Experimental Investigation of Freezing and Melting Characteristics of Graphene-Based Phase Change Nanocomposite for Cold Thermal Energy Storage Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene Nanocomposite
2.2. Stability Analysis
2.3. Thermal Conductivity Measurement
2.4. Experimental Test Facility for Freezing and Melting Characteristics
2.5. Test Matrix and Working Procedure
3. Results and Discussion
3.1. Thermal Conductivity
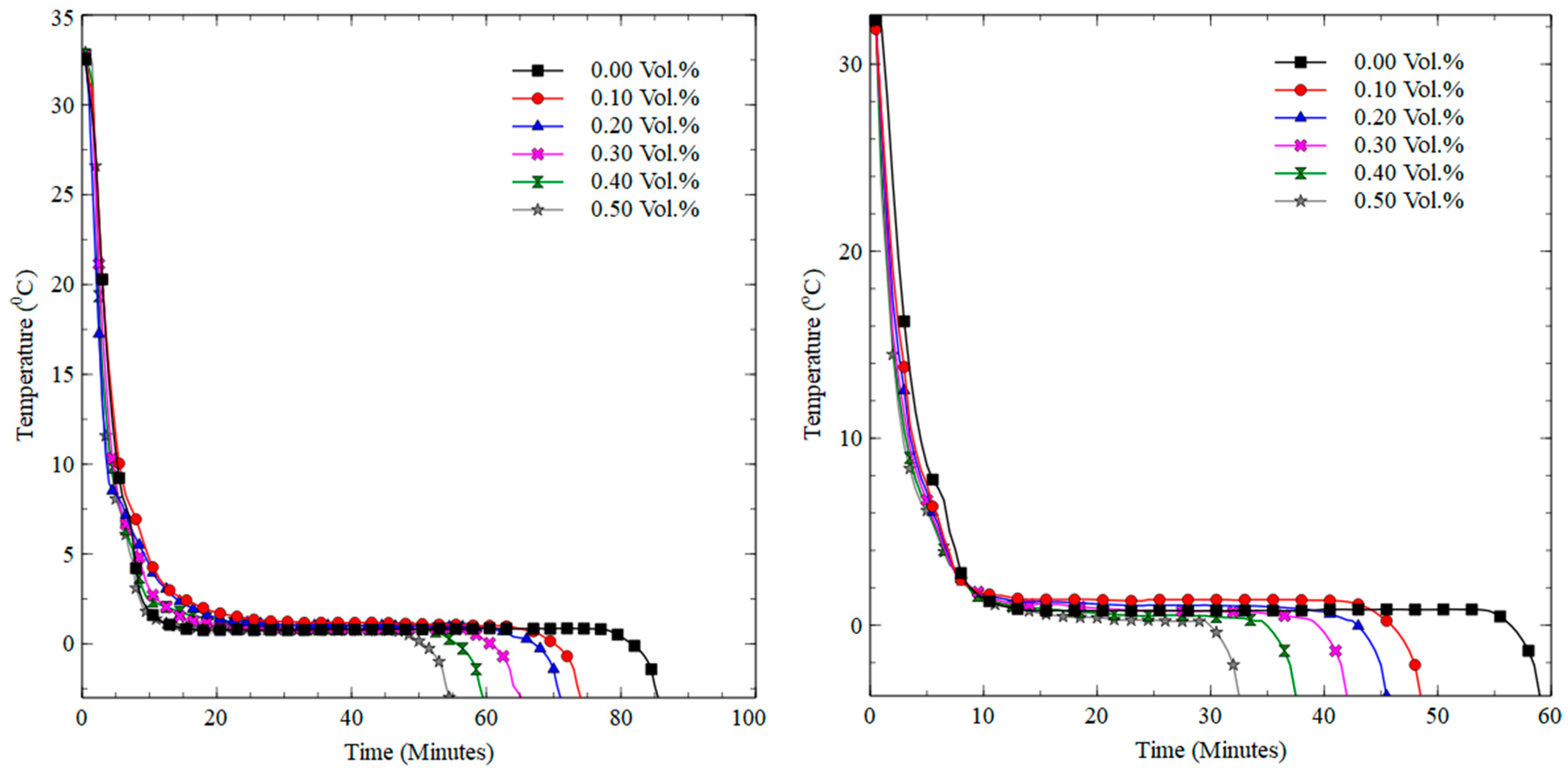
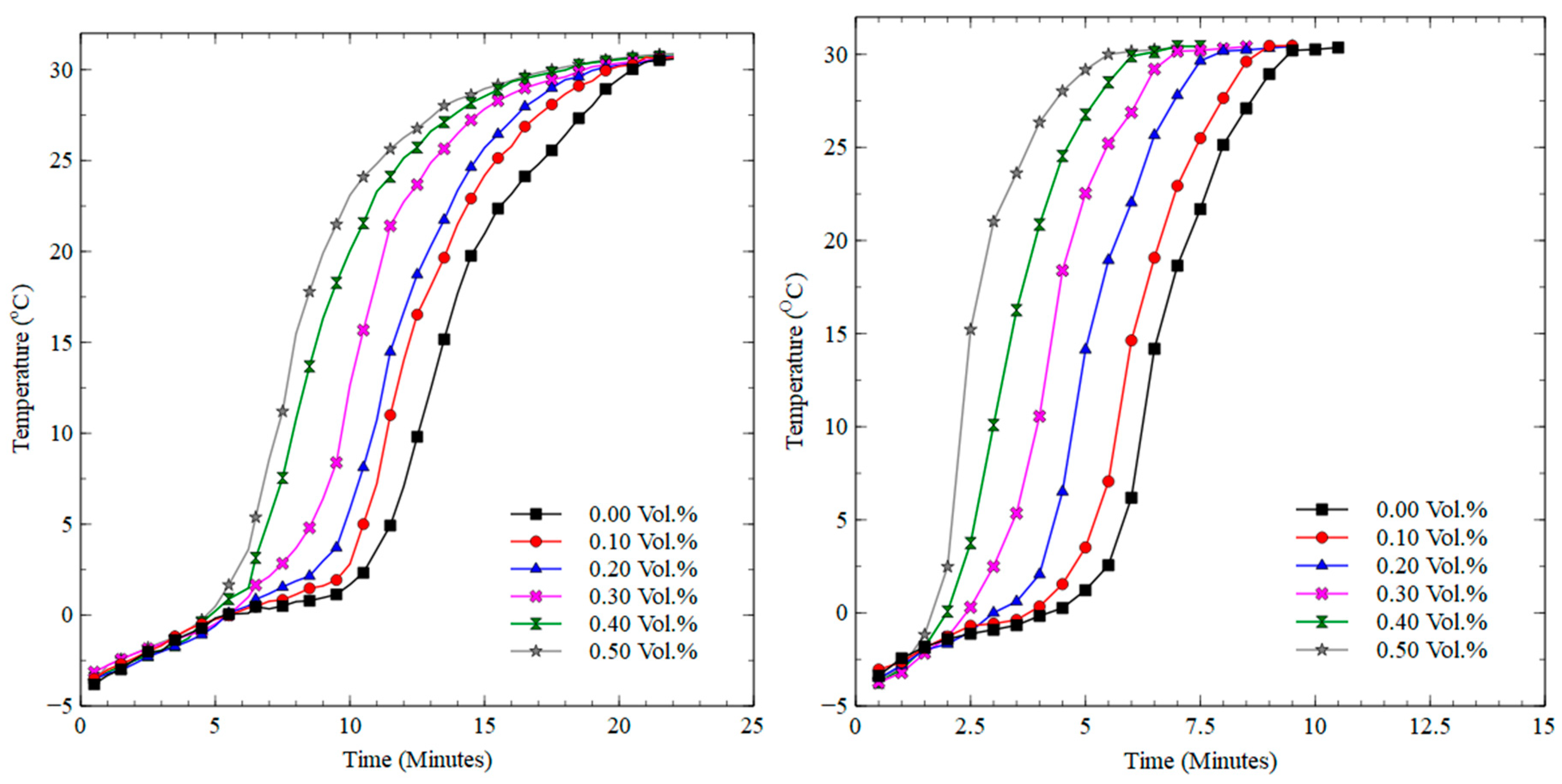
3.2. Freezing and Melting Characteristics
3.2.1. Freezing Process
3.2.2. Melting Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- de Blas, M.; Appelbaum, J.; Torres, J.L.; García, A.; Prieto, E.; Illanes, R. A Refrigeration Facility for Milk Cooling Powered by Photovoltaic Solar Energy. Prog. Photovolt. Res. Appl. 2003, 11, 467–479. [Google Scholar] [CrossRef]
- Torres-Toledo, V.; Meissner, K.; Coronas, A.; Müller, J. Performance characterisation of a small milk cooling system with ice storage for PV applications. Int. J. Refrig. 2015, 60, 81–91. [Google Scholar] [CrossRef]
- Pedersen, P.H.; Maté, J. SolarChill Vaccine Cooler and Refrigerator: A Breakthrough Technology. Industria Informatione. Refrig. Air Cond. 2006, 300 (Suppl. 1), 17–19. [Google Scholar]
