The Role of Substance P in Ischaemic Brain Injury
Abstract
:1. Introduction
2. Secondary Injury
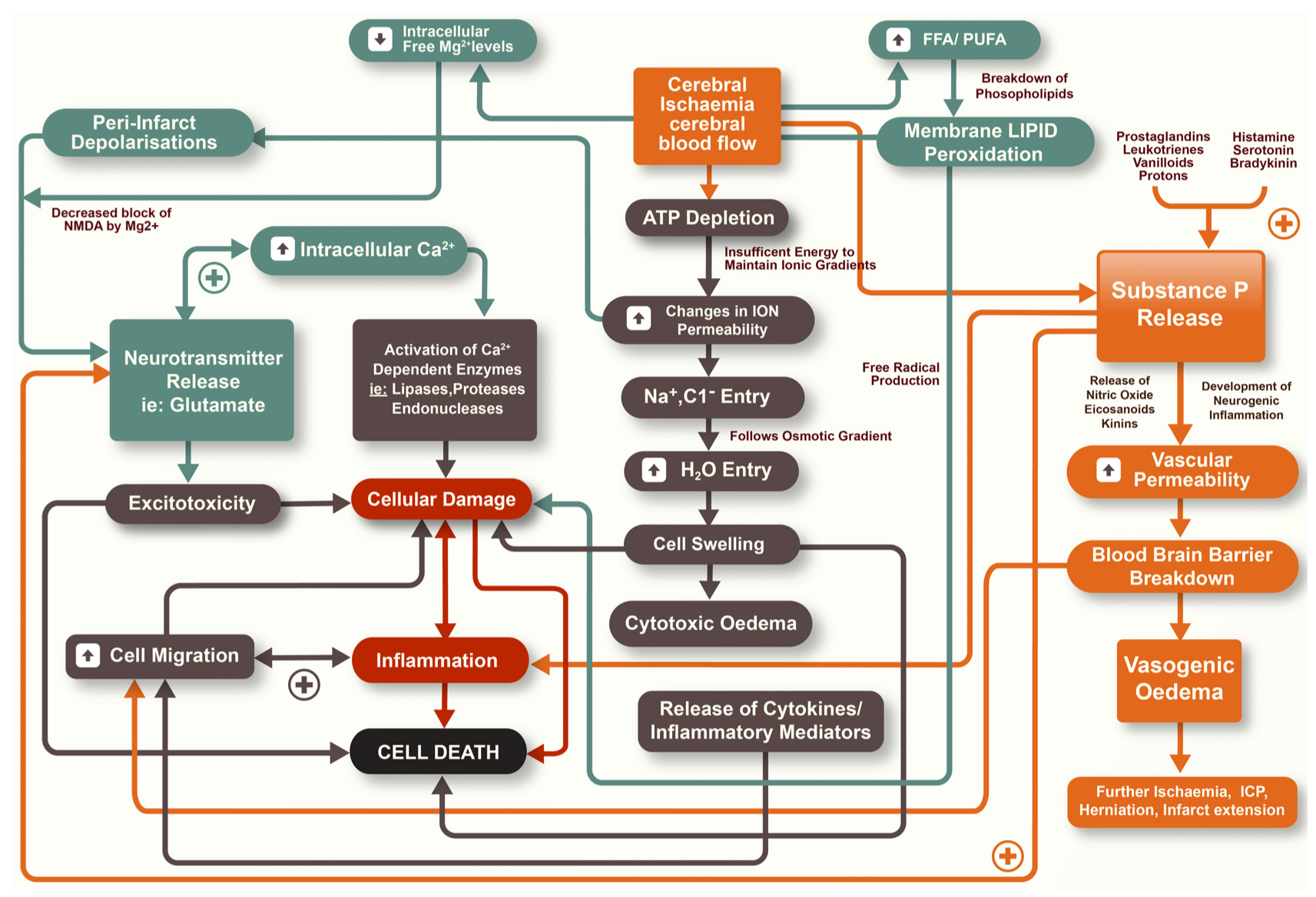
2.1. Blood-Brain Barrier (BBB)
2.2. Cerebral Oedema
3. Substance P
3.1. Synthesis
3.2. Localisation
3.3. Metabolism
3.4. Receptors
3.5. Functions
3.6. Trigeminovascular System
4. Neurogenic Inflammation
4.1. Neurogenic Inflammation in the Peripheral Nervous System
4.2. Neurogenic Inflammation in the Central Nervous System
4.3. Traumatic Brain Injury
4.4. Cerebral Ischemia
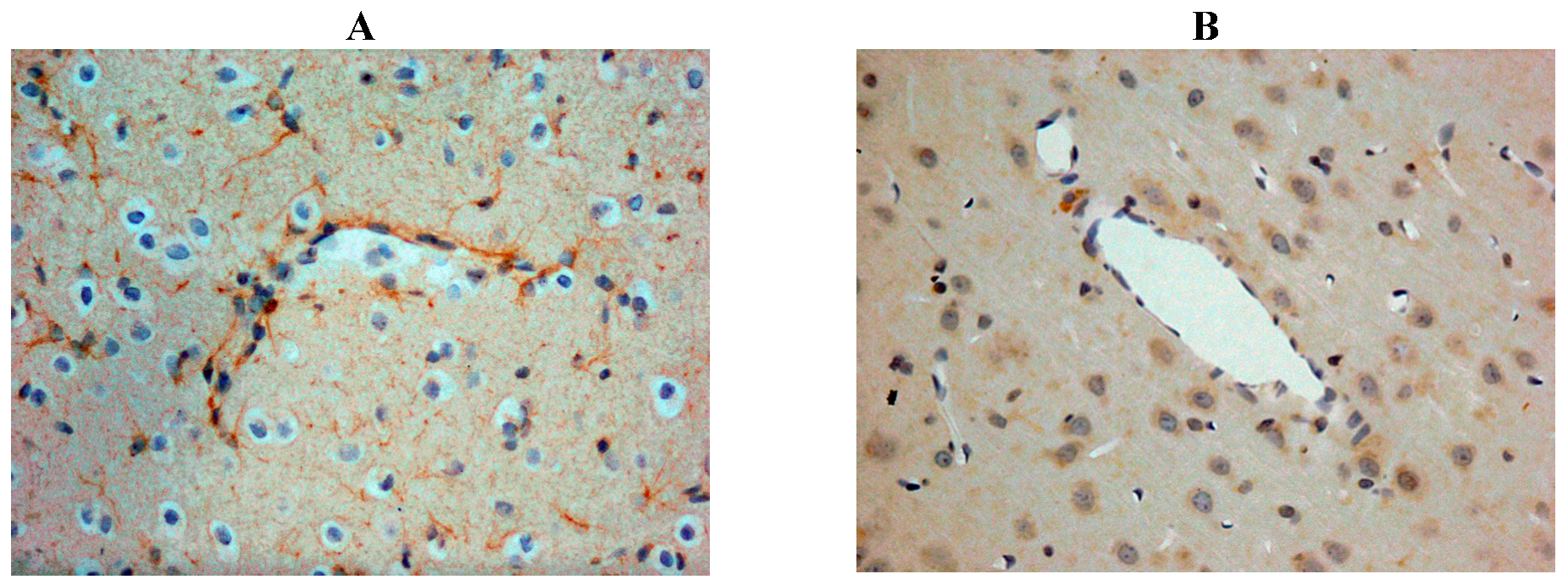
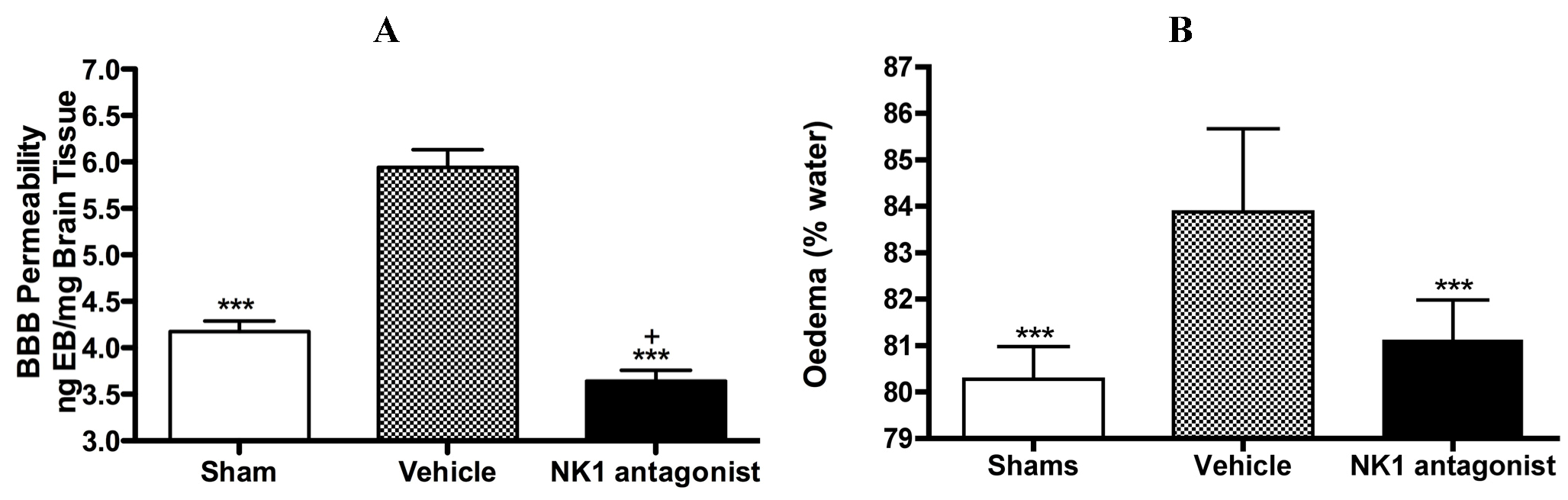
5. NK1 Tachykinin Receptor Antagonists
6. Classical Inflammation
7. Conclusions
Acknowledgments
Conflict of Interest
References
- Dewey, H.M.; Thrift, A.G.; Mihalopoulos, C.; Carter, R.; Macdonell, R.A.; McNeil, J.J.; Donnan, G.A. Cost of stroke in Australia from a societal perspective: Results from the North East Melbourne stroke incidence study (nemesis). Stroke 2001, 32, 2409–2416. [Google Scholar] [CrossRef]
- Zhang, J.B.; Ding, Z.Y.; Yang, Y.; Sun, W.; Hai, F.; Sui, X.N.; Li, X.Y.; Wang, H.Z.; Wang, X.T.; Zheng, J.L. Thrombolysis with alteplase for acute ischemic stroke patients with atrial fibrillation. Neurol. Res. 2010, 32, 353–358. [Google Scholar] [CrossRef]
- Lipton, P. Ischemic cell death in brain neurons. Physiol. Rev. 1999, 79, 1431–1568. [Google Scholar]
- Leker, R.R.; Shohami, E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: Neuroprotective opportunities. Brain Res. Brain Res. Rev. 2002, 39, 55–73. [Google Scholar] [CrossRef]
- Memezawa, H.; Minamisawa, H.; Smith, M.L.; Siesjo, B.K. Ischemic penumbra in a model of reversible middle cerebral artery occlusion in the rat. Exp. Brain Res. 1992, 89, 67–78. [Google Scholar]
- Lo, E.H.; Singhal, A.B.; Torchilin, V.P.; Abbott, N.J. Drug delivery to damaged brain. Brain Res. Brain Res. Rev. 2001, 38, 140–148. [Google Scholar]
