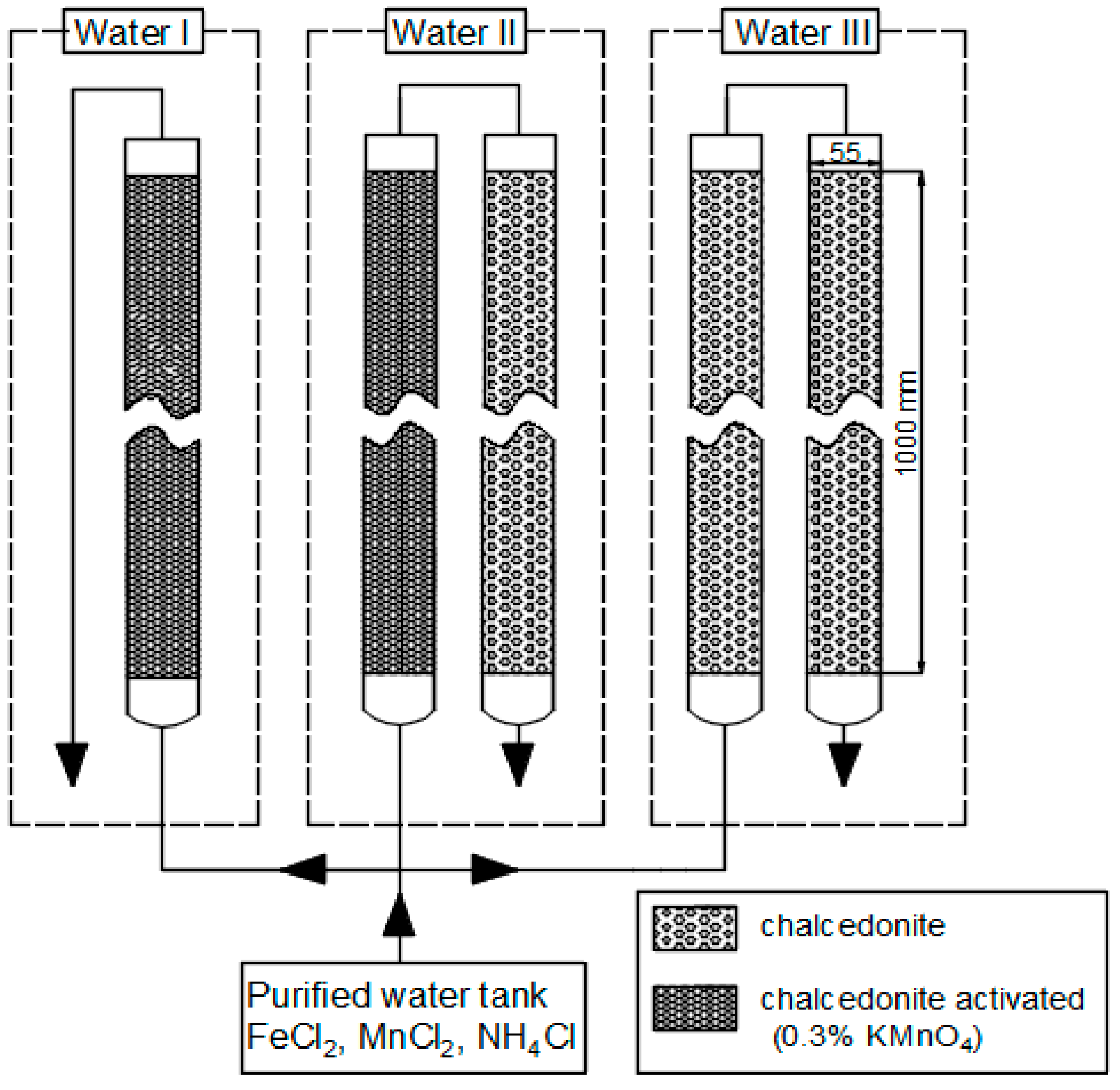


| Parameter | Value | |
|---|---|---|
| Height | 1.0 m | |
| Diameter | 0.055 m | |
| Filtration speed | 2–3 m/h | |
| Direction of water flow through the bed | I° filtration | anti-gravitational |
| Ii° filtration | gravitational | |
| Product Name | Chemical Formula | Grade | Source of Purchase |
|---|---|---|---|
| Ammonium chloride | NH4Cl | Pure p. a. | Avantor Performance Materials Poland S.A. (formerly POCH.S.A.) |
| Manganese (II) chloride tetrahydrate | MnCl2·4·H2O | ||
| Iron (II) chloride tetrahydrate | FeCl2·4·H2O | ||
| Potassium manganese (VII) | KMnO4 |
| Parameter | Min | Max | Mean | Median | SD | N |
|---|---|---|---|---|---|---|
| Ammonium Nitrogen | 0.50 | 3.70 | 2.49 | 2.50 | 0.62 | 57 |
| Nitrite Nitrogen | 0.00 | 2.26 | 0.37 | 0.06 | 0.61 | 57 |
| Nitrate Nitrogen | 0.02 | 3.58 | 2.01 | 2.00 | 0.85 | 57 |
| Manganese | 0.86 | 1.80 | 1.06 | 1.03 | 0.18 | 57 |
