Electrospun Nanometer to Micrometer Scale Biomimetic Synthetic Membrane Scaffolds in Drug Delivery and Tissue Engineering: A Review
Abstract
1. Introduction
2. Understanding the Basement Membrane
2.1. Influence of Age on the Basement Membrane
2.2. Influence of Disease on the Basement Membrane
2.3. Importance of Basement Membrane in Tissue Engineering
2.4. Importance of the Basement Membrane in Drug Delivery
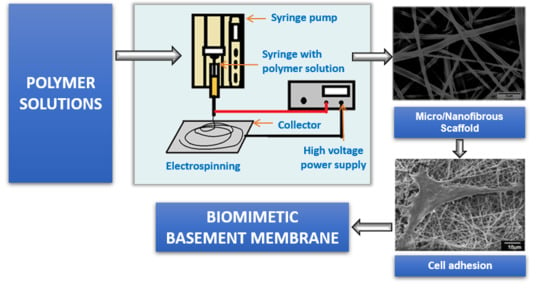
3. Electrospinning
3.1. Biomimetic Applications of Electrospun Nano Structures for Tissue Engineering Purposes
3.2. Applications of Electrospun Nano Structures for Drug Delivery Purposes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cruz-Acuña, R.; García, A. Synthetic hydrogels mimicking basement membrane matrices to promote cell-matrix interactions. Matrix Biol. 2017, 57–58, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Garland, S.; McKee, C.; Chang, Y.; Raghunathan, V.; Russell, P.; Murphy, C. A Cell Culture Substrate with Biologically Relevant Size-Scale Topography and Compliance of the Basement Membrane. Langmuir 2014, 30, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Falcone, F.; Stolnik, S.; Garnett, M. Basement membrane influences intestinal epithelial cell growth and presents a barrier to the movement of macromolecules. Exp. Cell. Res. 2014, 323, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Boivin, M.; Ye, D.; Pedram, A.; Said, H. Mechanism of TNF-α modulation of Caco-2 intestinal epithelial tight junction barrier: Role of myosin light-chain kinase protein expression. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005, 288, G422–G430. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M.; Urbano, M.; Valzasina, B.; Francolini, M.; Rotta, G.; Bonasio, R.; Granucci, F.; Kraehenbuhl, J.; Ricciardi-Castagnoli, P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001, 2, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Fowler, R.; Vllasaliu, D.; Trillo, F.; Garnett, M.; Alexander, C.; Horsley, H.; Smith, B.; Whitcombe, I.; Eaton, M.; Stolnik, S. Nanoparticle Transport in Epithelial Cells: Pathway Switching Through Bioconjugation. Small 2013, 9, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Hassan, I.; Obray, H.; Shah, R.; Wilson, G. The Transport of Vitamin B12 Through Polarized Monolayers of Caco-2 Cells. Gastroenterology 1990, 98, 1272–1279. [Google Scholar] [CrossRef]
- Tocce, E.; Liliensiek, S.; Wilson, M.; Yanez-Soto, B.; Nealey, P.; Murphy, C. Engineering the Biophysical Properties of Basement Membranes into Biomaterials: Fabrication and Effects on Cell Behavior. Compre. Biomater. 2011, 527–546. [Google Scholar]
- Bajaj, P.; Schweller, R.; Khademhosseini, A.; West, J.; Bashir, R. 3D Biofabrication Strategies for Tissue Engineering and Regenerative Medicine. Annu. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Merker, H. Morphology of the basement membrane. Microsc. Res. Tech. 1994, 28, 95–124. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, M.; Sherwood, D. An active role for basement membrane assembly and modification in tissue sculpting. J. Cell Sci. 2015, 128, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Fidler, A.; Vanacore, R.; Chetyrkin, S.; Pedchenko, V.; Bhave, G.; Yin, V.; Stothers, C.; Rose, K.; McDonald, W.; Clark, T.; et al. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. 2013, 111, 331–336. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Ponec, M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004, 12, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Glentis, A.; Gurchenkov, V.; Vignjevic, D. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adhes. Migrat. 2014, 8, 236–245. [Google Scholar] [CrossRef]
