Featured Application
The prepared hydrophobic gold nanomaterials show excellent surface-enhanced Raman scattering activity and, therefore, could be used as efficient organic-soluble SERS substrates for the detection of many hydrophobic food toxicants, such as 3,4-benzopyrene, and carcinogens, such as benzidine.
Abstract
Many previously reported syntheses of gold nanoparticles required lengthy reaction times, complicated operations, high temperatures, or multi-step manipulations. In this work, a morphology-controlled versatile one-pot synthesis of hydrophobic gold nanodots, nanobars, nanorods, and nanowires has been developed. A series of gold nanomaterials ranging from round nanodots, short nanobars, and long nanorods to ultrathin and ultralong nanowires (diameter <2 nm, length >2 μm) have been readily prepared by simply adjusting the feeding ratio of chloroauric acid to oleylamine, oleic acid, and triphenylsilane. The silk-like ultralong and ultrathin nanowires were found to have a single crystalline structure and may have significant potential applications in microelectronics and biosensors. Large sizes of gold spherical nanoparticles were obtained from gold nanodots via a seed-mediated growth approach. These nanoparticles and ultralong nanowires showed excellent surface-enhanced Raman scattering (SERS) activity in organic solvents and, therefore, were employed as efficient organic-soluble SERS substrates for the detection of hydrophobic food toxicants, such as 3,4-benzopyrene, and carcinogens, such as benzidine.
1. Introduction
In the past decades, gold nanomaterials have attracted considerable interest because of their wide application in catalysis [1,2,3,4,5,6], biomedicine [7,8,9], biology [10,11,12], optics [13,14,15], and electronics [16]. Particularly, colloidal gold nanoparticles have been employed as a highly efficient substrate for surface-enhanced Raman scattering (SERS) for almost 30 years—since 1979 [17]; this is due to their exceptional SERS enhancement factor, excellent stability, good biocompatibility, and wide commercial availability [18,19].
Gold spherical nanoparticles have been generally prepared via a citrate method as reported by Turkevich and Frens [20,21,22], biphasic method [23], and thermolysis method [24]. The citrate method produces nearly monodispersed colloidal gold nanoparticles with an average size of 10 to 100 nm, and these nanoparticles have served extensively as excellent substrates for SERS detection of a large variety of water-soluble analytes, such as dyes, protein, DNA/RNA, and food additives [25,26]. Small-sized gold nanoparticles (<10 nm) soluble in low-polar and non-polar organic solvents were obtained by a one-phase method using amine derivatives or hydrosilane as reductants [27,28,29,30] or by the thermolysis method [24]. Recently, ultralong gold nanowires and ultrathin gold nanorods have been successfully synthesized using oleylamine or hydrosilane as a reducing agent [31,32] in organic solvents. The fabrication of the nanowires into aligned arrays for potential use in microelectronic devices have been achieved [33,34]. In addition, the application of these nanowires as a SERS substrate in organic solvents has also been demonstrated [35], even though the SERS enhancement factor of these nanomaterials was quite limited. Since many hydrophobic toxicants and carcinogens, such as 3,4-benzopyrene, aflatoxin, and benzidine, are practically insoluble in water, those hydrophilic colloidal gold nanoparticles are not applicable because of their immiscibility with hydrophobic analytes. Therefore, it is still of considerable interest for chemists to develop a synthetic approach for the synthesis of hydrophobic gold nanomaterials with controllable and designable sizes and morphologies.
However, the intensive synthesis and use of gold nanomaterials may cause health risks due to potential nanoparticle exposure since the toxic effects of most commercial nanoparticles (e.g., Ag, Au, TiO2, etc.) on the human body have not been fully evaluated [36,37]. Morgeneyer recently observed the release of nanostructured objects in submicron size and their aggregates containing titanium dioxide from weathered industrial paints [38] and investigated the emission of nanoparticles from functional materials for medical applications and their aerosol formation [39]. Cases of nanoparticle exposure at workplaces during the synthesis of metal nanoparticles have also been reported [40]. Nano-safety and occupational health become a core subject in particle science [41]. As many previously reported syntheses of gold nanoparticles required lengthy reaction times, complicated operations, high temperatures, or multi-step manipulations, these approaches resulted in a greater chance of exposure to nanoparticles. Therefore, a fast and simple preparation of gold nanomaterials at mild conditions is highly desirable.
Herein, we report a versatile one-pot synthesis of hydrophobic gold nanomaterials including round nanodots, short nanobars, long nanorods, and silk-like ultralong and ultrathin nanowires by the reduction of chloroauric acid with hydrosilane in organic solvents at room temperature. The size and morphology of the gold nanomaterials are readily controlled by changing the feeding molar ratio of chloroauric acid to oleylamine (OAm), oleic acid (OA), and hydrosilane. The obtained gold nanoparticles are employed as efficient organic-soluble SERS substrates for the detection of hydrophobic toxicants, such as 3,4-benzopyrene, and carcinogens, such as benzidine. In addition, the long gold nanorods and silk-like ultralong nanowires are further used as solid SERS substrates for the measurement of strong polar toxicants, such as malachite green.
2. Materials and Methods
2.1. Instruments and Reagents
The morphology of prepared gold nanomaterials was observed on a transmission electron microscope (TEM, Tecnai G2 F30 S-Twin, FEI Company, Hillsboro, OR, The Netherlands) operating at an acceleration voltage of 300 kV. Dynamic light scattering (DLS) was also used to measure the size of the gold nanoparticles using a Nano ZS 90 Nanosizer (Malvern Instrument, Worcs, UK) equipped with a 628 nm laser source. The X-ray diffraction (XRD) patterns of Au nanomaterials were recorded on a PANalytical X’Pert PRO MRD X-Ray Diffraction System (PANalytical B. V., Almelo, The Netherlands) using a monochromatic Cu Kα source, λ = 1.5406 Å. SERS spectra were measured on a DeltaNu 785 Raman spectrometer (DeltaNu Inc., Laramie, WY, USA). The laser power of the spectrometer is 120 mW with an excitation wavelength at 785 nm and a spectral range of 200–2000 cm−1. The spectra were acquired with baseline off using NuSpec software (Copyright DeltaNu 2009) and analyzed using GRAMS/AI software (Ver 9.1, Thermo Fischer Scientific, Waltham, MA, USA).
Chloroauric acid (HAuCl4·4H2O, 99%) was purchased from Shanghai Siyu Chemical Technology Limited Company (Shanghai, China). The 3,4-benzopyrene (98%), benzidine (≥98%), and malachite green (AR) were supplied by Shanghai Macklin Biochemical Technology Limited Company (Shanghai, China). Chloroform (CHCl3, 99%), anhydrous methanol (CH3OH, 99.5%), anhydrous ethyl alcohol (CH3CH2OH, 99.5%), and acetone (99%) were obtained from Hangzhou Shuanglin Chemical Reagent Limited Company (Hangzhou, China). Triphenylsilane (TPS, 99%), oleic acid (AR), oleylamine (85–90%), hydroxylamine hydrochloride (98.5%), and dimethyl sulfoxide (DMSO, 99%) were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). All chemicals were used as received unless otherwise indicated.