- Axaopoulos, P.J.; Theodoridis, M.P. Design and experimental performance of a PV Ice-maker without battery. Sol. Energy 2009, 83, 1360–1369. [Google Scholar] [CrossRef]
- Direct Drive Solar Coolers Per Henrik Pedersen, Ivan Katic. 2016. Available online: https://www.dti.dk/_/media/56756_SolarChill_a_solar_pv_refrigerator_without_battery.pdf (accessed on 20 December 2018).
- Guruprasad, A.; Lingkun, L.; Xiang, H.; Fang, G. Thermal energy storage materials and systems for solar energy applications. Renew. Sustain. Energy Rev. 2017, 68, 693–706. [Google Scholar]
- Sathishkumar, A.; Kathirkaman, M.D.; Ponsankar, S.; Balasuthagar, C. Experimental investigation on solidification behaviour of water base nanocomposite pcm for building cooling applications. Indian J. Sci. Technol. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Ahammed, N.; Lazarus, G.A.; Titus, J.; Bose, J.R.; Wongwises, S. Measurement of thermal conductivity of graphene–water nanocomposite at below and above ambient temperatures. Int. Commun. Heat Mass Transf. 2016, 70, 66–74. [Google Scholar] [CrossRef]
- Harikrishnan, S.; Deepak, K.; Kalaiselvam, S. Thermal energy storage behavior of composite using hybrid nanomaterials as PCM for solar heating system. J. Therm. Anal. Calorim. 2014, 115, 1563–1571. [Google Scholar] [CrossRef]