- Kruegel, J.; Miosge, N. Basement membrane components are key players in specialized extracellular matrices. Cell. Mol. Life Sci. 2010, 67, 2879–2895. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.; Lohmer, L.; Hagedorn, E.; Sherwood, D. Traversing the basement membrane in vivo: A diversity of strategies. J. Cell Biol. 2014, 204, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P. Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harb. Perspect. Biol. 2010, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Wiradjaja, F.; DiTommaso, T.; Smyth, I. Basement membranes in development and disease. Birth Defects Res. Part C: Embryo Today: Rev. 2010, 90, 8–31. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.; Zoeller, J.; Nyström, A. Basement membrane proteoglycans: Modulators Par Excellence of cancer growth and angiogenesis. Mol. and Cells 2009, 27, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.; Roos, W. Elucidating the molecular mechanisms underlying cellular response to biophysical cues using synthetic biology approaches. Cell Adhes. Migrat. 2016, 10, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Flemming, R.; Murphy, C.; Abrams, G.; Goodman, S.; Nealey, P. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 1999, 20, 573–588. [Google Scholar] [CrossRef]
- Discher, D. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Schittny, J. High resolution immunoelectron microscopic localization of functional domains of laminin, nidogen, and heparan sulfate proteoglycan in epithelial basement membrane of mouse cornea reveals different topological orientations. J. Cell Biol. 1988, 107, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.; Patton, B. Developmental and Pathogenic Mechanisms of Basement Membrane Assembly. Curr. Pharm. Des. 2009, 15, 1277–1294. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Oertle, P.; Monnier, C.; Camenzind, L.; Reyes-Lua, M.; Hu, H.; Candiello, J.; Labilloy, A.; Balasubramani, M.; Henrich, P.; et al. New concepts in basement membrane biology. FEBS J. 2015, 282, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Cole, G.; Halfter, W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Balasubramani, M.; Schreiber, E.; Cole, G.; Mayer, U.; Halfter, W.; Lin, H. Biomechanical properties of native basement membranes. FEBS J. 2007, 274, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Henrich, P.; Monnier, C.; Halfter, W.; Haritoglou, C.; Strauss, R.; Lim, R.; Loparic, M. Nanoscale Topographic and Biomechanical Studies of the Human Internal Limiting Membrane. Invest. Opthalmol. Vis. Sci. 2012, 53, 2561. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Monnier, C.; Müller, D.; Oertle, P.; Uechi, G.; Balasubramani, M.; Safi, F.; Lim, R.; Loparic, M.; Henrich, P. The Bi-Functional Organization of Human Basement Membranes. PLoS ONE 2013, 8, e67660. [Google Scholar] [CrossRef] [PubMed]
- Behrens, D.; Villone, D.; Koch, M.; Brunner, G.; Sorokin, L.; Robenek, H.; Bruckner-Tuderman, L.; Bruckner, P.; Hansen, U. The Epidermal Basement Membrane Is a Composite of Separate Laminin- or Collagen IV-containing Networks Connected by Aggregated Perlecan, but Not by Nidogens. J. Biol. Chem. 2012, 287, 18700–18709. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, F.; Palacios, S.; Alemañ, N.; Guerrero, F. Changes of the basement membrane and type IV collagen in human skin during aging. Maturitas 1996, 25, 209–215. [Google Scholar] [CrossRef]