2.2. Synthesis of Gold Nanomaterials
2.2.1. Synthesis of Gold Nanodots
A typical procedure for the synthesis of gold nanodots is as follows: First, 5.0 mg of HAuCl4·4H2O, 3.0 μL of oleic acid, and 15.0 μL of oleylamine were dissolved in 8.0 mL of chloroform, followed by the addition of 2.0 mL of 180.0 mM triphenylsilane (TPS) in chloroform to the mixture. The solution was stirred at a speed of 300 rpm using a Teflon (PTFE)-coated magnetic stirring bar at room temperature for 2.5 h and a pink solution of gold nanodots with an average diameter of 7.5 ± 2.3 nm was obtained. The pink solution was evaporated at room temperature and completely dried under vacuum to produce a dark powder of gold nanoparticles (2.1 mg, yield: 87.8%).
2.2.2. Synthesis of Large Spherical Gold Nanoparticles Via a Seed-Growth Approach
A typical procedure for the synthesis of spherical gold nanoparticles is as follows: First, 5.0 mg of HAuCl4·H2O, 3.0 μL of oleic acid (OA), and 15.0 μL of oleylamine (OAm) were completely dissolved in 8.0 mL of chloroform by constant stirring in an ampere bottle. Then, 2.0 mL of 180.0 mM triphenylsilane (dissolved in chloroform) was added to the mixture. The mixture was stirred at a speed of 300 rpm using a Teflon (PTFE)-coated magnetic stirring bar at room temperature for 2 h and a pink solution of gold nanodots was obtained. This solution served as a seed for the growth of spherical gold nanoparticles.
The large spherical gold nanoparticles were synthesized via a seed-growth approach. Typically, 1.0 mg of HAuCl4·4H2O, 1.2 μL of OA, and 6.0 μL of OAm were mixed in 5.0 mL of chloroform and employed as a growth solution. Then, 1.0 mL of the above prepared gold seed solution and 4.0 mL of chloroform were added to the growth solution, followed by the addition of 18.0 μL of 100.0 mM hydroxylamine hydrochloride in DMSO while stirring. The mixture solution was stirred at a speed of 300 rpm using a Teflon (PTFE)-coated magnetic stirring bar at room temperature for one and a half hours; finally, a ruby red solution of grown spherical gold nanoparticles was obtained. In this situation, the amount of HAuCl4·4H2O in the growth solution was twice the amount of HAuCl4·4H2O in the seed solution and correspondingly the growth/seed ratio was simply referred to as 2:1. Double, triple, and quadruple the amounts of HAuCl4·4H2O, OA, OAm, and hydroxylamine hydrochloride in the growth solution facilitated the synthesis of larger and larger spherical gold nanoparticles, and in these cases, the growth/seed ratios were designated as 4:1, 6:1, and 8:1, respectively.
2.2.3. Synthesis of Gold Nanobars
The gold nanobars were synthesized at a molar ratio of HAuCl4·4H2O:oleic acid:oleylamine = 1:48:45.6. Typically, 5.0 mg of HAuCl4·4H2O, 180.0 μL of oleic acid, and 180.0 μL of oleylamine were dissolved in 8.0 mL of chloroform, followed by the addition of 2.0 mL of 18.0 mM triphenylsilane in chloroform to the mixture. The solution was stirred at a speed of 300 rpm using a Teflon (PTFE)-coated magnetic stirring bar at room temperature for 4 h. A wine-red solution of short gold nanobars with an aspect ratio of about 1.5–3 was obtained.
2.2.4. Synthesis of Long Gold Nanorods
The long gold nanorods were synthesized at a molar ratio of HAuCl4·4H2O:oleic acid:oleylamine = 1:24:45.6. Typically, 5.0 mg of HAuCl4·4H2O, 90.0 μL of oleic acid, and 180.0 μL of oleylamine were dissolved in 8.0 mL of chloroform, followed by the addition of 2.0 mL of 18.0 mM triphenylsilane in chloroform to the mixture. The solution was stirred at a speed of 300 rpm using a Teflon (PTFE)-coated magnetic stirring bar at room temperature for 4 h. A yellow–light brown solution of long gold nanorods with a length in the range of tenths of nanometers to several hundred nanometers was obtained.
2.2.5. Synthesis of Silk-Like Ultralong and Ultrathin Gold Nanowires
The silk-like ultralong and ultrathin gold nanowires were synthesized at a molar ratio of HAuCl4·4H2O:oleic acid:oleylamine = 1:24:114. Typically, 5.0 mg of HAuCl4·4H2O, 90.0 μL of oleic acid, and 450.0 μL of oleylamine were dissolved in 8.0 mL of chloroform, followed by the addition of 2.0 mL of 18.0 mM triphenylsilane in chloroform to the mixture. The solution was stirred at a speed of 300 rpm using a Teflon (PTFE)-coated magnetic stirring bar at room temperature for 6 hours. A deep brown solution of gold nanowires with ultralong length (>2 μm) and ultrathin diameter (<2 nm) was obtained.
2.3. SERS Measurements
2.3.1. SERS Detection of Benzidine in Organic Solvents Using Spherical Gold Nanoparticles as a Substrate
First, a solution of the above spherical gold nanoparticle (2.0 mL) was thoroughly mixed with 1 mL of acetone and 3 mL of anhydrous methanol. The mixture solution was centrifuged at 10,000 rpm for 5 min and then the dark precipitate was collected and re-dispersed in 1.0 mL of chloroform under ultra-sonication. This washing process was repeated twice, and the final precipitate was re-dispersed in 300.0 μL of chloroform under ultra-sonication for later use.
To a SERS spectrometer sample vial were added 300.0 μL of the above Au nanoparticle solution, 145.0 μL of methanol, and 155.0 μL of 0.8 mM benzidine in chloroform. The SERS spectrum was recorded at an integration time of 10 s, and each spectrum is an average of three independent readings.
2.3.2. SERS Detection of 3,4-Benzopyrene in Organic Solvents Using Spherical Gold Nanoparticles as a Substrate
First, a solution of the above spherical gold nanoparticle (2.0 mL) was thoroughly mixed with 1 mL of acetone and 3 mL of anhydrous methanol. The mixture was centrifuged at 10,000 rpm for 5 min, and then the precipitate was collected and re-dispersed in 1.0 mL of chloroform under ultra-sonication. This washing process was repeated twice, and the final precipitate was re-dispersed in 300.0 μL of chloroform.
To a SERS spectrometer sample vial were added 300.0 μL of the above Au nanoparticle solution and 300.0 μL of 0.3 mM 3,4-benzopyrene in chloroform. The SERS spectrum was recorded at an integration time of 10 s, and each spectrum is an average of three independent readings.
2.3.3. SERS Detection of Malachite Green Using Long Gold Nanorods or Silk-Like Ultralong Nanowires as a Solid Substrate
First, a solution of the above-prepared long gold nanorod (5.0 mL) was added drop by drop to a depression area of a concave glass slide at room temperature. After the solvent had completely evaporated, a layer of dark gold nanorods (5 × 5 mm2) was obtained. Afterward, the slide was carefully dipped into 10.0 mL of acetone for 1 min, dried at room temperature, and then the layer of gold nanorods was used as a solid substrate for further SERS detection. Then, 20.0 μL of 1.0 mM malachite green in chloroform was added to the center of the gold nanorod layer by a microsyringe. The laser irradiation from the SERS spectrometer was adjusted to focus on the surface of the center of the layer. The SERS spectrum was recorded at an integration time of 10 s, and each spectrum is an average of 10 independent readings. The SERS detection of malachite green using silk-like ultralong nanowires as a solid substrate was completed in a similar manner except that the gold nanorod solution was replaced with the above-prepared ultralong gold nanowire solution.