| Iron | 0.78 | 1.17 | 1.02 | 1.00 | 0.08 | 57 |
| Dissolved Oxygen | 5.10 | 10.84 | 8.26 | 8.45 | 1.31 | 55 |
| pH | 7.13 | 7.70 | 7.43 | 7.46 | 0.16 | 55 |
| Physical Properties | Chemical Composition | ||||
|---|---|---|---|---|---|
| Parameter | Unit | Range | Parameter | Unit | Range |
| Specific density | t/m3 | 2.62–2.67 | SiO2 | % | 94.0–99.0 |
| Bulk density | t/m3 | 1.0–2.0 | Al2O3 | % | 0.4–3.6 |
| Porosity | % | 15–30 | Fe2O3 | % | 0.1–0.8 |
| Impregnability | % | 4–10 | CaO | % | 0.1–1.2 |
| Compressive strength | MPa | 60–120 | MgO | % | 0.0–0.3 |
| Abrasion in the Devala drum | % | 6–15 | Na2O | % | 0.04–0.2 |
| Oil number | g/100 g meal | 26 | K2O | % | 0.1–0.5 |
| Parameter | Unit | Analytical Method/Standard |
|---|---|---|
| Ammonium nitrogen | g N-NH4+/m3 | Colorimetric method with Nessler’s reagent using UV-Vis Spectrophotometer (DR 5000, Hach-Lange, Germany, Europe), method number 8155 |