- Rosenberg, G.A. Neurological diseases in relation to the blood-brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1139–1151. [Google Scholar] [CrossRef]
- Jiao, H.; Wang, Z.; Liu, Y.; Wang, P.; Xue, Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J. Mol. Neurosci. 2011, 44, 130–139. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Ting, P.; Martinez, H.; Klatzo, I. The biphasic opening of the blood-brain barrier to proteins following temporary middle cerebral artery occlusion. Acta Neuropathol. 1985, 68, 122–129. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Ischemic brain edema. Prog. Cardiovasc. Dis. 1999, 42, 209–216. [Google Scholar] [CrossRef]
- Rosenberg, G.A.; Yang, Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg. Focus 2007, 22, E4. [Google Scholar]
- Petty, M.A.; Wettstein, J.G. Elements of cerebral microvascular ischaemia. Brain Res. Brain Res. Rev. 2001, 36, 23–34. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Cahn, R.; Juhler, M.; Goping, G.; Campbell, G.; Klatzo, I. Role of extracellular proteins in the dynamics of vasogenic brain edema. Acta Neuropathol. 1985, 66, 3–11. [Google Scholar]
- Hacke, W.; Schwab, S.; Horn, M.; Spranger, M.; de Georgia, M.; von Kummer, R. Malignant’ middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch. Neurol. 1996, 53, 309–315. [Google Scholar] [CrossRef]
- Ayata, C.; Ropper, A.H. Ischaemic brain oedema. J. Clin. Neurosci. 2002, 9, 113–124. [Google Scholar] [CrossRef]
- Klatzo, I. Pathophysiological aspects of brain edema. Acta Neuropathol. 1987, 72, 236–239. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Ueki, M.; Chen, Q.; Suemasu, H.; Taniguchi, I.; Okeda, R. Biomechanical characteristics of brain edema: The difference between vasogenic-type and cytotoxic-type edema. Acta Neurochir. Suppl. (Wien.) 1994, 60, 158–161. [Google Scholar]