- Harish, S.; Daniel, O.; Yasuyuki, T.; Masamichi, K. Thermal conductivity enhancement of lauric acid phase change nanocomposite with graphene nanoplatelets. Appl. Therm. Eng. 2015, 80, 205–211. [Google Scholar] [CrossRef]
- Sakr, M.H.; Abdel-Aziz, R.M.; Ghorab, A.A.E. Experimental and theoretical study on freezing and melting in capsules for thermal storage. ERJ Soubra Fac. Eng 2008, 9, 48–65. [Google Scholar]
- Kaygusuz, K.; Sari, A. Thermal energy storage system using a technical grade paraffin wax as latent heat energy storage material. Energy Sources 2005, 27, 1535–1546. [Google Scholar] [CrossRef]
- Kaygusuz, K. Experimental and theoretical investigation of latent heat storage for water based solar heating systems. Energy Convers Manag. 1995, 36, 315–323. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.C.; Saini, J.S. Latent heat thermal energy storage using cylindrical capsule: Numerical and experimental investigations. Renew. Energy 2006, 31, 2025–2041. [Google Scholar] [CrossRef]
- Zalba, B.; Sánchez-valverde, B.; Marín, J.M. An experimental study of thermal energy storage with phase change materials by design of experiments. J. Appl. Stat. 2005, 32, 321–332. [Google Scholar] [CrossRef]
- TEAP Energy Products. 2004. Available online: http://www.teappcm.com (accessed on 5 September 2018).