3. Results and Discussion
3.1. Characteristics of Au Nanomaterials by TEM and XRD
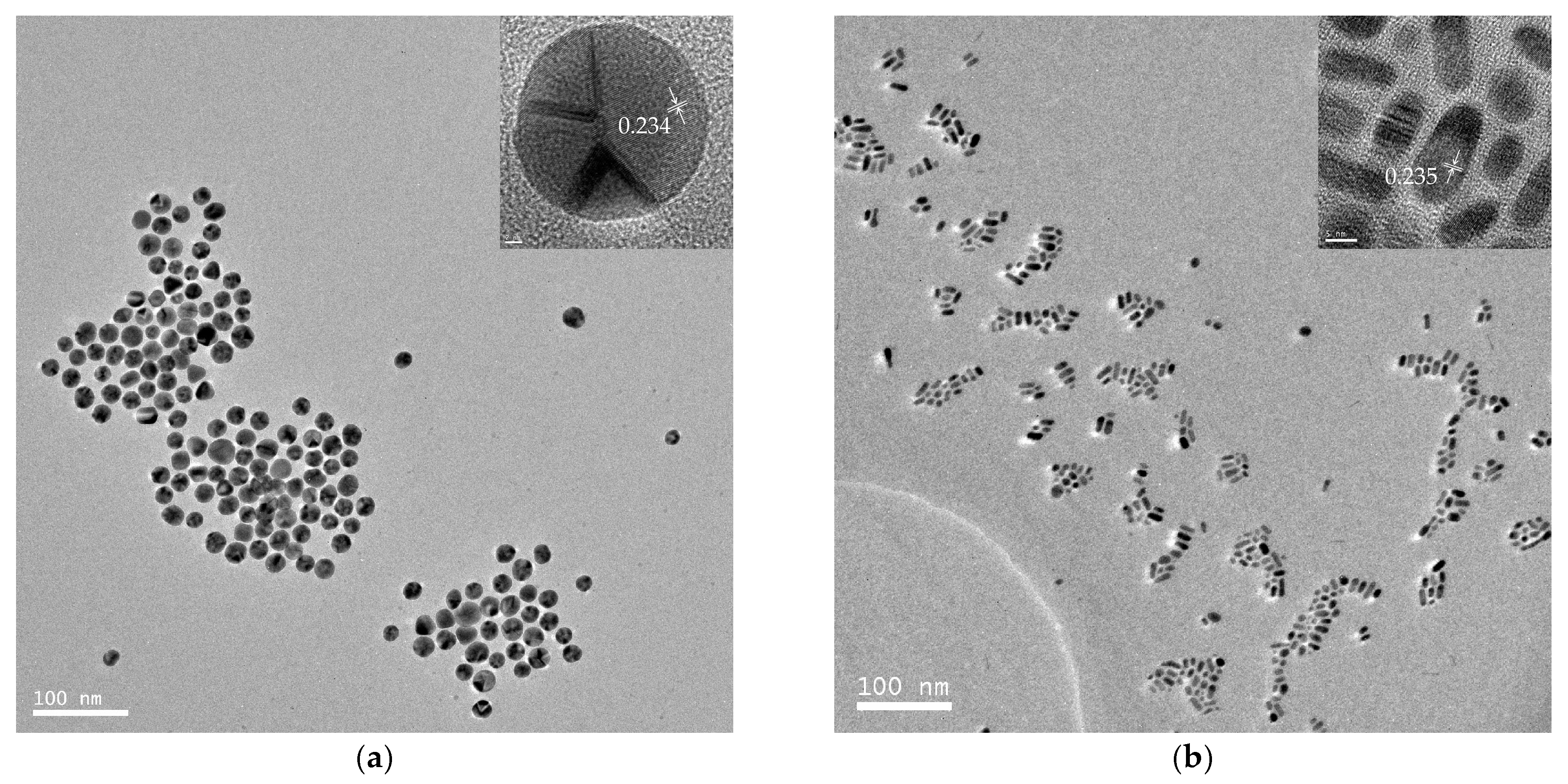
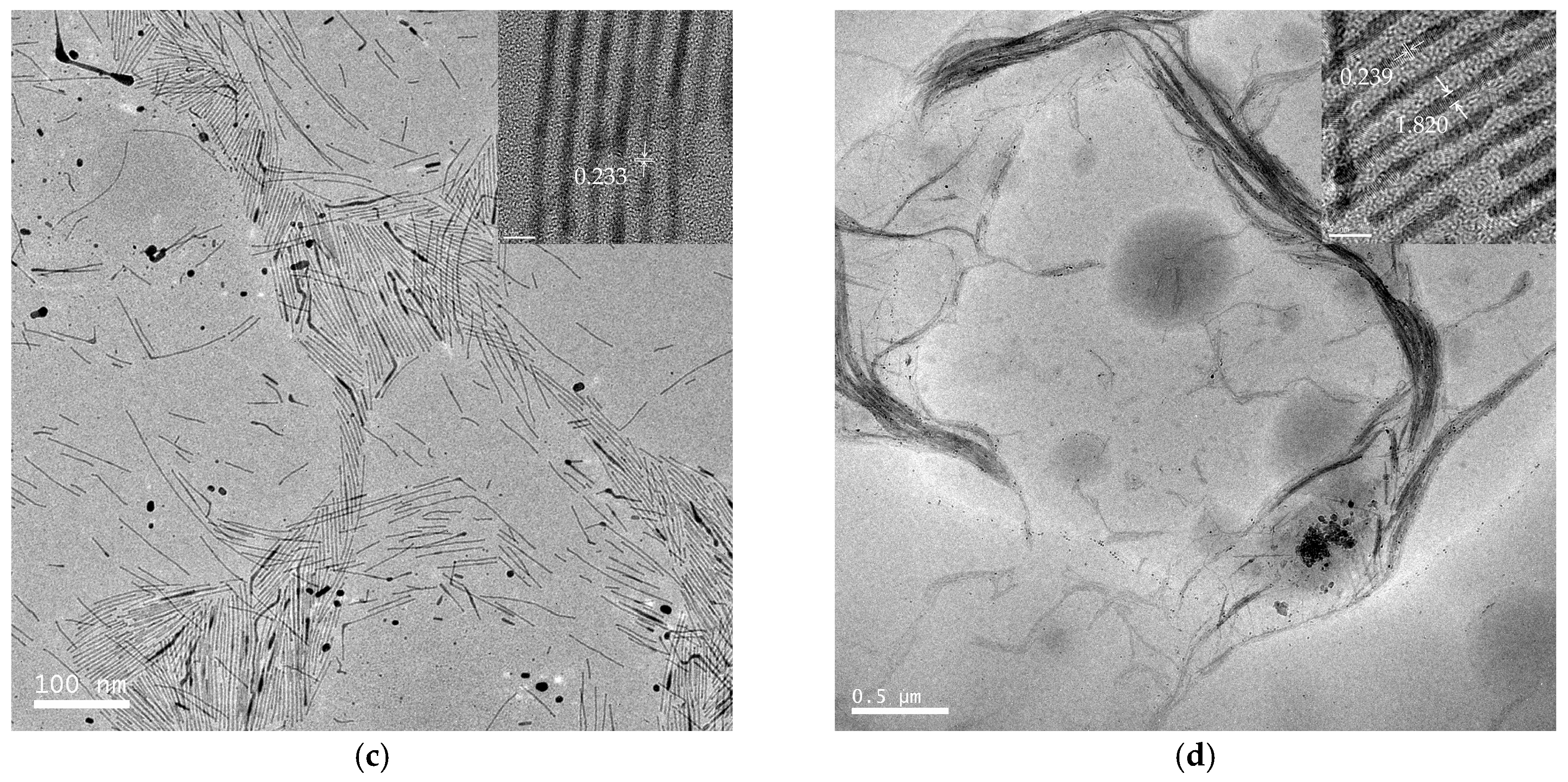
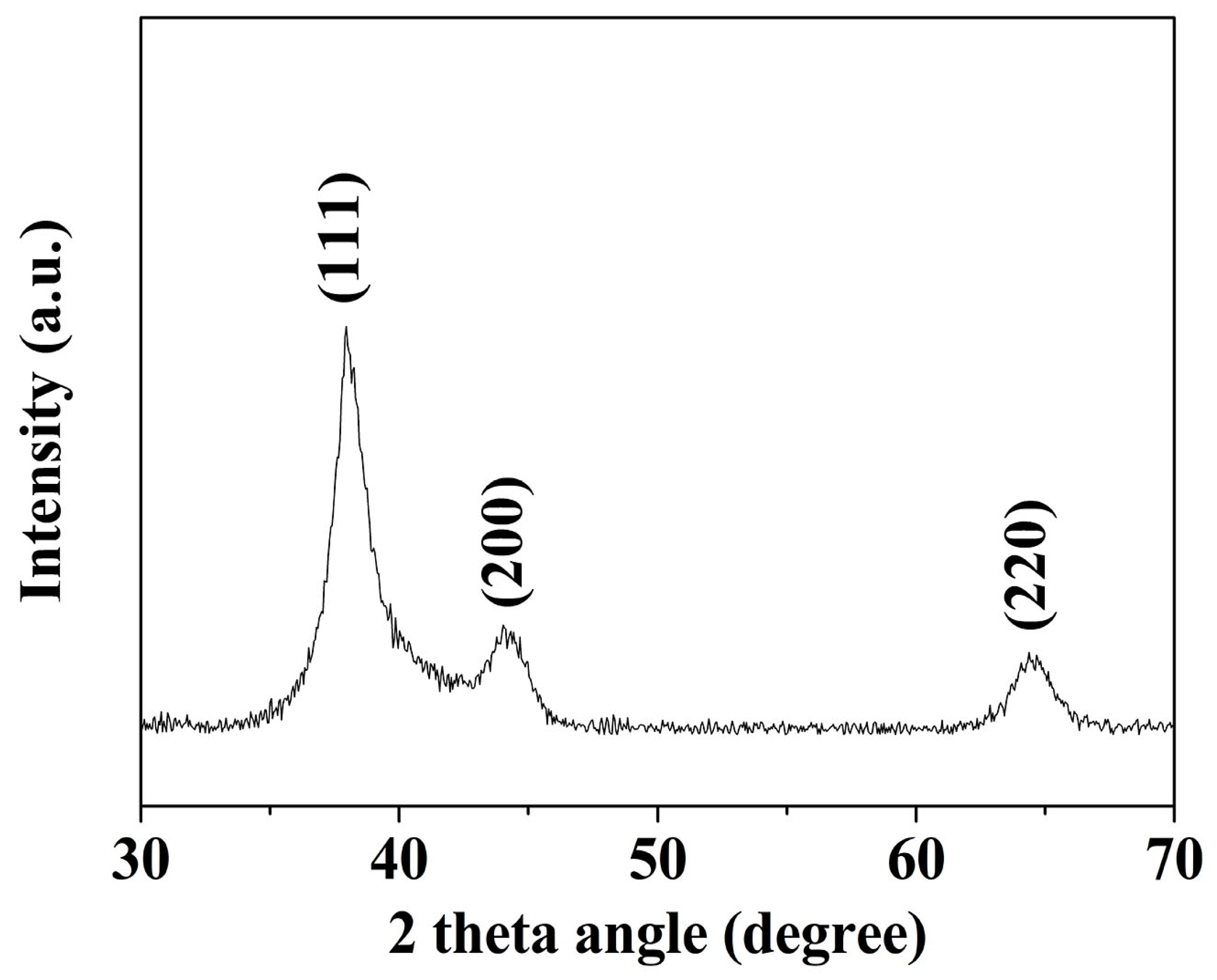
TEM images of a series of round nanodots, short nanobars, long nanorods, and ultralong nanowires prepared with different molar ratios of [TPS]:[Au]:[OA]:[OAm] are shown in Figure 1. Obviously, the morphologies of the gold nanomaterials could be controlled by changing the ratio of [OA]:[OAm]:[Au]. The aspect ratio of the gold nanoparticles increased with an increase in the ratio of OAm and OA to chloroauric acid. For example, at a low molar ratio of OAm and OA to chloroauric acid ([OA]:[OAm]:[Au] = 0.8:3.8:1), round nanodots with an average diameter of 18.8 nm were obtained (Figure 1a). Increasing the ratio of [OA]:[OAm]:[Au] to 48:45.6:1 resulted in the formation of short nanobars with an aspect ratio of about 1.5–3 (Figure 1b). Long nanorods with a length in the range of tenths of nanometers to several hundred nanometers appeared when the ratio of oleic acid was decreased ([OA]:[OAm]:[Au] = 24:45.6:1), as shown in Figure 1c. When the ratio was further increased to [OA]:[OAm]:[Au] = 24:114:1, silk-like nanowires with ultralong lengths (>2 μm) and ultrathin diameters (<2 nm) were produced (Figure 1d). These silk-like ultralong and ultrathin nanowires may offer significant potential applications in microelectronics and biosensors. From the high resolution transmission electron microscope (HRTEM) image in Figure 1, the interfringe distances of the Au nanodots, Au nanobars, Au nanorods, and Au nanowires were measured to be 0.234 nm, 0.235 nm, 0.233 nm, and 0.239 nm, respectively, corresponding to (111) lattice spacing (0.235 nm) of face-centered cubic (fcc) Au (JCPDS89-3697), and the diameter of the nanowires was determined to be 1.82 nm. The crystallographic structure of the ultralong nanowires was further confirmed by the X-ray diffraction pattern in Figure 2. The diffraction peaks at 2θ = 38.2°, 44.0°, and 64.7° were respectively ascribed to (111), (200), and (220) planes of fcc gold lattice, which agrees well with the diffraction standard of fcc gold.
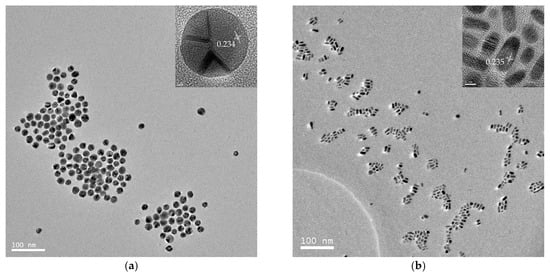
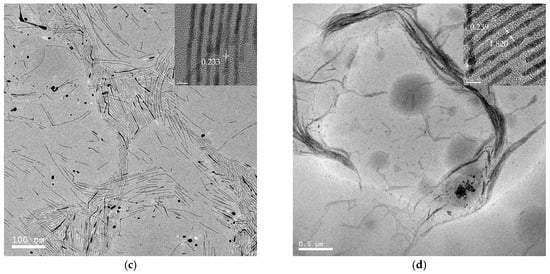
Figure 1.
Transmission electron microscope (TEM) and high resolution transmission electron microscope (HRTEM) images of gold round nanodots (a); short nanobars (b); long nanorods (c); and silk-like ultralong and ultrathin nanowires (d) prepared at [TPS]:[Au]:[OA]:[OAm] = 3:1:0.8:3.8, 3:1:48:45.6, 3:1:24:45.6, and 3:1:24:114, respectively.
