Luminescence Tuning of Fluorinated Bistolanes via Electronic or Aggregated-Structure Control
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis
2.3. Preparation of AcO-Substituted Bistolane 1aB
2.3.1. 1-Acetoxy-2,3,5,6-tetrafluoro-4-[2-[4-[2-(4-methoxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (1aB)
2.3.2. 1-Acetoxy-2,3,5,6-tetrafluoro-4-[2-[4-[2-(4-hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (1bB)
2.4. Preparation of CF3-Substituted Bistolane 1aC
2.4.1. 1-Trifluoromethyl-2,3,5,6-tetrafluoro-4-[2-[4-[2-(4-methoxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (1aC)
2.4.2. 1-Trifluoromethyl-2,3,5,6-tetrafluoro-4-[2-[4-[2-(4-hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (1bC)
2.5. Preparation of CN-Substituted Bistolane 1aD
2.5.1. 1-Cyano-2,3,5,6-tetrafluoro-4-[2-[4-[2-(4-methoxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (1aD)
2.5.2. 1-Cyano-2,3,5,6-tetrafluoro-4-[2-[4-[2-(4-hexyloxyphenyl)ethyn-1-yl]phenyl]ethyn-1-yl]benzene (1bD)
2.6. X-ray Crystallography
2.7. Computations
2.8. Phase Transition Behavior
2.9. Photophysical Behavior
3. Results and Discussion
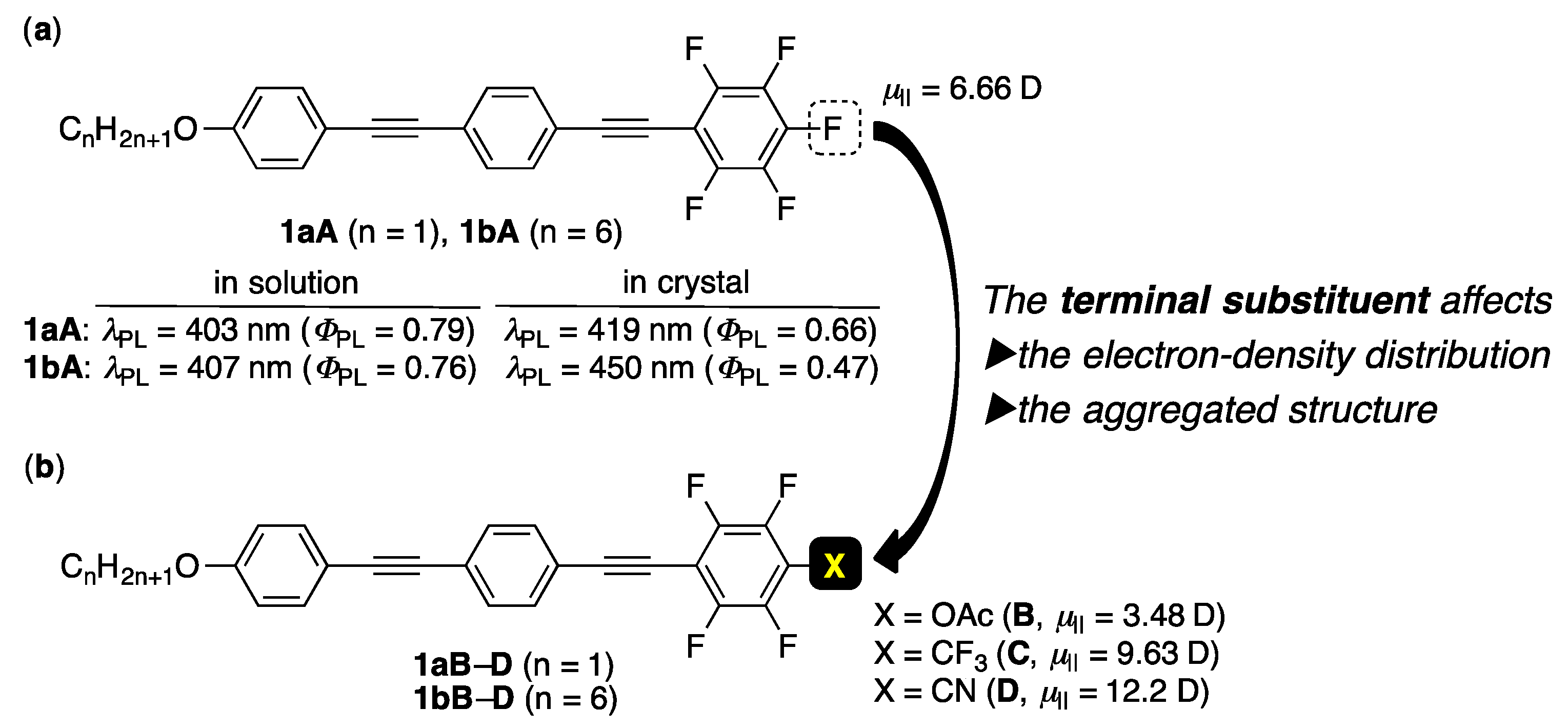
3.1. Molecular Design
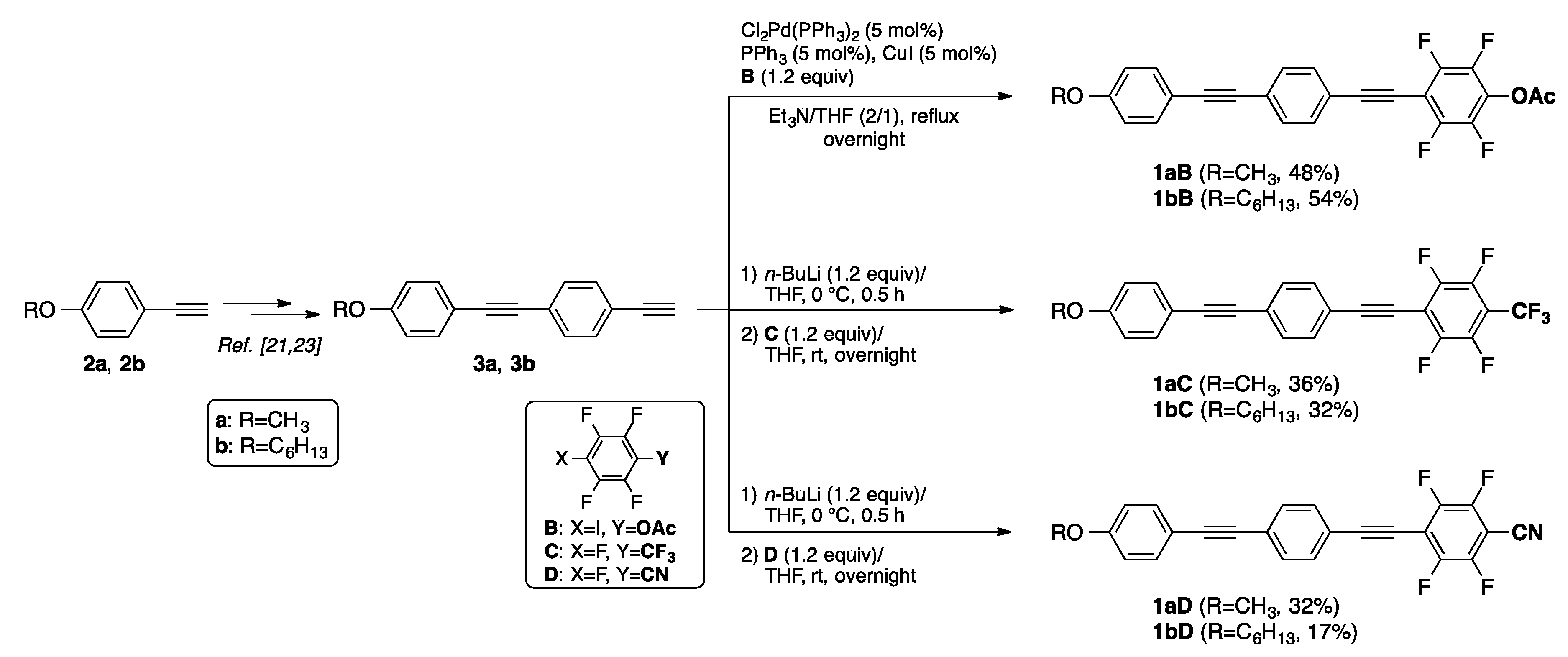
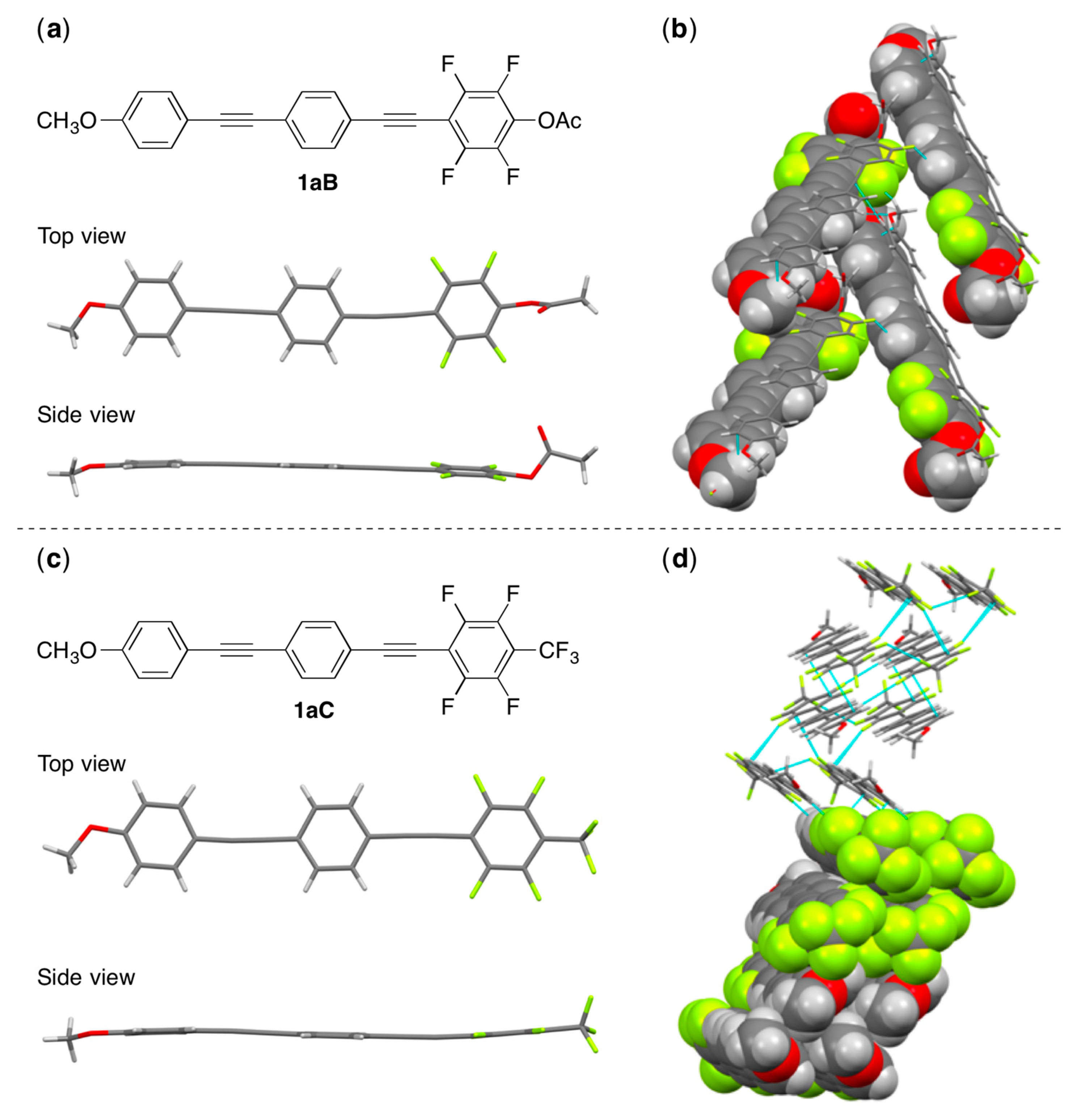
3.2. Synthesis and Crystal Structure
