Biomechanical Evaluation of a New Fixation Type in 3D-Printed Periacetabular Implants using a Finite Element Simulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Information
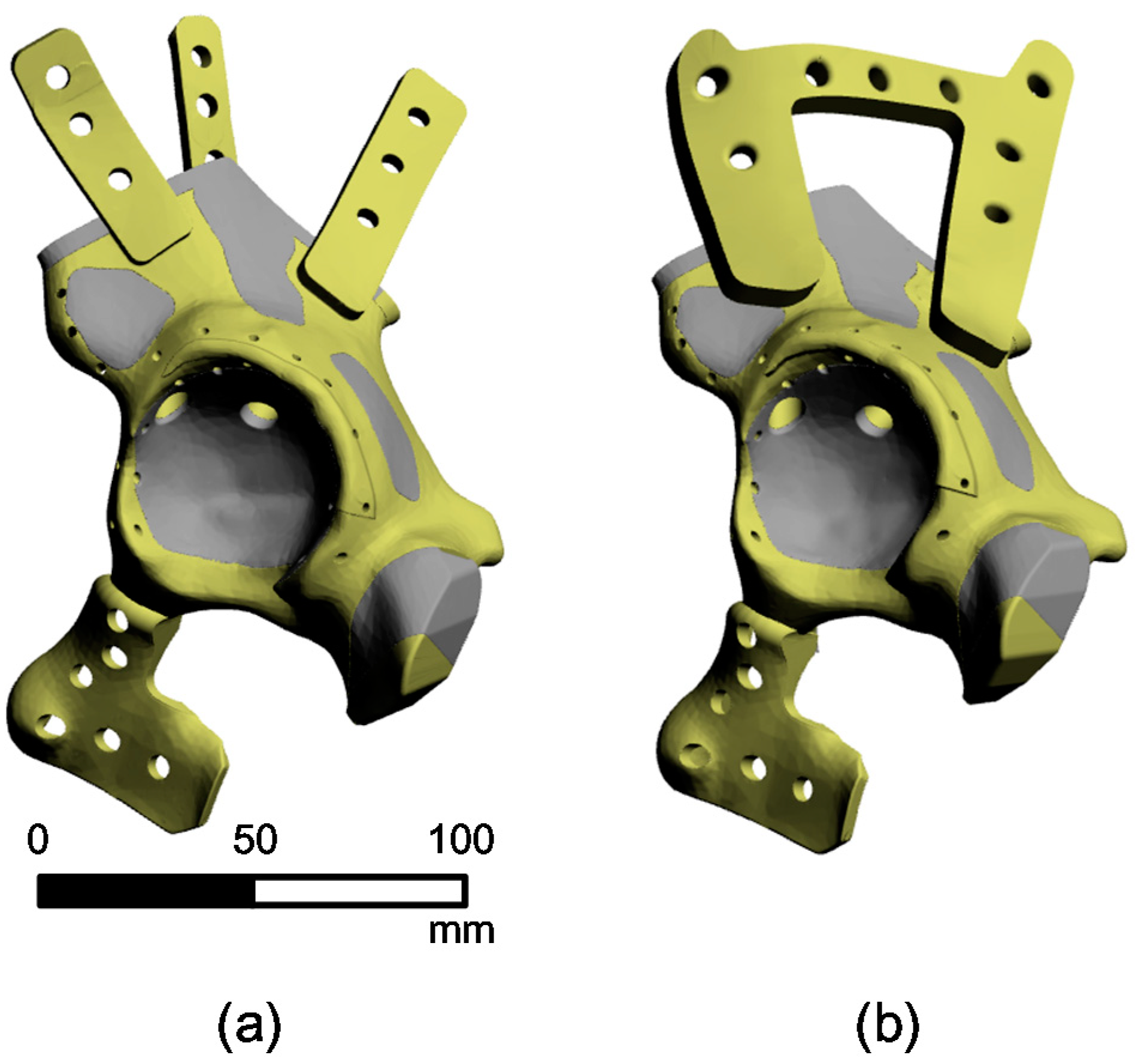
2.2. 3D-Reconstruction of the Pelvic Implant Model
2.3. Finite Element Simulation of a Pelvic Implant Model
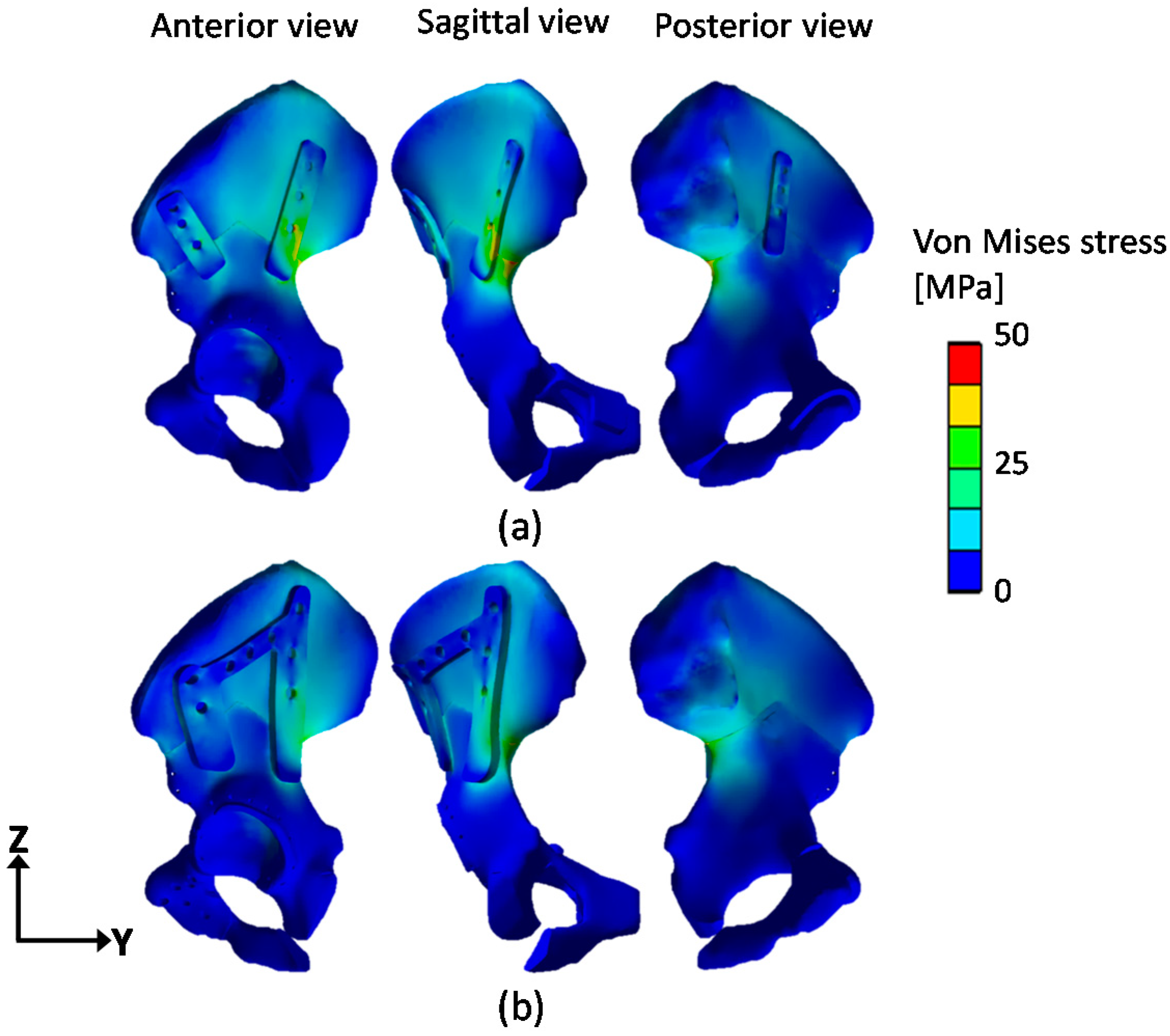
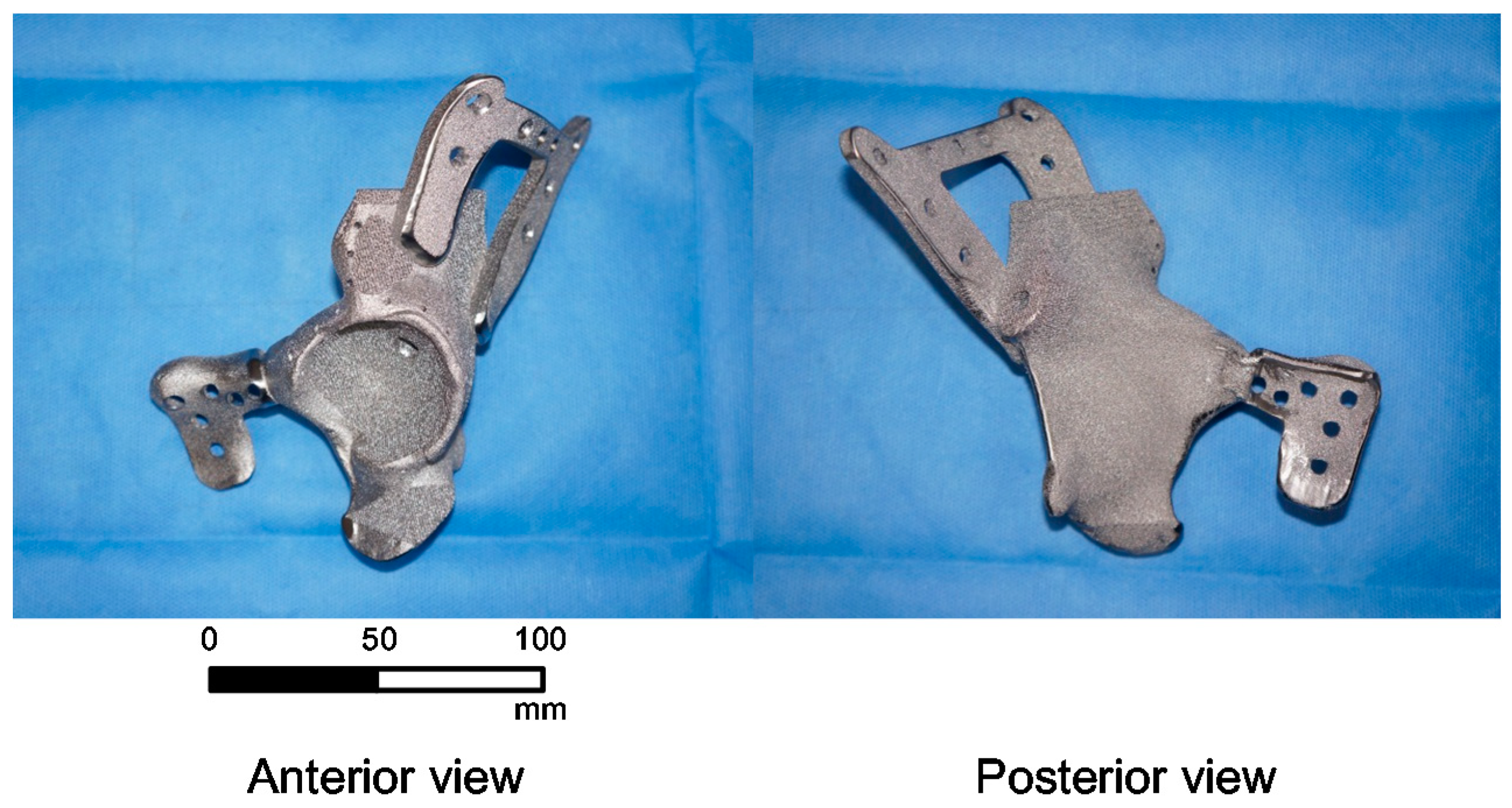
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, K.C.; Kumta, S.M.; Geel, N.V.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided. Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Fu, J.; Li, X.; Pei, Y.; Li, X.; Pei, G.; Guo, Z. Implantation of customized 3-D printed titanium prosthesis in limb salvage surgery: A case series and review of the literature. World J. Surg. Oncol. 2015, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. 2015, 34, 369–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Cronskär, M.; Bäckström, M.; Rännar, L.-E. Production of customized hip stem prostheses—A comparison between conventional machining and electron beam melting (EBM). Rapid Prototyp. J. 2013, 19, 365–372. [Google Scholar]
- Mazzoli, A.; Germani, M.; Raffaeli, R. Direct fabrication through electron beam melting technology of custom cranial implants designed in a PHANToM-based haptic environment. Mater. Des. 2009, 30, 3186–3192. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lamber, C.S. Customised titanium implant fabricated in additive manufacturing for craniomaxillofacial surgery. Virtual Phys. Prototyp. 2014, 9, 115–125. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosaemail, M.A.; Filho, R.M.; de Carvalho Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Craniomaxillofac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Dong, P.; Li, Z.; Zhu, F.; Wang, Z.; Cai, X. Biomechanical analysis of the fixation systems for anterior column and posterior hemi-transverse acetabular fractures. Acta Orthopaedica et Traumatologica Turcica 2017, 51, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H.; Wieland, T.; Pajenda, G.; Vecsei, V.; Mousavi, M. Minimally invasive ilioinguinal approach to the acetabulum. Injury 2007, 38, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.V.; Nork, S.E.; Chip, R.M.L. Perioperative complications associated with operative treatment of acetabular fractures. J. Trauma Acute Care Surg. 2001, 51, 1098–1103. [Google Scholar] [CrossRef]
- Ebraheim, N.A.; Savolaine, E.R.; Hoeflinger, M.J.; Jackson, W.T. Radiological diagnosis of screw penetration of the hip joint in acetabular fracture reconstruction. J. Orthop. Trauma 1989, 3, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Sawaguchi, T.; Brown, T.D.; Rubash, H.E.; Mears, D.C. Stability of acetabular fractures after internal fixation: A cadaveric study. Acta Orthop. 1984, 55, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Schopfer, A.; DiAngelo, D.; Hearn, T.; Powell, J.; Tile, M. Biomechanical comparison of methods of fixation of isolated osteotomies of the posterior acetabular column. Int. Orthop. 1994, 18, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.A.; Elbahri, H.; Shanmugam, R. Biomechanical analysis of a novel acetabulum reconstruction technique with acetabulum reconstruction cage and threaded rods after type II pelvic resections. Sarcoma 2016, 2016, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Plessers, K.; Mau, H. Stress analysis of a Burch-Schneider cage in an acetabular bone defect: A case study. Reconstr. Rev. 2016, 6, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Atik, F.; Ataç, M.S.; Özkan, A.; Kilinç, Y.; Arslan, M. Biomechanical analysis of titanium fixation plates and screws in mandibular angle fractures. Niger. J. Clin. Pract. 