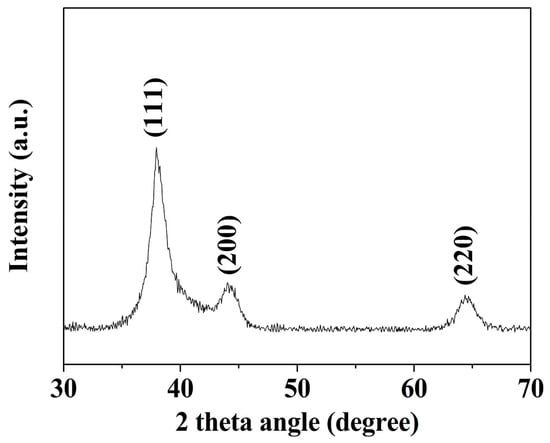
Figure 2.
X-ray diffraction (XRD) pattern of silk-like ultralong and ultrathin nanowires.
The solutions of round nanodots, short nanobars, long nanorods, and ultralong nanowires in chloroform were clear and transparent, displaying pink, wine red, light brown, and deep brown colors, respectively, at room temperature (Supplementary Material Figure S1). These solutions showed no perceptible color change and aggregation or precipitation of gold nanoparticles even after they had been stored in a fridge at 4 °C for two months, indicating that these gold nanomaterials have excellent long-term stability.
3.2. Characteristics of Large Spherical Au Nanoparticles by TEM and XRD
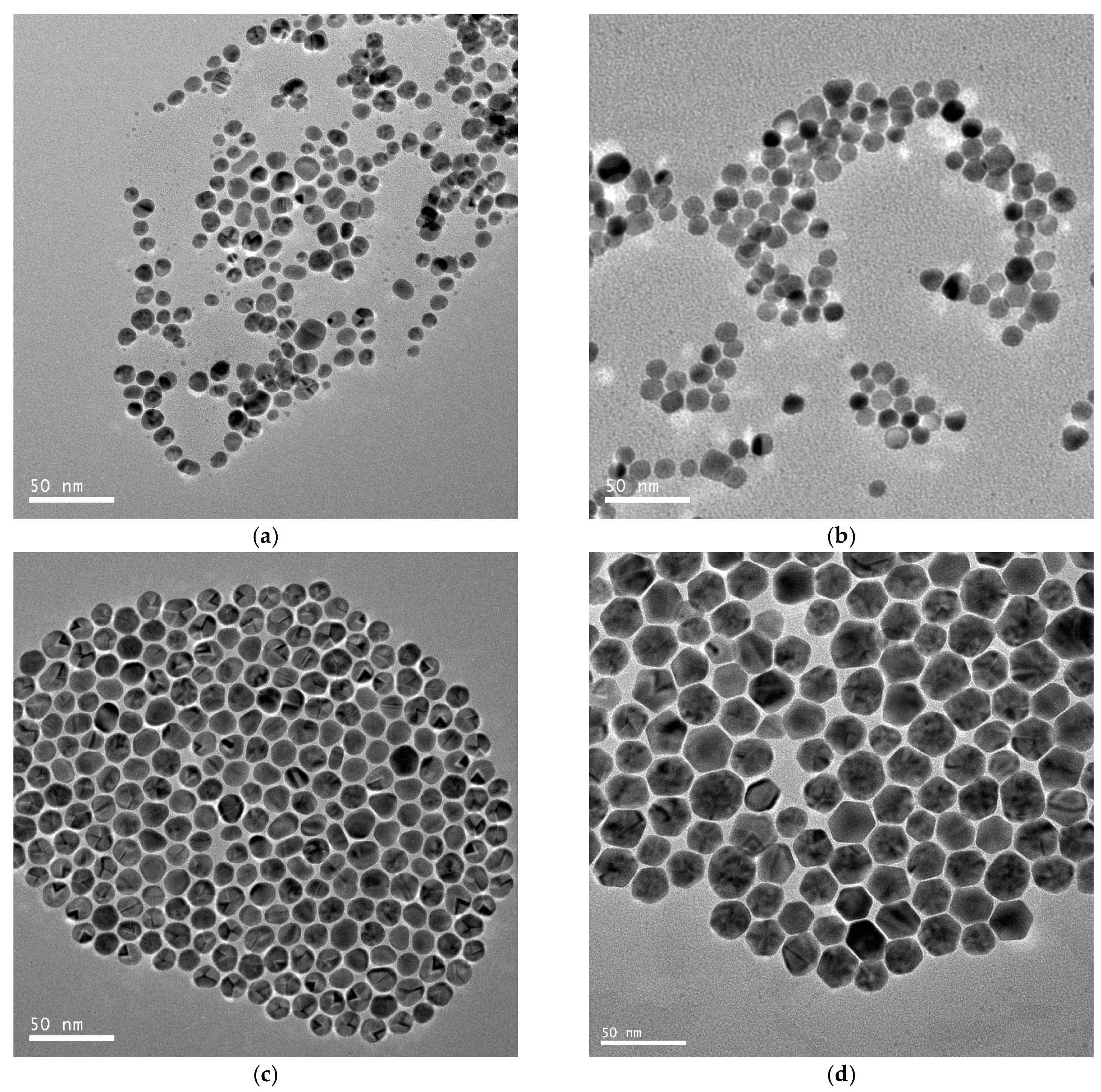
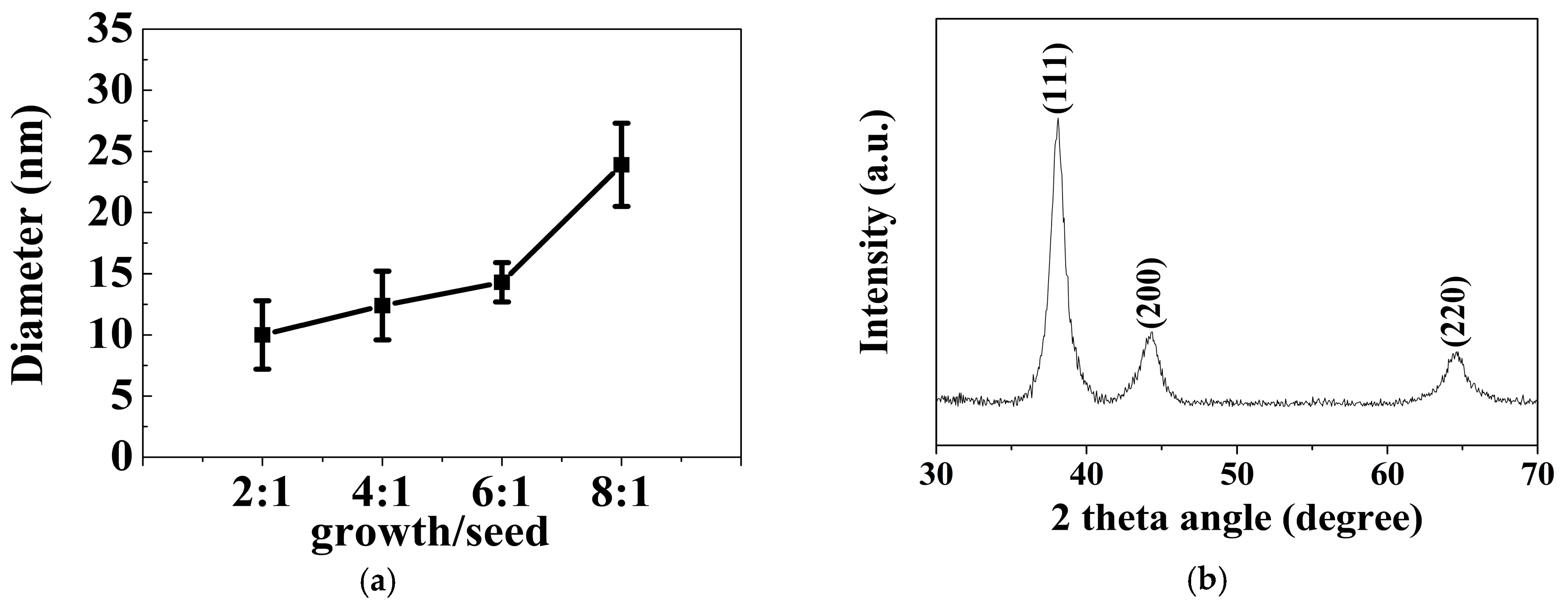
Typical TEM images of different-sized gold spherical nanoparticles resulting from the gold nanodots via a seed-directed growth approach are presented in Figure 3. Figure 3a–d show that the Au nanoparticles prepared at a growth/seed ratio of 2, 4, 6, and 8 produced nanoparticles with mean diameters of 10.0 ± 2.8 nm, 10.7 ± 2.2 nm, 14.3 ± 1.6 nm, and 23.9 ± 3.4 nm, respectively. Obviously, the size of the gold nanoparticles was controlled by the ratio of the seed to the growth precursor. The gold nanoparticles increased in size with an increase in the growth/seed ratio, as shown in Figure 4a. The increasing tendency in the size of the gold nanoparticles was also confirmed by dynamic light scattering (DLS) measurements. The mean diameters of the Au nanoparticles were measured to be 14.8 ± 2.4 nm, 17.0 ± 1.2 nm, 21.2 ± 3.9 nm, and 37.2 ± 9.3 nm, respectively, at the growth/seed ratios of 2, 4, 6, and 8, as shown in the Supplementary Material Figure S2. By carefully adjusting the growth/seed ratio, spherical gold nanoparticles in the size of 10–25 nm could be obtained. The crystallographic structure of the largest Au nanoparticles (growth/seed ratio = 8) was also confirmed by X-ray diffraction pattern (Figure 4b). The diffraction peaks at 2θ = 38.1°, 44.2°, and 64.7° were respectively ascribed to (111), (200), and (220) planes of fcc Au, which agree well with the diffraction standard of fcc Au.