- Yoon, J.I.; Moon, C.G.; Kim, E.; Son, Y.S.; Kim, J.D.; Kato, T. Experimental study on freezing of water with supercooled region in a horizontal cylinder. Appl. Eng. 2001, 21, 657–668. [Google Scholar] [CrossRef]
- Kalaiselvam, S.; Veerappan, M.; Arul Aaron, A.; Iniyan, S. Experimental and analytical investigation of solidification and melting characteristics of PCMs inside cylindrical encapsulation. Int. J. Sci. 2008, 47, 858–874. [Google Scholar] [CrossRef]
- Novoselov, K.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Mehrali, M.; Tahan Latibari, S.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C.; Naghavi, M.S. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Mehrali, M.; Sadeghinezhad, E.; Latibari, S.T.; Kazi, S.N.; Mehrali, M.; Zubi, M.N.B.M.; Metselaar, H.S.C. Investigation of thermal conductivity and rheological properties of nanofluids containing graphene nanoplatelets. Nanoscale Res. Lett. 2014, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.D.; Li, X.; Hu, P.F.; Hu, G.H. Study on the supercooling degree and nucleation behavior of water-based graphene oxide nanofluids PCM. Int. J. Refrig. 2015, 50, 80–86. [Google Scholar] [CrossRef]
- Liu, J.; Ye, Z.C.; Zhang, L.; Fang, X.M.; Zhang, Z.G. A combined numerical and experimental study on graphene/ionic liquid nanofluid based direct absorption solar collector. Sol. Energy Mater. Sol. Cells 2015, 136, 177–186. [Google Scholar] [CrossRef]
- Hadadian, M.; Goharshadi, E.K.; Youssefi, A. Electrical conductivity, thermal conductivity, and rheological properties of graphene oxide-based nanofluids. J. Nanopart. Res. 2014, 16, 2788. [Google Scholar] [CrossRef]
- Sudeep, P.M.; Taha-Tijerina, J.; Ajayan, P.M.; Narayananc, T.N.; Anantharaman, M.R. Nanofluids based on fluorinated graphene oxide for efficient thermal management. RSC Adv. 2014, 4, 24887. [Google Scholar] [CrossRef]
- Mehrali, M.; Deghinezhad, E.S.; Latibari, S.T.; Mehrali, M.; Togun, H.; Zubir, M.N.M.; Kazi, S.N.; Metselaar, H.S.C. Preparation, characterization, viscosity, and thermal conductivity of nitrogen doped graphene aqueous nanofluids. J. Mater. Sci. 2014, 49, 7156–7171. [Google Scholar] [CrossRef]
- Liu, J.; Wang, F.X.; Zhang, L.; Fang, X.M.; Zhang, Z.G. Thermodynamic properties and thermal stability of ionic liquid-based nanofluids containing graphene as advanced heat transfer fluids for medium-to-high-temperature applications. Renew Energy 2014, 63, 519–523. [Google Scholar] [CrossRef]
- Selvam, C.; Mohan Lal, D.; Harish, S. Thermal conductivity enhancement of ethylene glycol and water with grapheme nanoplatelets. Thermochim. Acta 2016, 642, 32–38. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Harr, L.; Gallagher, J.S.; Kell, G.S. NBS/NRC Steam Tables; Hemisphere Publishing Corporation: Washington, WA, USA, 1984. [Google Scholar]
- Marsh, K.N. Recommended Reference Materials for the Realization of Physicochemical Propertie; Blackwell Scientific Publications: Oxford, UK, 1987. [Google Scholar]
- Sengers, J.V.; Watson, J.T.R. Improved international formulations for the viscosity and thermal conductivity of water substance. J. Phys. 1986, 15, 1291. [Google Scholar] [CrossRef]