3.3. Phase Transition Behavior
3.4. PL Behavior in the Solution Phase
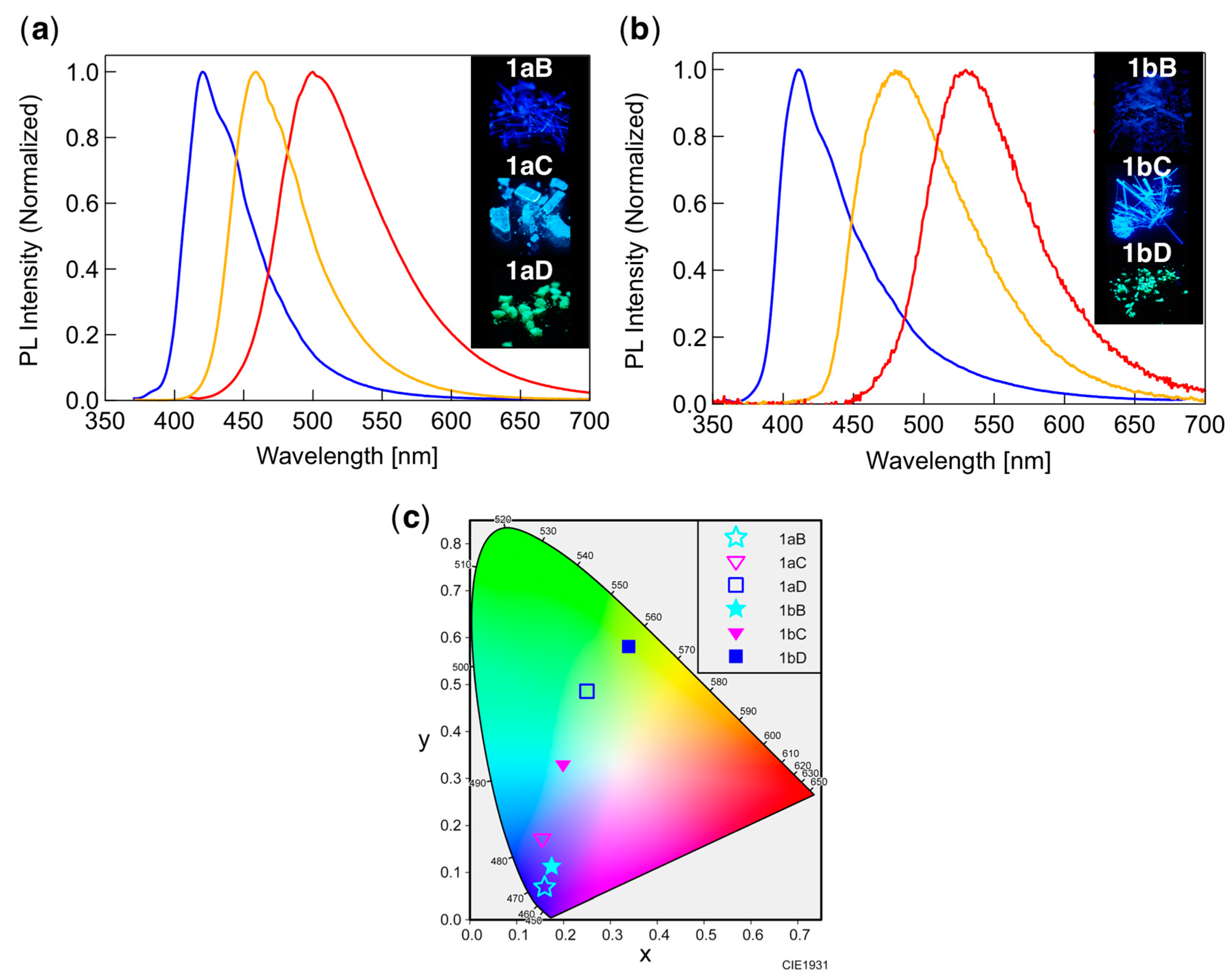
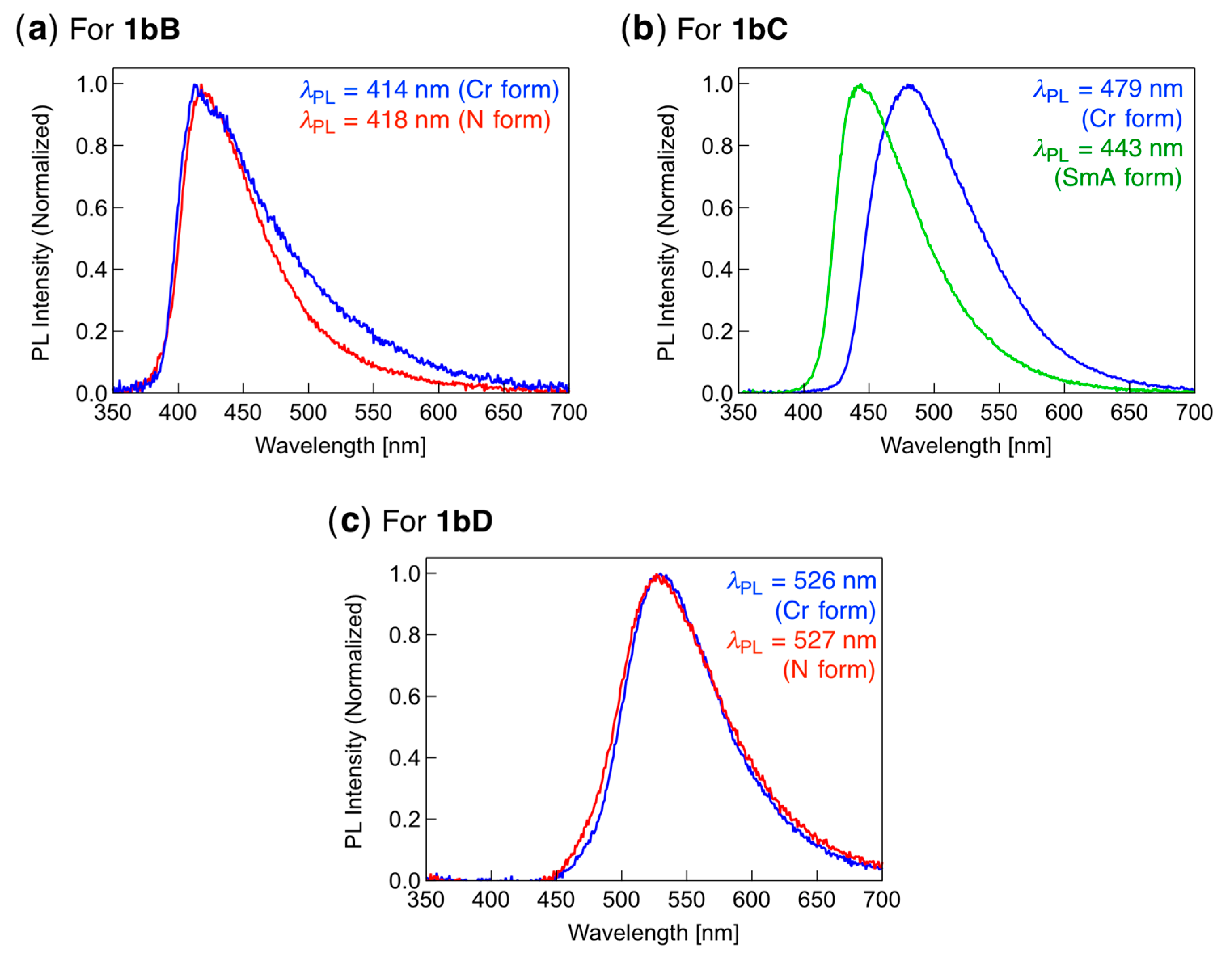
3.5. PL Behavior in the Crystal Phase
3.6. PL Behavior in the LC Phase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- Hemmer, E.; Acosta-Mora, P.; Méndez-Ramos, J.; Fischer, S. Optical nanoprobes for biomedical applications: Shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef]
- Hou, J.-T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.-Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Bui, T.-T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs). Beilstein J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [Green Version]
- Jhulki, S.; Moorthy, J.N. Small molecular hole-transporting materials (HTMs) in organic light-emitting diodes (OLEDs): Structural diversity and classification. J. Mater. Chem. C 2018, 6, 8280–8325. [Google Scholar] [CrossRef]
- Grimsdale, A.C.; Chan, K.L.; Martin, R.E.; Jokisz, P.G.; Holmes, A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009, 109, 897–1091. [Google Scholar] [CrossRef]
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of intramolecular motions: The general mechanism behind aggregation-induced emission. Chem. Eur. J. 2014, 20, 15349–15353. [Google Scholar] [CrossRef]