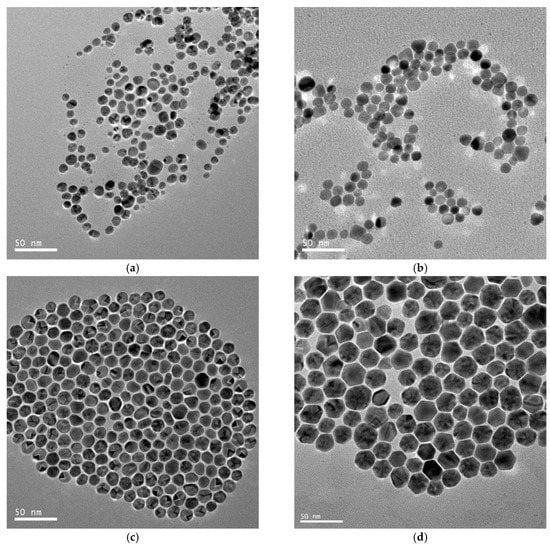
Figure 3.
TEM images of the hydrophobic gold spherical nanoparticles resulting from the growth of seed gold nanodots at growth/seed ratios = 2:1 (a); 4:1 (b); 6:1 (c); and 8:1 (d).
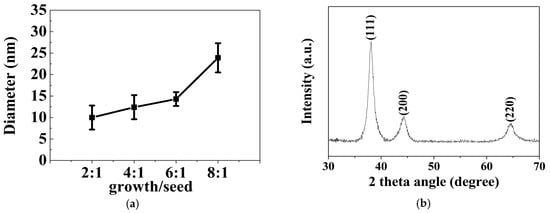
Figure 4.
(a) The dependence of the size of the spherical gold nanoparticle on the growth/seed ratio; (b) XRD pattern of the gold nanoparticles prepared at a growth/seed ratio of 8:1.
3.3. Detection of 3,4-Benzopyrene and Benzidine in Chloroform
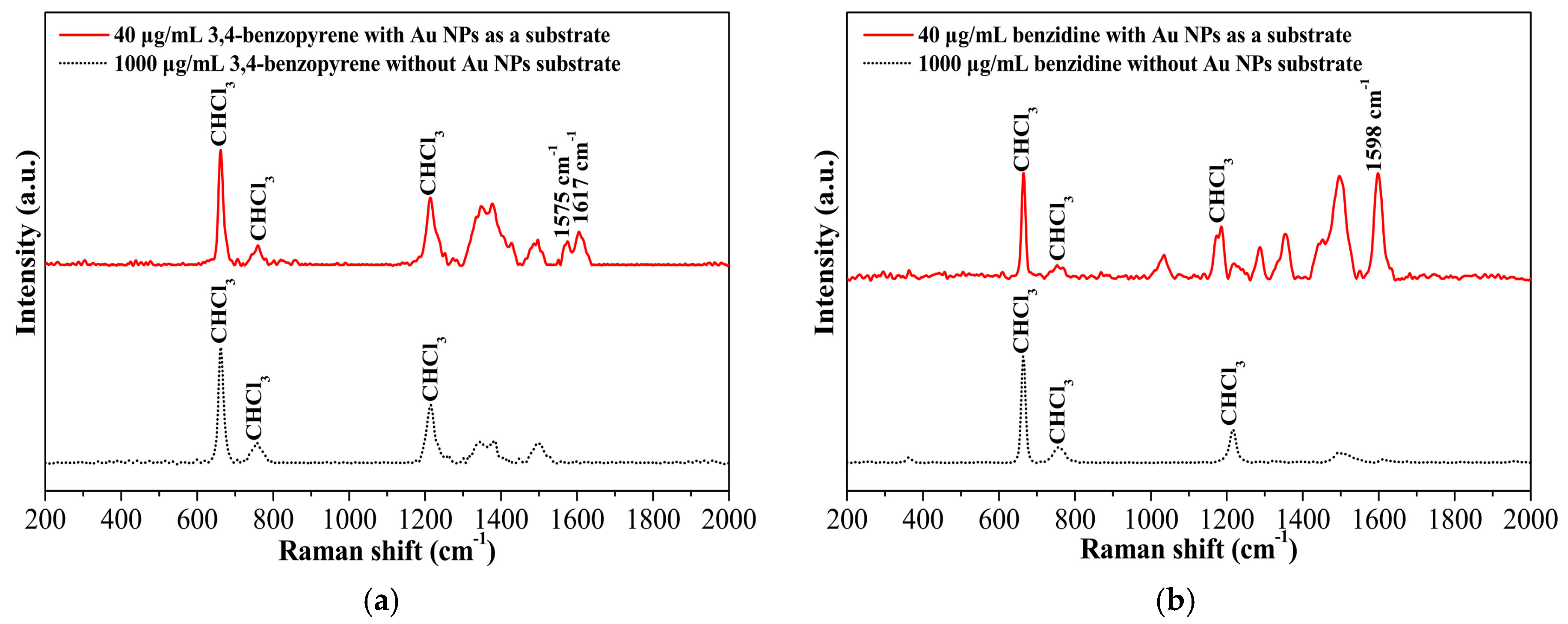
Since many toxicants, such as 3,4-benzopyrene, quintozene, and aflatoxin, are practically insoluble in water, it is difficult to use the conventional hydrophilic colloidal gold nanoparticles as efficient SERS substrates to detect the hydrophobic analytes. The hydrophobic gold nanomaterials in the current work provide a new option for the substrate used in hydrophobic organic solvents such hexane, toluene, and chloroform. Figure 5 demonstrates the SERS spectra of 3,4-benzopyrene and benzidine in chloroform using synthesized hydrophobic spherical gold nanoparticles as a substrate. As shown in Figure 5a, the characteristic Raman signals of 3,4-benzopyrene at 1617 cm−1 and 1575 cm−1 were hardly observed in chloroform at a concentration as high as 1000 μg/mL in the absence of the spherical gold nanoparticles. A significant increase in signal intensity was detected when the gold nanoparticles were mixed with 3,4-benzopyrene and used as a substrate even at a substantially lower concentration of 3,4-benzopyrene (40 μg/mL). Similar results were obtained for benzidine, as shown in Figure 5b.