2016, 19, 386–390. [Google Scholar] [PubMed]
- Zhang, B.C.; Liu, H.B.; Kai, X.H.; Wang, Z.H.; Xu, F.; Kang, H.; Ding, R.; Luo, X.Q. Biomechanical comparison of a novel transoral atlantoaxial anchored cage with established fixation technique—A finite element analysis. BMC Musculoskelet. Disord. 2015, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.K.; Mehta, A.; Jangra, J.; Faizan, A.; Kiapour, A.; Hoy, R.W.; Fauth, A.R. Anatomic facet replacement system (AFRS) restoration of lumbar segment mechanics to intact: A finite element study and in vitro cadaver investigation. SAS J. 2007, 1, 46–54. [Google Scholar] [CrossRef]
- Chang, J.K.; Gill, S.S.; Zura, R.D.; Krause, W.R.; Wang, G.J. Comparative strength of three methods of fixation of transverse acetabular fractures. Clin. Orthop. Relat. Res. 2001, 392, 433–441. [Google Scholar] [CrossRef]
- Shazar, N.; Brumback, R.J.; Novak, V.P.; Belkoff, S.M. Biomechanical evaluation of transverse acetabular fracture fixation. Clin. Orthop. Relat. Res. 1998, 352, 215–222. [Google Scholar] [CrossRef]
- Xin-wei, L.; Shuo-gui, X.; Chun-cai, Z.; Qing-ge, F.; Pan-feng, W. Biomechanical study of posterior wall acetabular fracture fixation using acetabular tridimensional memory alloy-fixation system. Clin. Biomech. 2010, 25, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.A.; Bay, B.K.; Chapman, M.W.; Sharkey, N.A. Biomechanical consequences of fracture and repair of the posterior wall of the acetabulum. J. Bone Joint Surg. Am. 1995, 77, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.S.R.; Dessouki, O.; Samiezadeh, S.; Bougherara, H.; Schemitsch, E.H.; Zero, R. Biomechanical analysis using FEA and experiments of a standard plate method versus three cable methods for fixing acetabular fractures with simultaneous THA. Med. Eng. Phys. 2017, 46, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip joint loading during walking and running, measured in two patients. J. Biomech. 1993, 26, 969–990. [Google Scholar] [CrossRef]
- Harrysson, O.L.A.; Cansizoglu, O.; Marcellin-Little, D.J.; Cormier, D.R.; West II, H.A. Direct metal fabrication of titanium implants with tailored materials and mechanical properties using electron beam melting technology. Mater. Sci. Eng. C 2008, 28, 366–373. [Google Scholar] [CrossRef]
- Shen, F.H.; Mason, J.R.; Shimer, A.L.; Arlet, V.M. Pelvic fixation for adult scoliosis. Eur. Spine J. 2013, 22 (Suppl. 2), 265–275. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.S.; Northmore-Ball, M.D.; Tanner, K.E. Effects of acetabular resurfacing component material and fixation on the strain distribution in the pelvis. Proc. Inst. Mech. Eng. 2002, 216, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cai, X.; Liu, X.; Zhang, H. Biomechanical analysis of the acetabular buttressplate: Are complex acetabular fractures in the quadrilateral area stable after treatment with anterior construct plate-1/3 tube buttress plate fixation? Clinics 2013, 68, 1028e1033. [Google Scholar] [CrossRef]
- Andersen, R.C.; O’Toole, R.V.; Nascone, J.W.; Sciadini, M.F.; Frisch, H.M.; Turen, C.W. Modified stoppa approach for acetabular fractures with anterior and posterior column displacement: Quantification of radiographic reduction and analysis of interobserver variability. J. Orthop. Trauma 2010, 24, 271e278. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Black, J. Biological Performance of Materials, Fundamentals of Biocompatibility, 3rd ed.; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Thelen, S.; Barthelat, F.; Brinson, L.C. Mechanics considerations for microporous titanium as an orthopedic implant material. J. Biomed. Mater. Res. A 2004, 69A, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Dalstra, M.; Huiskes, R.; van Erning, L. Development and validation of a three-dimensional finite element model of the pelvic bone. J. Biomech. Eng. 1995, 117, 272–278. [Google Scholar] [CrossRef] [PubMed]



| Material | Density (g/cm3) | Young’s Modulus (MPa) | Poisson’s Ratio (v) | Tensile Strength (MPa) | Compressive Strength (MPa) |
|---|---|---|---|---|---|
| Cortical bone | 1.64 | 16,700 | 0.26 | 106 | 157 |
| Cancellous bone | 0.16 | 155 | 0.30 | 6 | 6 |
| Ti-6Al-4V | 4.62 | 96,000 | 0.36 | 1070 | 1070 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, D.W.; Lim, A.; Park, J.W.; Lim, K.M.; Kang, H.G. Biomechanical Evaluation of a New Fixation Type in 3D-Printed Periacetabular Implants using a Finite Element Simulation. Appl. Sci. 2019, 9, 820. https://doi.org/10.3390/app9050820
Park DW, Lim A, Park JW, Lim KM, Kang HG. Biomechanical Evaluation of a New Fixation Type in 3D-Printed Periacetabular Implants using a Finite Element Simulation. Applied Sciences. 2019; 9(5):820. https://doi.org/10.3390/app9050820
Chicago/Turabian StylePark, Dae Woo, Aekyeong Lim, Jong Woong Park, Kwon Mook Lim, and Hyun Guy Kang. 2019. "Biomechanical Evaluation of a New Fixation Type in 3D-Printed Periacetabular Implants using a Finite Element Simulation" Applied Sciences 9, no. 5: 820. https://doi.org/10.3390/app9050820