- Shen, P.; Zhuang, Z.; Zhao, Z.; Tang, B.Z. AIEgens based on main group heterocycles. J. Mater. Chem. C 2018, 6, 11835–11852. [Google Scholar] [CrossRef]
- He, Z.; Ke, C.; Tang, B.Z. Journey of aggregation-induced emission research. ACS Omega 2018, 3, 3267–3277. [Google Scholar] [CrossRef]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2013; pp. 7–21. [Google Scholar]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Konno, T. Trifluoromethylated internal alkynes: Versatile building blocks for the preparation of various fluorine-containing molecules. Synlett 2014, 25, 1350–1370. [Google Scholar] [CrossRef]
- Konno, T. Trifluoromethyl (CF3) group insertion methods in stereoselective synthesis. In Stereoselective Synthesis of Drugs and Natural Products; Andrunshko, V., Andrunshko, N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 769–806. [Google Scholar]
- Yamada, S.; Konno, T. New types of negative dielectric anisotropy liquid crystals: Synthesis of CF2CF2-carbocyclic mesogens and an evaluation of their liquid crystal characteristics. In Chemical Elements (Fluorine, Rhodium and Rubidium): Properties, Synthesis and Applications; Huff, A., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 53–92. [Google Scholar]
- Tamamoto, K.; Yamada, S.; Konno, T. Practical tetrafluoroethylene fragment installation through a coupling reaction of (1,1,2,2-tetrafluorobut-3-en-1-yl)zinc bromide with various electrophiles. Beilstein J. Org. Chem. 2018, 14, 2375–2383. [Google Scholar] [CrossRef]
- Yamada, S.; Mitsuda, A.; Miyano, K.; Tanaka, T.; Morita, M.; Agou, T.; Kubota, T.; Konno, T. Development of novel solid-state light-emitting materials based on pentafluorinated tolane fluorophores. ACS Omega 2018, 3, 9105–9113. [Google Scholar] [CrossRef]
- Yamada, S.; Miyano, K.; Agou, T.; Kubota, T.; Konno, T. 2-Chloroalkoxy-substituted pentafluorinated bistolanes as novel light-emitting liquid crystals. Crystals 2019, 9, 195. [Google Scholar] [CrossRef]
- Yamada, S.; Tanaka, T.; Ichikawa, T.; Konno, T. Novel V- and Y-shaped light-emitting liquid crystals with pentafluorinated bistolane-based luminophores. ACS Omega 2019, 4, 3922–3932. [Google Scholar] [CrossRef]
- Yamada, S.; Morita, M.; Agou, T.; Kubota, T.; Ichikawa, T.; Konno, T. Thermoresponsive luminescence properties of polyfluorinated bistolane-type light-emitting liquid crystals. Org. Biomol. Chem. 2018, 16, 5609–5617. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Morita, M.; Konno, T. Multi-color photoluminescence induced by electron-density distribution of fluorinated bistolane derivatives. J. Fluorine Chem. 2017, 202, 54–64. [Google