- Alvarado, J.; Murphy, C.; Juster, R. Age-related changes in the basement membrane of the human corneal epithelium. Invest. Ophthalmol. Visual Sci. 1983, 24, 1015–1028. [Google Scholar]
- Richardson, LL.; Kleinman, HK.; Dym, M. The effects of aging on basement membrane in the testis. J. Androl. 1995, 16, 118–126. [Google Scholar] [PubMed]
- Neumann, K.; Kellner, C.; Kuhn, K.; Stolte, H.; Schurek, H. Age-dependent thickening of glomerular basement membrane has no major effect on glomerular hydraulic conductivity. Nephrol. Dial. Transpl. 2004, 19, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Van Agtmael, T.; Bruckner-Tuderman, L. Basement membranes and human disease. Cell Tissue Res. 2009, 339, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Gubler, M. Inherited diseases of the glomerular basement membrane. Nat. Clin. Pract. Nephrol. 2008, 4, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Kayaselcuk, F.; Serin, E.; Gumurdulu, Y.; Ozer, B.; Tuncer, I.; Boyacioglu, S. Subepithelial basement membrane thickness in patients with normal colonic mucosal appearance in colonoscopy: Results from southern Turkey. World J. Gastroenterol. 2004, 10, 1056. [Google Scholar] [CrossRef]
- Eltboli, O.; Mistry, V.; Barker, B.; Brightling, C. Relationship between blood and bronchial submucosal eosinophilia and reticular basement membrane thickening in chronic obstructive pulmonary disease. Respirology 2015, 20, 667–670. [Google Scholar] [CrossRef] [PubMed]
- McAteer, JA.; Dougherty, GS.; Gardner, JK.; Evan, AP. Polarized epithelial cysts in vitro: A review of cell and explant culture systems that exhibit epithelial cyst formation. Scanning Microsc. 1988, 2, 1739–1763. [Google Scholar] [PubMed]
- Haigo, S.; Bilder, D. Global Tissue Revolutions in a Morphogenetic Movement Controlling Elongation. Science 2011, 331, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Harunaga, J.; Doyle, A.; Yamada, K. Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev. Biol. 2014, 394, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Thanou, M.; Stolnik, S.; Fowler, R. Recent advances in oral delivery of biologics: Nanomedicine and physical modes of delivery. Expert Opin. Drug Discovery 2018, 15, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Mantaj, J.; Abu-Shams, T.; Enlo-Scott, Z.; Swedrowska, M.; Vllasaliu, D. Role of the Basement Membrane as an Intestinal Barrier to Absorption of Macromolecules and Nanoparticles. Mol. Pharm. 2018, 15, 5802–5808. [Google Scholar] [CrossRef] [PubMed]
- Alfano, M.; Chasens, A.; Masi, C. Autoradiographic study of the penetration of radiolabelled dextrans and inulin through non-keratinized oral mucosain vitro. J. Periodontal Res. 1977, 12, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Doshi, J.; Reneker, D. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Ahmed, F.; Lalia, B.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalin. 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Lu, W.; Sun, J.; Jiang, X. Recent advances in electrospinning technology and biomedical applications of electrospun fibers. J. Mater. Chem. B 2014, 2, 2369. [Google Scholar] [CrossRef]