- Lazovic, J.; Basu, A.; Lin, H.W.; Rothstein, R.P.; Krady, J.K.; Smith, M.B.; Levison, S.W. Neuroinflammation and both cytotoxic and vasogenic edema are reduced in interleukin-1 type 1 receptor-deficient mice conferring neuroprotection. Stroke 2005, 36, 2226–2231. [Google Scholar] [CrossRef]
- Kuroiwa, T.; Miyasaka, N.; Fengyo, Z.; Yamada, I.; Nakane, M.; Nagaoka, T.; Tamura, A.; Ohno, K. Experimental ischemic brain edema: Morphological and magnetic resonance imaging findings. Neurosurg. Focus 2007, 22, E11. [Google Scholar]
- Gartshore, G.; Patterson, J.; Macrae, I.M. Influence of ischemia and reperfusion on the course of brain tissue swelling and blood-brain barrier permeability in a rodent model of transient focal cerebral ischemia. Exp. Neurol. 1997, 147, 353–360. [Google Scholar] [CrossRef]
- Hanley, D.F. Review of critical care and emergency approaches to stroke. Stroke 2003, 34, 362–364. [Google Scholar] [CrossRef]
- Broderick, J.P.; Hacke, W. Treatment of acute ischemic stroke: Part II: Neuroprotection and medical management. Circulation 2002, 106, 1736–1740. [Google Scholar] [CrossRef]
- Maggi, C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995, 26, 911–944. [Google Scholar] [CrossRef]
- Leeman, S.E.; Ferguson, S.L. Substance P: An historical perspective. Neuropeptides 2000, 34, 249–254. [Google Scholar] [CrossRef]
- Otsuka, M.; Yoshioka, K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993, 73, 229–308. [Google Scholar]
- Hokfelt, T.; Broberger, C.; Xu, Z.Q.; Sergeyev, V.; Ubink, R.; Diez, M. Neuropeptides—An overview. Neuropharmacology 2000, 39, 1337–1356. [Google Scholar] [CrossRef]
- Harrison, S.; Geppetti, P. Substance P. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Lundy, F.T.; Linden, G.J. Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation. Crit. Rev. Oral Biol. Med. 2004, 15, 82–98. [Google Scholar] [CrossRef]
- Ribeiro-da-Silva, A.; Hokfelt, T. Neuroanatomical localisation of substance P in the CNS and sensory neurons. Neuropeptides 2000, 34, 256–271. [Google Scholar] [CrossRef]