Scholar] [CrossRef]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Frish, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Peterson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Okuno, K.; Shigeta, Y.; Kishi, R.; Nakano, M. Non-empirical tuning of CAM-B3LYP functional in time-dependent density functional theory for excitation energies of diarylethene derivatives. Chem. Phys. Lett. 2013, 585, 201–206. [Google Scholar] [CrossRef]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Misra, R.; Bhattacharyya, S.P. Intramolecular Charge Transfer: Theory and Applications; Wiley-VCH: Weinheim, Germany, 2018; pp. 4–19. [Google Scholar]
- Liu, C.; Zhao, H.; Zhao, H.; Wang, Z.; Zhang, B. Base-promoted direct and highly selective alkynylation of electron-deficient ocatafluorotoluene. RSC Adv. 2015, 5, 31993–31997. [Google Scholar] [CrossRef]
- Levitus, M.; Schmieder, K.; Ricks, H.; Shimizu, K.D.; Bunz, U.H.F.; Garcia-Garibay, M.A. Steps to demarcate the effects of chromophore aggregation and planarization in poly(phenyleneethynylene)s. 1. Rotationally interrupted conjugation in the excited states of 1,4-bis(phenylethynyl)benzene. J. Am. Chem. Soc. 2001, 123, 4259–4265. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Mooibroek, T.J.; Gamez, P.; Reedijk, J. Lone pair–π interactions: A new supramolecular bond? CrystEngComm 2008, 10, 1501–1515. [Google Scholar] [CrossRef]