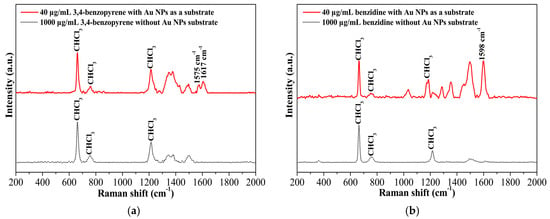
Figure 5.
(a) Surface-enhanced Raman scattering (SERS) spectra of 3,4-benzopyrene with and without spherical gold nanoparticles (Au NPs) as a hydrophobic substrate; (b) SERS spectra of benzidine with and without Au NPs as a hydrophobic substrate.
3.4. Detection of Malachite Green Using Gold Nanorods or Nanowires as a Solid Substrate
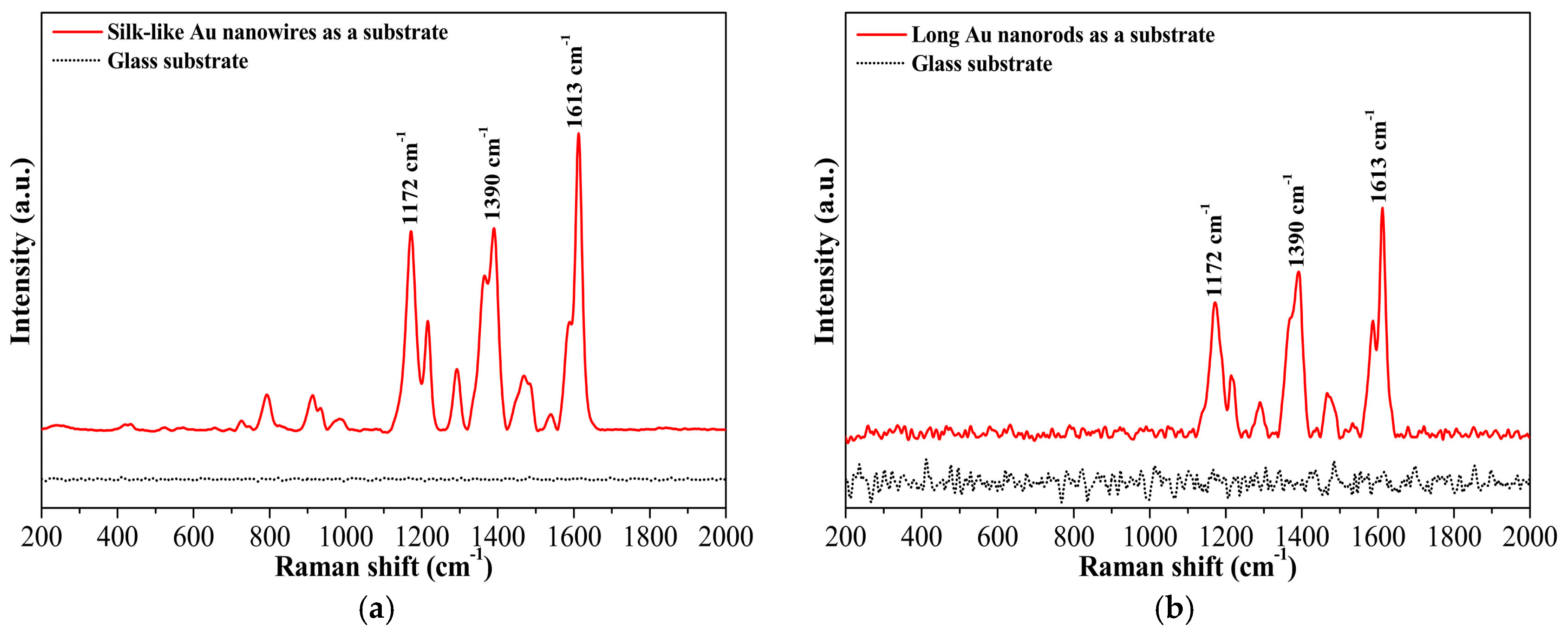
In addition to non-polar hydrophobic analytes (e.g., 3,4-benzopyrene), the gold nanomaterials in the current work could also serve as solid substrates for the detection of strong polar analytes, such as malachite green. Figure 6a,b demonstrates the SERS spectra of malachite green using the ultralong gold nanowires or long nanorods deposited on a piece of glass as a solid SERS substrate. Apparently, without the nanowires or nanorods as a substrate, no typical SERS signals of malachite green at 1613 cm−1, 1390 cm−1, and 1172 cm−1 were observed on glass. A substantial increase in signal intensity was detected when the nanowires or nanorods was used as a solid substrate. These results indicate that all gold nanoparticles, long nanorods, and silk-like ultralong nanowires exhibited excellent surface-enhanced Raman scattering activities and can be employed as efficient SERS substrates for the detection of many hydrophobic toxicants and strong polar analytes.
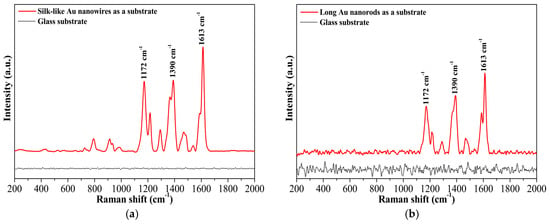
Figure 6.
(a) SERS spectra of malachite green using silk-like ultralong Au nanowires as a solid substrate; (b) SERS spectra of malachite green using long Au nanorods as a solid substrate.
4. Conclusions
In summary, this work presents a simple and versatile approach for a one-pot synthesis of size-controlled and morphology-controlled gold nanomaterials, including round nanodots, short nanobars, and long nanorods, as well as silk-like ultralong and ultrathin nanowires, in a safe and productive way. The size and morphology of the gold nanoparticles were found to be controlled by the molar ratios of chloroauric acid to oleylamine, oleic acid, and hydrosilane. The obtained gold spherical nanoparticles, nanorods and nanowires showed excellent surface-enhanced Raman scattering activity and, therefore, were used as an efficient organic-soluble SERS substrate or a solid SERS substrate for the detection of many hydrophobic toxicants, such as 3,4-benzopyrene and benzidine, and strong polar analytes, such as malachite green.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/9/5/935/s1, Figure S1: Photo images of the solutions of gold round nanodots, short nanobars, long nanorods, and silk-like ultralong and ultrathin nanowires in chloroform; Figure S2: The size of Au nanoparticles measured by dynamic light scattering (DLS) at the growth/seed ratio of 2, 4, 6, and 8, respectively.