- Thavasi, V.; Singh, G.; Ramakrishna, S. Electrospun nanofibers in energy and environmental applications. Energy Environ. Sci. 2008, 1, 205. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Zarghami, N.; Melat Yar, H.; Akbarzadeh, A. Nanofiber: Synthesis and biomedical applications. Artif. Cells Nanomed. Biotechnol. 2014, 44, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Pham, Q.; Sharma, U.; Mikos, A. Electrospinning of Polymeric Nanofibers for Tissue Engineering Applications: A Review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Laurencin, C.; Caterson, E.; Tuan, R.; Ko, F. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Xu, C.; Kotaki, M.; Ramakrishna, S. Electrospun P(LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials 2004, 25, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.; Boland, G.; Tuli, R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 2003, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tuli, R.; Huang, X.; Laquerriere, P.; Tuan, R. Multilineage differentiation of human mesenchymal stem cells in a three-dimensional nanofibrous scaffold. Biomaterials 2005, 26, 5158–5166. [Google Scholar] [CrossRef] [PubMed]
- Manea, L.; Hristian, L.; Leon, A.; Popa, A. Recent advances of basic materials to obtain electrospun polymeric nanofibers for medical applications. IOP Conf. Ser. Mater. Sci. Eng. 2016, 145, 032006. [Google Scholar] [CrossRef]
- Sell, S.; Wolfe, P.; Garg, K.; McCool, J.; Rodriguez, I.; Bowlin, G. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Matthews, J.; Wnek, G.; Simpson, D.; Bowlin, G. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.; Sell, S.; Boland, E.; Simpson, D.; Bowlin, G. Nanofiber technology: Designing the next generation of tissue engineering scaffolds. Adv. Drug Delivery Rev. 2007, 59, 1413–1433. [Google Scholar] [CrossRef] [PubMed]
- Zahari, N.; Idrus, R.; Chowdhury, S. Laminin-Coated Poly(Methyl Methacrylate) (PMMA) Nanofiber Scaffold Facilitates the Enrichment of Skeletal Muscle Myoblast Population. Int. J. Mol. Sci. 2017, 18, 2242. [Google Scholar] [CrossRef] [PubMed]
- Kidoaki, S.; Kwon, I.; Matsuda, T. Mesoscopic spatial designs of nano- and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 2005, 26, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Fesharaki, M.; Razavi, S.; Ghasemi-Mobarakeh, L.; Behjati, M.; Yarahmadian, R.; Kazemi, M.; Hejazi, H. Differentiation of Human Scalp Adipose-Derived Mesenchymal Stem Cells into Mature Neural Cells on Electrospun Nanofibrous Scaffolds for Nerve Tissue Engineering Applications. Cell J. (Yakhteh). 2018, 20. [Google Scholar]
- Binulal, N.; Natarajan, A.; Menon, D.; Bhaskaran, V.; Mony, U.; Nair, S. PCL–gelatin composite nanofibers electrospun using diluted acetic acid–ethyl acetate solvent system for stem cell-based bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 25, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Jun, I.; Shin, Y.; Jang, W.; Kim, S.; Shin, H. The Development of Genipin-Crosslinked Poly(caprolactone) (PCL)/Gelatin Nanofibers for Tissue Engineering Applications. Macromol. Biosci. 2010, 10, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yan, S.; Dai, J.; Lu, Y.; Wang, Y.; Sun, M.; Gong, J.; Yao, Y. Human lung epithelial cells A549 epithelial-mesenchymal transition induced by PVA/Collagen nanofiber. Colloids Surf. B 2018, 162, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Haddad, T.; Noel, S.; Liberelle, B.; El Ayoubi, R.; Ajji, A.; De Crescenzo, G. Fabrication and surface modification of poly lactic acid (PLA) scaffolds with epidermal growth factor for neural tissue engineering. Biomatter 2016, 6, e1231276. [Google Scholar] [CrossRef] [PubMed]