- Neel, A.J.; Hilton, M.J.; Sigman, M.S.; Toste, F.D. Exploiting non-covalent π interactions for catalyst design. Nature 2017, 543, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Hird, M.; Toyne, K.J.; Goodby, J.W.; Gray, G.W.; Minter, V.; Tuffin, R.P.; McDonnell, D.G. Synthesis, mesomorphic behaviour and optical anisotropy of some novel materials for nematic mixtures of high birefringence. J. Mater. Chem. 2004, 14, 1731–1743. [Google Scholar] [CrossRef]
- Seed, A.J.; Toyne, K.J.; Goodby, J.W.; Hird, M. Synthesis, transition temperatures, and optical properties of various 2,6-disubstituted naphthalenes and related 1-benzothiophenes with butylsulfanyl and cyano or isothiocyanato terminal groups. J. Mater. Chem. 2000, 10, 2069–2080. [Google Scholar] [CrossRef]
- Seed, A.J.; Toyne, K.J.; Goodby, J.W. Synthesis of the series of monofluoro-substituted 4-butylsulfanyl-4’-cyanobiphenyls and effect of the position of fluorine within the core on refractive indices, optical anisotropies, polarisabilities and order parameters. J. Mater. Chem. 1995, 5, 2201–2207. [Google Scholar] [CrossRef]
- Misra, R.; Bhattacharyya, S.P. Intramolecular Charge Transfer: Theory and Applications; Wiley-VCH: Weinheim, Germany, 2018; pp. 115–144. [Google Scholar]