- Severini, C.; Improta, G.; Falconieri-Erspamer, G.; Salvadori, S.; Erspamer, V. The tachykinin peptide family. Pharmacol. Rev. 2002, 54, 285–322. [Google Scholar] [CrossRef]
- Cao, T.; Gerard, N.P.; Brain, S.D. Use of NK(1) knockout mice to analyze substance P-induced edema formation. Am. J. Physiol. 1999, 277, R476–R481. [Google Scholar]
- Kashiba, H.; Ueda, Y.; Senba, E. Systemic capsaicin in the adult rat differentially affects gene expression for neuropeptides and neurotrophin receptors in primary sensory neurons. Neuroscience 1997, 76, 299–312. [Google Scholar]
- Freed, A.L.; Cooper, J.D.; Davies, M.I.; Lunte, S.M. Investigation of the metabolism of substance P in rat striatum by microdialysis sampling and capillary electrophoresis with laser-induced fluorescence detection. J. Neurosci. Methods 2001, 109, 23–29. [Google Scholar] [CrossRef]
- Matsas, R.; Kenny, A.J.; Turner, A.J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem. J. 1984, 223, 433–440. [Google Scholar]
- Skidgel, R.A.; Erdos, E.G. Cleavage of peptide bonds by angiotensin I converting enzyme. Agents Actions Suppl. 1987, 22, 289–296. [Google Scholar]
- Skidgel, R.A.; Erdos, E.G. The broad substrate specificity of human angiotensin I converting enzyme. Clin. Exp. Hypertens A 1987, 9, 243–259. [Google Scholar] [CrossRef]
- Probert, L.; Hanley, M.R. The immunocytochemical localisation of ‘substance-P-degrading enzyme’ within the rat spinal cord. Neurosci. Lett. 1987, 78, 132–137. [Google Scholar] [CrossRef]
- Blumberg, S.; Teichberg, V.I.; Charli, J.L.; Hersh, L.B.; McKelvy, J.F. Cleavage of substance P to an N-terminal tetrapeptide and a C-terminal heptapeptide by a post-proline cleaving enzyme from bovine brain. Brain Res. 1980, 192, 477–486. [Google Scholar] [CrossRef]
- Azaryan, A.V.; Galoyan, A.A. Substrate specificity of cerebral cathepsin D and high-Mr aspartic endopeptidase. J. Neurosci. Res. 1988, 19, 268–271. [Google Scholar] [CrossRef]