- Lafond, E.; Lawson, A.; Niemeier, R.; Cady, C.; Nair, K. Biocompatibility of human Whartons Jelly Mesenchymal Stem Cells on poly-caprolactone and collagen based nanofiber mats. In Proceedings of the 2015 41st Annual Northeast Biomedical Engineering Conference (NEBEC), Troy, NY, USA, 17–19 April 2015; pp. 1–2. [Google Scholar]
- Shalumon, K.; Deepthi, S.; Anupama, M.; Nair, S.; Jayakumar, R.; Chennazhi, K. Fabrication of poly (l-lactic acid)/gelatin composite tubular scaffolds for vascular tissue engineering. Int. J. Biol. Macromol. 2015, 72, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Asadollahi, M.; Ghaedi, K.; Salehi, H.; Arpanaei, A. Electrospun aligned PLGA and PLGA/gelatin nanofibers embedded with silica nanoparticles for tissue engineering. Int. J. Biol. Macromol. 2015, 79, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.; Lenz, S.; Wang, T.; Abebayehu, D.; Brooks, B.; Ogle, R.; Botchwey, E. Laminin- and basement membranepolycaprolactone blend nanofibers as a scaffold for regenerative medicine. Nanomater. Environ. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Binulal, N.; Deepthy, M.; Selvamurugan, N.; Shalumon, K.; Suja, S.; Mony, U.; Jayakumar, R.; Nair, S. Role of Nanofibrous Poly(Caprolactone) Scaffolds in Human Mesenchymal Stem Cell Attachment and Spreading for In Vitro Bone Tissue Engineering—Response to Osteogenic Regulators. Tissue Eng. Part A 2010, 16, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.; Park, C. Human endothelial cell growth on mussel-inspired nanofiber scaffold for vascular tissue engineering. Biomaterials 2010, 31, 9431–9437. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.; McClugage, S.; Link, M.; Sefcik, L.; Ogle, R.; Botchwey, E. Laminin Nanofiber Meshes That Mimic Morphological Properties and Bioactivity of Basement Membranes. Tissue Eng. Part C 2009, 15, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Bye, F.; Bullock, A.; Singh, R.; Sefat, F.; Roman, S.; MacNeil, S. Development of a Basement Membrane Substitute Incorporated Into an Electrospun Scaffold for 3D Skin Tissue Engineering. J. Biomater. Tissue Eng. 2014, 4, 686–692. [Google Scholar] [CrossRef]
- Kanie, K.; Narita, Y.; Zhao, Y.; Kuwabara, F.; Satake, M.; Honda, S.; Kaneko, H.; Yoshioka, T.; Okochi, M.; Honda, H.; et al. Collagen type IV-specific tripeptides for selective adhesion of endothelial and smooth muscle cells. Biotechnol. Bioeng. 2012, 109, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Dohle, E.; Singh, S.; Nishigushi, A.; Fischer, T.; Wessling, M.; Möller, M.; Sader, R.; Kasper, J.; Ghanaati, S.; Kirkpatrick, C. Human Co- and Triple-Culture Model of the Alveolar-Capillary Barrier on a Basement Membrane Mimic. Tissue Eng. Part C 2018, 24, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, A.; Singh, S.; Wessling, M.; Kirkpatrick, C.; Möller, M. Basement Membrane Mimics of Biofunctionalized Nanofibers for a Bipolar-Cultured Human Primary Alveolar-Capillary Barrier Model. Biomacromolecules 2017, 18, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Böttjer, R.; Grothe, T.; Wehlage, D.; Ehrmann, A. Electrospraying poloxamer/(bio-)polymer blends using a needleless electrospinning machine. J. Textiles Fibrous Mater. 2018, 1, 2515221117743079. [Google Scholar] [CrossRef]
- Aytimur, A.; Koçyiğit, S.; Uslu, İ. Synthesis and Characterization of Poly(vinyl alcohol)/Poly(vinyl pyrrolidone)-Iodine Nanofibers with Poloxamer 188 and Chitosan. Polym. Plast. Technol. Eng. 2013, 52, 661–666. [Google Scholar] [CrossRef]
- Kerleta, V.; Andrlik, I.; Braunmuller, S.; Franke, T.; Wirth, M.; Gabor, F. Poloxamer 188 supplemented culture medium increases the vitality of Caco-2 cells after subcultivation and freeze/thaw cycles. Altex 2010, 27, 191–197. [Google Scholar] [CrossRef]