- Li, M.; Han, J.; Zhao, H.; Liu, Z.; Ma, L.; Sun, C.; Yin, H.; Shi, Y. Investigation of the intermolecular hydrogen bonding effects on the intramolecular charge transfer process of coumarin 340 in tetrahydrofuran solvent. J. Cluster Sci. 2018, 29, 585–592. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Volyniuk, D.; Grazulevicius, J.V.; Sini, G. Blue versus yellow emission in bipolar fluorenone derivatives: The impact of aggregation and hydrogen bonding. J. Mater. Chem. C 2018, 6, 1679–1692. [Google Scholar] [CrossRef]
- Kukhta, N.A.; da Silva Filho, D.A.; Volyniuk, D.; Grazulevicius, J.V.; Sini, G. Can fluorenone-based compounds emit in the blue region? Impact of the conjugation length and the ground-state aggregation. Chem. Mater. 2017, 29, 1695–1707. [Google Scholar] [CrossRef]




| λabs (nm) 1 (ε) 2 | Ground (S0) State 3 | λPL (nm) 4 (ФPL) 4,5 | Excited (S1) State 6 | |||
|---|---|---|---|---|---|---|
| μ|| (D) | HOMO/LUMO (eV) | μ|| (D) | HOMO/LUMO (eV) | |||
| 1aB | 333 (55.2) | 1.91 | −7.09/−1.30 | 410 (0.85) | 3.48 | −6.74/−1.66 |
| 1aC | 341 (48.1) | 7.22 | −7.13/−1.58 | 444 (0.99) | 9.63 | −6.83/−1.94 |
| 1aD | 356 (46.7) | 9.67 | −7.15/−1.87 | 488 (0.91) | 12.2 | −6.86/−2.19 |
| 1bB | 335 (54.5) | 2.27 | −7.07/−1.29 | 414 (0.86) | 3.93 | −6.72/−1.65 |