- Kageyama, T. Rabbit procathepsin E and cathepsin E. Nucleotide sequence of cDNA, hydrolytic specificity for biologically active peptides and gene expression during development. Eur. J. Biochem. 1993, 216, 717–728. [Google Scholar] [CrossRef]
- Hooper, N.M.; Turner, A.J. Isolation of two differentially glycosylated forms of peptidyl-dipeptidase a (angiotensin converting enzyme) from pig brain: A re-evaluation of their role in neuropeptide metabolism. Biochem. J. 1987, 241, 625–633. [Google Scholar]
- Sakurada, T.; Hara, A.; Matsumura, H.; Yamada, H.; Sakurada, S.; Kisara, K. A substance P analogue reduces amino acid contents in the rat spinal cord. Pharmacol. Toxicol. 1990, 66, 75–76. [Google Scholar] [CrossRef]
- Wang, L.H.; Ahmad, S.; Benter, I.F.; Chow, A.; Mizutani, S.; Ward, P.E. Differential processing of substance P and neurokinin A by plasma dipeptidyl(amino)peptidase IV, aminopeptidase M and angiotensin converting enzyme. Peptides 1991, 12, 1357–1364. [Google Scholar] [CrossRef]
- Kavelaars, A.; Broeke, D.; Jeurissen, F.; Kardux, J.; Meijer, A.; Franklin, R.; Gelfand, E.W.; Heijnen, C.J. Activation of human monocytes via a non-neurokinin substance P receptor that is coupled to Gi protein, calcium, phospholipase D, MAP kinase, and IL-6 production. J. Immunol. 1994, 153, 3691–3699. [Google Scholar]
- Regoli, D.; Boudon, A.; Fauchere, J.L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994, 46, 551–599. [Google Scholar]
- Hardwick, J.C.; Mawe, G.M.; Parsons, R.L. Tachykinin-induced activation of non-specific cation conductance via NK3 neurokinin receptors in guinea-pig intracardiac neurones. J. Physiol. 1997, 504, 65–74. [Google Scholar] [CrossRef]
- Black, P.H. Stress and the inflammatory response: A review of neurogenic inflammation. Brain Behav. Immun. 2002, 16, 622–653. [Google Scholar] [CrossRef]