- Rabinataj, A.D.; Hassanzadeh, H.A.; Khaki, M.; Abbasi, M. Unconstrained melting and solidification inside rectangular enclosure. J. Fundam. Appl. Sci. 2015, 7, 436–451. [Google Scholar] [CrossRef]




| Volume Percentage of Functionalised GnP | Initial Temperature of the Sample | Bath Temperatures for Freezing | Sample Temperature after Freezing | Bath Temperature for Melting | Sample Temperature after Melting |
|---|---|---|---|---|---|
| 0%, 0.1%, 0.2%, 0.3%, 0.4% & 0.5% | 32 °C | −6 °C & −10 °C | −3 °C | 31 °C & 36 °C | 30 °C |
| Temp (°C) | DI Water Standard K (W/mK) | DI Water Measured K (W/mK) | Nanocomposite (0.1%) | Nanocomposite (0.2%) | Nanocomposite (0.3%) | Nanocomposite (0.4%) | Nanocomposite (0.5%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K (W/mK) | Percentage Increase (%) | K (W/mK) | Percentage Increase (%) | K (W/mK) | Percentage Increase (%) | K (W/mK) | Percentage Increase (%) | K (W/mK) | Percentage Increase (%) | |||
| −10 | 2.30 | 2.317 | 2.771 | 19.59 | 2.930 | 26.46 | 3.156 | 36.21 | 3.371 | 45.51 | 3.565 | 53.87 |
| −5 | 2.25 | 2.27 | 2.694 | 18.67 | 2.851 | 25.59 | 3.055 | 34.58 | 3.292 | 45.02 | 3.479 | 53.24 |
| 0 | 2.22 | 2.233 | 2.63 | 17.77 | 2.792 | 25.05 | 2.980 | 33.46 | 3.232 | 44.73 | 3.395 | 52.04 |
| 5 | 0.57 | 0.576 | 0.644 | 11.80 | 0.656 | 13.87 | 0.679 | 17.8 | 0.700 | 21.53 | 0.717 | 24.42 |
| 10 | 0.58 | 0.589 | 0.656 | 11.37 | 0.669 | 13.57 | 0.692 | 17.52 | 0.715 | 21.34 | 0.731 | 24.19 |
| 15 | 0.589 | 0.596 | 0.662 | 11.07 | 0.674 | 13.03 | 0.698 | 17.1 | 0.720 | 20.83 | 0.738 | 23.85 |
| 20 | 0.598 | 0.604 | 0.669 | 10.76 | 0.682 | 12.95 | 0.706 | 16.81 | 0.729 | 20.71 | 0.748 | 23.79 |
| 25 | 0.607 | 0.609 | 0.676 | 11.00 | 0.690 | 13.33 | 0.713 | 17.05 | 0.736 | 20.85 | 0.755 | 23.94 |
| 30 | 0.615 | 0.616 | 0.682 | 10.71 | 0.697 | 13.2 | 0.720 | 16.89 | 0.744 | 20.7 | 0.762 | 23.78 |
| 35 | 0.623 | 0.623 | 0.690 | 10.75 | 0.706 | 13.3 | 0.729 | 16.95 | 0.750 | 20.45 | 0.770 | 23.54 |
| 40 | 0.63 | 0.635 | 0.699 | 10.07 | 0.720 | 13.45 | 0.744 | 17.15 | 0.766 | 20.62 | 0.784 | 23.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidney, S.; Dhasan, M.L.; C., S.; Harish, S. Experimental Investigation of Freezing and Melting Characteristics of Graphene-Based Phase Change Nanocomposite for Cold Thermal Energy Storage Applications. Appl. Sci. 2019, 9, 1099. https://doi.org/10.3390/app9061099
Sidney S, Dhasan ML, C. S, Harish S. Experimental Investigation of Freezing and Melting Characteristics of Graphene-Based Phase Change Nanocomposite for Cold Thermal Energy Storage Applications. Applied Sciences. 2019; 9(6):1099. https://doi.org/10.3390/app9061099
Chicago/Turabian StyleSidney, Shaji, Mohan Lal Dhasan, Selvam C., and Sivasankaran Harish. 2019. "Experimental Investigation of Freezing and Melting Characteristics of Graphene-Based Phase Change Nanocomposite for Cold Thermal Energy Storage Applications" Applied Sciences 9, no. 6: 1099. https://doi.org/10.3390/app9061099
APA StyleSidney, S., Dhasan, M. L., C., S., & Harish, S. (2019). Experimental Investigation of Freezing and Melting Characteristics of Graphene-Based Phase Change Nanocomposite for Cold Thermal Energy Storage Applications. Applied Sciences, 9(6), 1099. https://doi.org/10.3390/app9061099