- Jun, I.; Han, H.; Edwards, J.; Jeon, H. Electrospun Fibrous Scaffolds for Tissue Engineering: Viewpoints on Architecture and Fabrication. Int. J. Mol. Sci. 2018, 19, 745. [Google Scholar] [CrossRef] [PubMed]
- Levorson, E.; Raman Sreerekha, P.; Chennazhi, K.; Kasper, F.; Nair, S.; Mikos, A. Fabrication and characterization of multiscale electrospun scaffolds for cartilage regeneration. Biomed. Mater. 2013, 8, 014103. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, K.E.; Kim, M.H.; You, H.K.; Lee, J.; Park, W.H. Effect of nanofiber content on bone regeneration of silk fibroin/poly(epsilon-caprolactone) nano/microfibrous composite scaffolds. Int. J. Nanomed. 2015, 10, 485–502. [Google Scholar]
- Lu, Y.; Huang, J.; Yu, G.; Cardenas, R.; Wei, S.; Wujcik, E.; Guo, Z. Coaxial electrospun fibers: Applications in drug delivery and tissue engineering. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 654–677. [Google Scholar] [CrossRef] [PubMed]
- Bulysheva, A.A.; Bowlin, G.L.; Klingelhutz, A.J.; Yeudall, W.A. Low-temperature electrospun silk scaffold for in vitro mucosal modeling. J. Biomed. Mater. Res. A 2012, 100A, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Tourlomousis, F.; Ding, H.; Kalyon, D.M.; Chang, R.C. Melt Electrospinning Writing Process Guided by a “Printability Number”. J. Manuf. Sci. Eng. 2017, 139, 081004. [Google Scholar] [CrossRef]
- Jenkins, T.L.; Meehan, S.; Pourdeyhimi, B.; Little, D. Meltblown Polymer Fabrics as Candidate Scaffolds for Rotator Cuff Tendon Tissue Engineering. Tissue Eng. Part A 2017, 23, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.X.; Teh, K.S.; Zang, X.N.; Wu, D.Z.; Wen, Z.Y.; Lin, L.W. High Aspect-Ratio 3d Microstructures Via near-Field Electrospinning for Energy Storage Applications. In Proceedings of the 2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; pp. 29–32. [Google Scholar]
- Fattahi, P.; Dover, J.T.; Brown, J.L. 3D Near-Field Electrospinning of Biomaterial Microfibers with Potential for Blended Microfiber-Cell-Loaded Gel Composite Structures. Adv. Healthc. Mater. 2017, 6, 1700456. [Google Scholar] [CrossRef] [PubMed]
- Jordahl, J.; Solorio, L.; Sun, H.; Ramcharan, S.; Teeple, C.; Haley, H.; Lee, K.; Eyster, T.; Luker, G.; Krebsbach, P.; et al. 3D Jet Writing: Functional Microtissues Based on Tessellated Scaffold Architectures. Adv. Mater. 2018, 30, 1707196. [Google Scholar] [CrossRef] [PubMed]
- Smith-Freshwater, A.; Bowlin, G.; Yang, H. A Novel Electrospun Dendrimer-Gelatin Hybrid Nanofiber Scaffold for Tissue Regeneration and Drug Delivery. MRS Proc. 2008, 1094, DD1009–DD1007. [Google Scholar] [CrossRef]
- Chen, D.W.C.; Liao, J.Y.; Liu, S.J.; Chan, E.C. Novel biodegradable sandwich-structured nanofibrous drug-eluting membranes for repair of infected wounds: An in vitro and in vivo study. Int. J. Nanomed. 2012, 7, 763–771. [Google Scholar]
- Ding, Z.; Fan, Z.; Huang, X.; Lu, Q.; Xu, W.; Kaplan, D. Silk–Hydroxyapatite Nanoscale Scaffolds with Programmable Growth Factor Delivery for Bone Repair. ACS Appl. Mater. Interfaces 2016, 8, 24463–24470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, L.; Yan, Z.; Yu, Q.; Mo, X. Electrospun Biomimic Nanofibrous Scaffolds of Silk Fibroin/Hyaluronic Acid for Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2012, ahead-of-print, 1–14. [Google Scholar] [CrossRef] [PubMed]

| Composition of Nanofibers | Applications | Property | Author | Year |
|---|---|---|---|---|