| 1bC | 343 (52.5) | 7.51 | −7.12/−1.58 | 451 (0.95) | 10.0 | −6.81/−1.94 |
| 1bD | 360 (44.8) | 9.95 | −7.13/−1.86 | 493 (0.87) | 12.6 | −6.84/−2.19 |
| Solvent (ε) 1 | Toluene (2.38) | THF (7.58) | CH2Cl2 (8.93) | MeCN (35.9) | DMF (36.7) |
|---|---|---|---|---|---|
| λPL (nm) 2 | 409 | 482 | 451 | 518 | 523 |
| ФPL 3 | 0.79 | 0.55 | 0.95 | 0.58 | 0.40 |
| Compound | λPL (nm) 1 | ФPL 2 |
|---|---|---|
| 1aB | 420 | 0.48 |
| 1aC | 459 | 0.80 |
| 1aD | 500 | 0.36 |
| 1bB | 414 (418) | 0.17 |
| 1bC | 479 (443) | 0.73 |
| 1bD | 526 (527) | 0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, M.; Yamada, S.; Agou, T.; Kubota, T.; Konno, T. Luminescence Tuning of Fluorinated Bistolanes via Electronic or Aggregated-Structure Control. Appl. Sci. 2019, 9, 1905. https://doi.org/10.3390/app9091905
Morita M, Yamada S, Agou T, Kubota T, Konno T. Luminescence Tuning of Fluorinated Bistolanes via Electronic or Aggregated-Structure Control. Applied Sciences. 2019; 9(9):1905. https://doi.org/10.3390/app9091905
Chicago/Turabian StyleMorita, Masato, Shigeyuki Yamada, Tomohiro Agou, Toshio Kubota, and Tsutomu Konno. 2019. "Luminescence Tuning of Fluorinated Bistolanes via Electronic or Aggregated-Structure Control" Applied Sciences 9, no. 9: 1905. https://doi.org/10.3390/app9091905