| Nitrite nitrogen | g N-NO2−/m3 | Colorimetric method by Nitrite Test Merck 1.14408 (Germany, Europe), method number 8507 |
| Nitrate nitrogen | g N-NO3−/m3 | Colorimetric method with sodium salicylate using UV-Vis Spectrophotometer (DR 5000, Hach-Lange, Germany, Europe), method number 8039 |
| Dissolved oxygen | g O2/m3 | Electrochemical method using a HQ30D Dissolved Oxygen Meter (Hach-Lange, Germany, Europe) |
| Manganese | g Mn/m3 | Colorimetric method using UV-Vis Spectrophotometer (DR 5000, Hach-Lange, Germany, Europe), method number 8149 |
| Iron | g Fe/m3 | Spectrophotometric method by the rhododendron method using UV-Vis Spectrophotometer (DR 5000, Hach-Lange, Germany, Europe), method number 8008 |
| Filtration Materials | The Sorption Capacity | References | |
|---|---|---|---|
| g Fe /dm3 deposits | g Mn /dm3 deposits | ||
| MTM® | 1,3 | 0,6 | [36,37] |
| GreensandPlusTM | 1,3 | 0,6 | [38,39] |
| Parameters | Water I | Water II | Water III |
|---|---|---|---|
| Beds Pre-activated | Bed without Pre-activation | ||
| The amount of nitrogen removed Δ N-NH4+ [g N-NH4+/m3] | 0–3.70 | 0.1–3.5 | 1.34–3.00 |
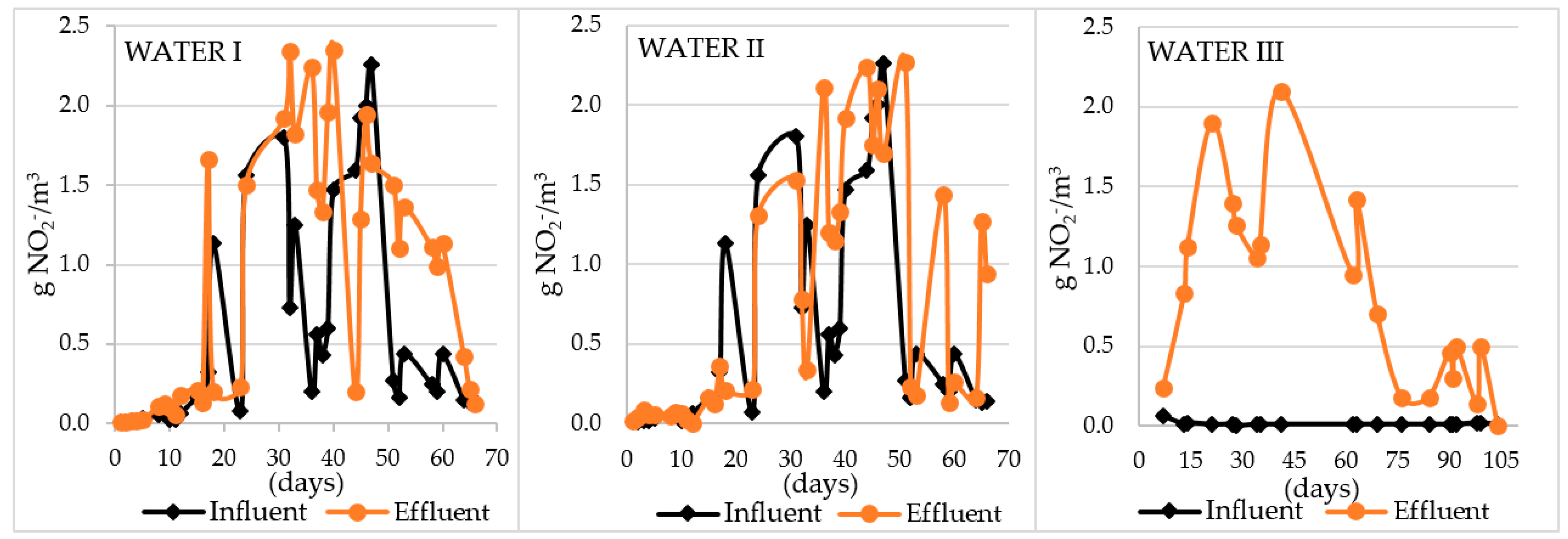
| Amount of nitrite nitrogen formed Δ N-NO2− [g N-NO2−/m3] | (−1.39)–2.04 | (−0.923)–2 | (−0.01)–2.09 |
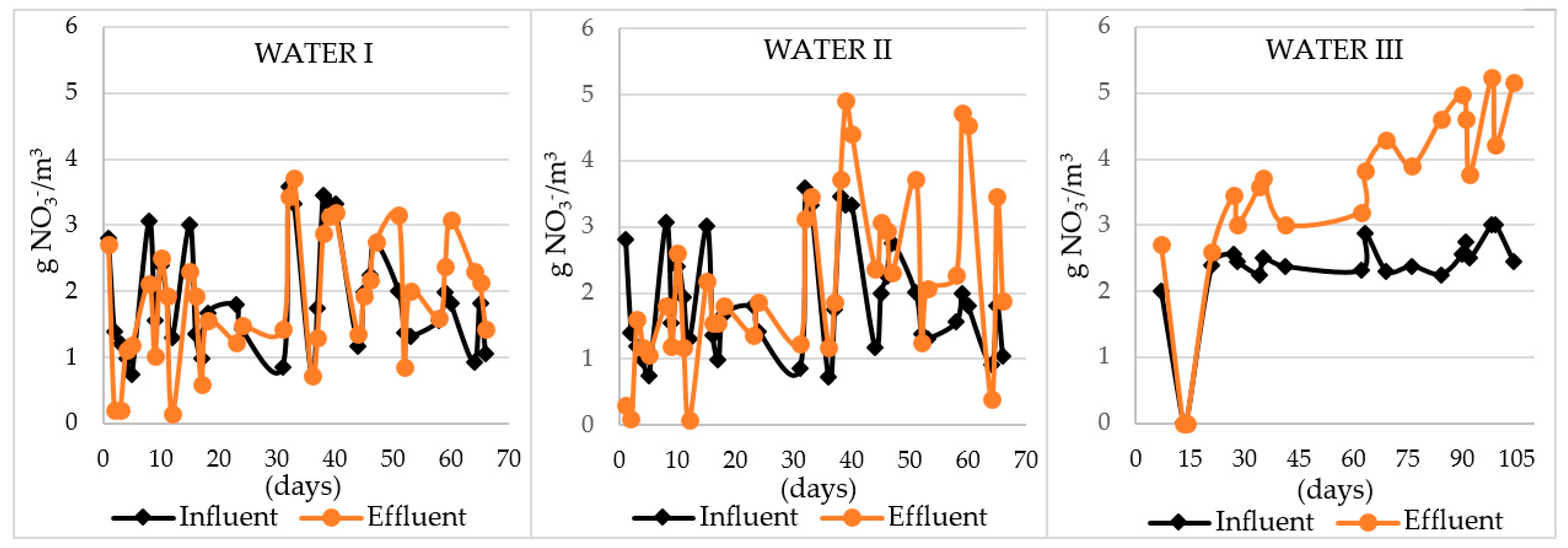
| Amount of nitrate nitrogen formed Δ N-NO3− [g N-NO3−/m3] | (−1.20)–1.38 | (−2.5)–2.73 | (−0.02)–2.73 |
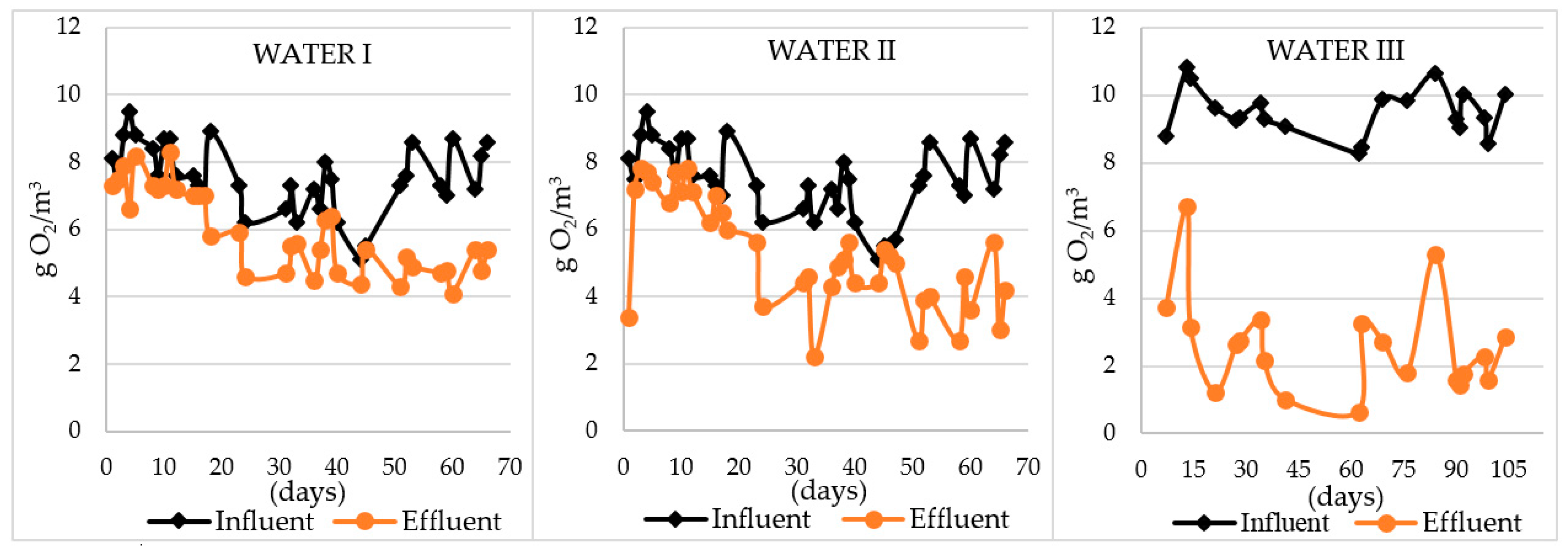
| Oxygen consumption Δ O2 [g O2/m3] | 0–4.06 | 0.52 | 4.12–8.43 |
| Efficiency of N-NH4+ removal [%] | 0–100 | 2.85–100 | 78–96 |
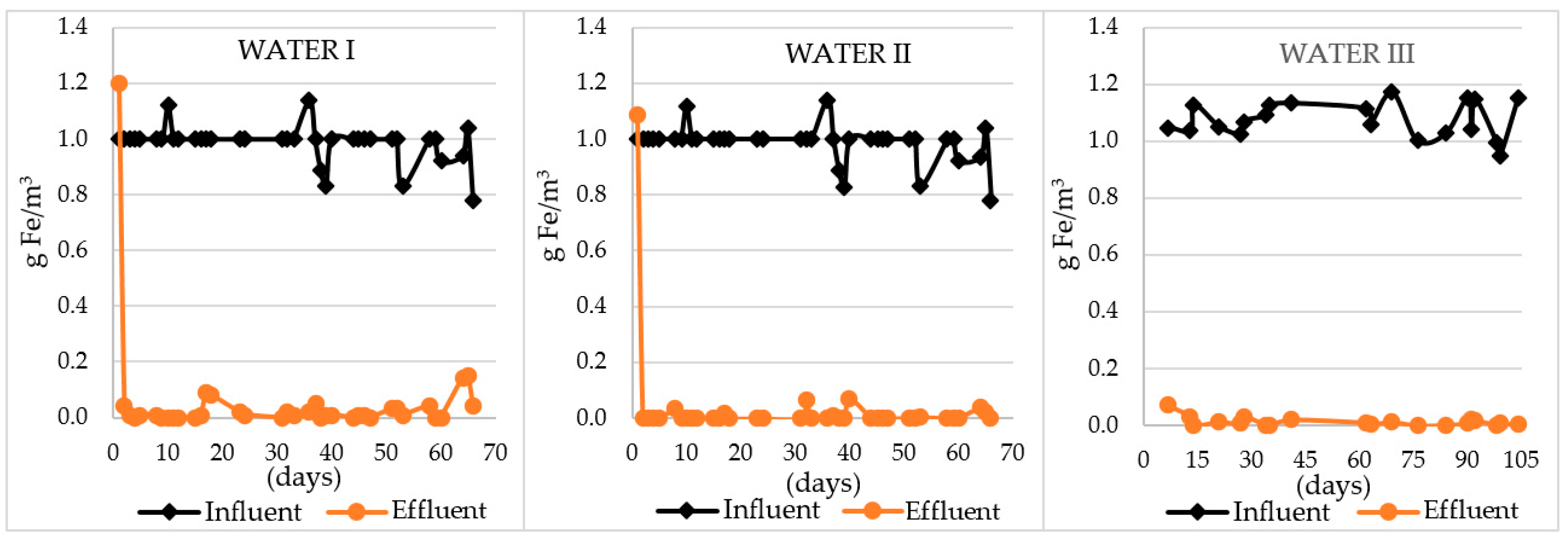
| Efficiency of Fe removal [%] | 85–100 | 93–100 | 93–100 |
| Efficiency of Mn removal [%] | (−34)–97% from 57 days, washing out Mn to the filtrate | 52–99% | from 28 days 0, washing out Mn to the filtrate |
| Time to obtain a normative value (0.39 g N-NH4+/m3) [day] | 23 | 23 | 14 |
| Oxygen consumption per 1g of nitrogen [g O2/1 g N] | 2.02 | 2.43 | 3.20 |
| The start time of the I phase of nitrification [day] | 31 | 1 | 7 |
| The start time of the II phase of nitrification [day] | 51 | 24 | 21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papciak, D.; Domoń, A.; Puszkarewicz, A.; Kaleta, J. The Use of Chalcedonite as a Biosorption Bed in the Treatment of Groundwater. Appl. Sci. 2019, 9, 751. https://doi.org/10.3390/app9040751
Papciak D, Domoń A, Puszkarewicz A, Kaleta J. The Use of Chalcedonite as a Biosorption Bed in the Treatment of Groundwater. Applied Sciences. 2019; 9(4):751. https://doi.org/10.3390/app9040751
Chicago/Turabian StylePapciak, Dorota, Andżelika Domoń, Alicja Puszkarewicz, and Jadwiga Kaleta. 2019. "The Use of Chalcedonite as a Biosorption Bed in the Treatment of Groundwater" Applied Sciences 9, no. 4: 751. https://doi.org/10.3390/app9040751