| PCL/gelatin composite | Nerve tissue engineering—Human Scalp Adipose-Derived Mesenchymal Stem Cells (SADS) | Nanofibrous scaffold coated with platelet rich plasma. Cell proliferation and differentiation into neural cells observed. | Fesharaki et al. [65] | 2018 |
| PVA/collagen composite | Human lung epithelial cell A549 | Glutaraldehyde crosslinking, epithelial mesenchymal transition observed in nanofibers with a 170 nm average diameter | Li et al. [68] | 2018 |
| PMMA coated with collagen and laminin | Human myoblast and fibroblast cell growth | PMMA average fiber diameter = 360 nm, Genipin was used as a crosslinking agent, collagen promoted fibroblast proliferation and laminin promoted myoblast proliferation | Zahari et al. [63] | 2017 |
| PLA | Neural tissue engineering | PLA functionalized with polyallylamine and epidermal growth factor was covalently grafted to the amine group | Haddad et a l. [69] | 2016 |
| PCL/collagen composite | Human Wharton’s jelly mesenchymal stem cells | Fiber diameter =542–633 nm | Lafond et al. [70] | 2015 |
| poly (L-lactic acid)/gelatin | Vascular tissue engineering—Human umbilical vein endothelial cells, smooth muscle cells | Fiber diameter = 100–500 nm | Shalumon et al. [71] | 2015 |
| PLGA/gelatin | Stem cell culture | Fiber diameter = 267 nm, gelatin enhanced the hydrophilicity, the scaffold was imbedded with mesoporous silica nanoparticles to improve tensile strength, cell attachment and proliferation. | Mehrasa et al. [72] | 2015 |
| PCL/gelatin composite | Bone tissue engineering- Human mesenchymal stem cells | 30% to 40% gelatin in nanofibers provided optimum hydrophilicity, degradability and promoted cell growth | Binulal et al. [66] | 2014 |
| PCL/laminin | Human embryonic stem cell culture | Fiber diameter = 31–780 nm, promoted peripheral nerve regeneration | Neal et al. [73] | 2014 |
| PCL/gelatin composite | Muscle tissue engineering- myoblasts | Genipin as a crosslinking agent, average fiber diameter = 250–350 nm, promising cell proliferation | Kim et al. [67] | 2010 |
| PCL | Human mesenchymal cell growth | Fiber average diameter = 277 nm, Nanofibers promote cell attachment, spreading and differentiation compared to microfibers | Binulal et al. [74] | 2010 |
| PCL | Human umbilical vein endothelial cells | 700 nm fibers coated with poly(dopamine)(PDA), enhanced cell adhesion with an expression of cell markers | Ku et al. [75] | 2010 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazhanimala, S.K.; Vllasaliu, D.; Raimi-Abraham, B.T. Electrospun Nanometer to Micrometer Scale Biomimetic Synthetic Membrane Scaffolds in Drug Delivery and Tissue Engineering: A Review. Appl. Sci. 2019, 9, 910. https://doi.org/10.3390/app9050910
Pazhanimala SK, Vllasaliu D, Raimi-Abraham BT. Electrospun Nanometer to Micrometer Scale Biomimetic Synthetic Membrane Scaffolds in Drug Delivery and Tissue Engineering: A Review. Applied Sciences. 2019; 9(5):910. https://doi.org/10.3390/app9050910
Chicago/Turabian StylePazhanimala, Shaleena K., Driton Vllasaliu, and Bahijja T. Raimi-Abraham. 2019. "Electrospun Nanometer to Micrometer Scale Biomimetic Synthetic Membrane Scaffolds in Drug Delivery and Tissue Engineering: A Review" Applied Sciences 9, no. 5: 910. https://doi.org/10.3390/app9050910
APA StylePazhanimala, S. K., Vllasaliu, D., & Raimi-Abraham, B. T. (2019). Electrospun Nanometer to Micrometer Scale Biomimetic Synthetic Membrane Scaffolds in Drug Delivery and Tissue Engineering: A Review. Applied Sciences, 9(5), 910. https://doi.org/10.3390/app9050910