Author Contributions
J.N. is responsible for the conceptualization, funding acquisition, and manuscript editing. C.X. performed the synthesis of the gold nanomaterials, investigated applications of the nanomaterials as SERS substrates, and wrote the original draft; K.J. developed the method for the synthesis of the gold nanomaterials and used GRAMS/AI software to process the SERS data. X.N. analyzed the SERS spectra and verified the SERS data; P.S. contributed to the project administration and supervision and provided chemical reagents and materials.
Funding
This research was funded by the National Natural Science Foundation of China (grant number: 31301483) and Natural Science Foundation of Zhejiang Province (grant number: LY17C200016).
Conflicts of Interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the manuscript submitted. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Bond, C.G.; Thompson, D.T. Catalysis by Gold. Catal. Rev. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Haruta, M.; Daté, M. Advances in the catalysis of Au nanoparticles. Appl. Catal. A Gen. 2001, 222, 427–437. [Google Scholar] [CrossRef]
- Porta, F.; Prati, L.; Rossi, M.; Scari, G. New Au(0) sols as precursors for heterogeneous liquid-phase oxidation catalysts. J. Catal. 2002, 211, 464–469. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold Catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Goodman, D.W. Catalytically active gold: From nanoparticles to ultrathin films. Acc. Chem. Res. 2006, 39, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Garcia, H. Support gold nanoparticles as catalysts for organic reactions. Chem. Soc. Rev. 2008, 37, 2096–2126. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.C.; Grow, M.E.; Pan, H.; Bednarek, M.; Ghann, W.E.; Zabetakis, K.; Cornish, J. Gold nanoparticle-cored poly(propyleneimine) dendrimers as a new platform for multifunctional drug delivery systems. New. J. Chem. 2011, 35, 2366–2374. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; EI-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.; Heller, D.A.; Winslow, M.M.; Dahlman, J.E.; Pratt, G.W.; Langer, R.; Jacks, T.; Anderson, D.G. Treating metastatic cancer with nanotechnology. Nat. Rev. Cancer 2011, 12, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Gil, P.R.; Zhang, F.; Zanella, M.; Parak, W.J. Biological applications of gold nanoparticles. Chem. Soc. Rev. 2008, 37, 1896–1908. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic nanoshells: From probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Schatz, G.C. Using theory and computation to model nanoscale properties. Proc. Natl. Acad. Sci. USA 2007, 104, 6885–6892. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Goodman, P. Current and future uses of gold in electronics. Gold Bull. 2002, 35, 21–26. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 2 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of gold nanoparticles and their endocytotic fate inside the cellular compartment: A microscopic overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; EI-Sayed, I.H.; EI-Sayed, M.A. Au nanoparticles target cancer. Nano Today 2007, 2, 18–29. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phy. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Park, H.; Schadt, M.J.; Lim, I.S.; Njoki, P.N.; Kim, S.H.; Jang, M.; Luo, J.; Zhong, C. Fabrication of magnetic core@shell Fe oxide@Au nanoparticles for interfacial bioactivity and bio-separation. Langmuir 2007, 23, 9050–9056. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Harpster, M.H.; Park, H.J.; Johnson, P.A.; Wilson, W.C. Surface-enhanced Raman scattering detection of DNA derived from the west nile virus genome using magnetic capture of Raman-active gold nanoparticles. Anal. Chem. 2011, 83, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Neng, J.; Tan, J.; Kan, J.; Sun, P. A fast and cost-effective detection of melamine by surface enhanced Raman spectroscopy using a novel hydrogen bonding-assisted supramolecular matrix and gold-coated magnetic nanoparticles. Appl. Sci. 2017, 7, 475. [Google Scholar] [CrossRef]
- Subramaniam, C.; Tom, R.T.; Pradeep, T. On the formation of protected gold nanoparticles from AuCl4- by the reduction using aromatic amines. J. Nanopart. Res. 2005, 7, 209–217. [Google Scholar] [CrossRef]
- Newman, J.D.S.; Blanchard, G.J. Formation of gold nanoparticles using amine reducing agents. Langmuir 2006, 22, 5882–5887. [Google Scholar] [CrossRef] [PubMed]
- Hiramatsu, H.; Osterloh, F.E. A simple large-scale synthesis of nearly monodisperse gold and silver nanoparticles with adjustable sizes and with exchangeable surfactants. Chem. Mater. 2004, 16, 2509–2511. [Google Scholar] [CrossRef]
- Sugie, A.; Somete, T.; Kanie, K.; Muramatsu, A.; Mori, A. Triethylsilane as a mild and efficient reducing agent for the preparation of alkanethiol-capped gold nanoparticles. Chem. Commun. 2008, 33, 3882–3884. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yavuz, M.S.; Tuan, H.; Korgel, B.A.; Xia, Y. Ultrathin gold nanowires can be obtained by reducing polymeric stands of oleylamine-AuCl complexes formed via aurophilic interaction. J. Am. Chem. Soc. 2008, 130, 8900–8901. [Google Scholar] [CrossRef] [PubMed]
- Takahata, R.; Yamazoe, S.; Koyasu, K.; Imura, K.; Tsukuda, T. Gold ultrathin nanorods with controlled aspect ratios and surface modifications: Formation mechanism and localized surface plasmon. J. Am. Chem. Soc. 2018, 12, 6640–6647. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A.; Nordquist, C.D.; Jackson, T.N.; Mayer, B.R.; Mbindyo, J.; Mallouk, T.E. Electric-field assisted assembly and alignment of metallic nanowires. Appl. Phys. Lett. 2000, 77, 1399–1401. [Google Scholar] [CrossRef]
- Sánchez-Iglesias, A.; Rivas-Murias, B.; Grzelczak, M.; Pérez-Juste, J.; Liz-Marzán, L.M.; Rivadulla, F.; Correa-Duarte, M.A. Highly transparent and conductive films of densely aligned ultrathin Au nanowire monolayers. Nano Lett. 2012, 12, 6066–6070. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Yang, Y.; You, Y.; Li, G.; Guo, J.; Yu, T.; Shen, Z.; Wu, T.; Xing, B. Simple and rapid synthesis of ultrathin gold nanowires, their self-assembly and application in surface-enhanced Raman scattering. Chem. Commun. 2009, 15, 1984–1986. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Handy, R.D.; Shaw, B.J. Toxic effects of nanoparticles and nanomaterials: Implications for public health, risk assessment and the public perception of nanotechnology. Health Risk Soc. 2007, 9, 125–144. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Aguerre-Chariol, O.; Bressot, C. STEM imaging to characterize nanoparticle emissions and help to design nanosafer paints. Chem. Eng. Res. Des. 2018, 136, 663–674. [Google Scholar] [CrossRef]
- Bressot, C.; Aubry, A.; Pagnoux, C.; Aguerre-Chariol, O.; Morgeneyer, M. Assessment of functional nanomaterials in medical applications: Can time mend public and occupational health risks related to the products’ fate? J. Toxicol. Environ. Health A 2018, 81, 957–973. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, B.; Reverberi, A.P.; Varbanov, P.S. Safety opportunities for the synthesis of metal nanoparticles and short-cut approach to workplace risk evaluation. J. Clean. Prod. 2019, 209, 297–308. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Ramirez, A.; Poletto, M.; Smith, S.W.; Tweedie, R.; Heng, J.; Maass, S.; Bressot, C. Particle technology as a uniform discipline? Towards a holistic approach to particles, their creation, characterisation, handling and processing! Chem. Eng. Res. Des. 2018. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).