- Carrasco, G.A.; van de Kar, L.D. Neuroendocrine pharmacology of stress. Eur. J. Pharmacol. 2003, 463, 235–272. [Google Scholar] [CrossRef]
- Kalsner, S. The question of feedback at the somadendritic region and antidepressant drug action. Brain Res. Bull. 2000, 52, 467–473. [Google Scholar] [CrossRef]
- Levesque, M.; Wallman, M.J.; Parent, R.; Sik, A.; Parent, A. Neurokinin-1 and neurokinin-3 receptors in primate substantia nigra. Neurosci. Res. 2007, 57, 362–371. [Google Scholar] [CrossRef]
- Malcangio, M.; Bowery, N.G. Peptide autoreceptors: Does an autoreceptor for substance P exist? Trends Pharmacol. Sci. 1999, 20, 405–407. [Google Scholar] [CrossRef]
- Patacchini, R.; Maggi, C.A.; Holzer, P. Tachykinin autoreceptors in the gut. Trends Pharmacol. Sci. 2000, 21, 166. [Google Scholar] [CrossRef]
- Dam, T.V.; Quirion, R. Pharmacological characterization and autoradiographic localization of substance p receptors in guinea pig brain. Peptides 1986, 7, 855–864. [Google Scholar] [CrossRef]
- Campos, M.M.; Calixto, J.B. Neurokinin mediation of edema and inflammation. Neuropeptides 2000, 34, 314–322. [Google Scholar] [CrossRef]
- Gavioli, E.C.; Canteras, N.S.; de Lima, T.C. The role of lateral septal NK1 receptors in mediating anxiogenic effects induced by intracerebroventricular injection of substance P. Behav. Brain Res. 2002, 134, 411–415. [Google Scholar] [CrossRef]
- Boyce, S.; Smith, D.; Carlson, E.; Hewson, L.; Rigby, M.; O’Donnell, R.; Harrison, T.; Rupniak, N.M. Intra-amygdala injection of the substance P [NK(1) receptor] antagonist L-760735 inhibits neonatal vocalisations in guinea-pigs. Neuropharmacology 2001, 41, 130–137. [Google Scholar] [CrossRef]
- Palecek, J.; Paleckova, V.; Willis, W.D. Postsynaptic dorsal column neurons express NK1 receptors following colon inflammation. Neuroscience 2003, 116, 565–572. [Google Scholar] [CrossRef]
- Atalay, B.; Bolay, H.; Dalkara, T.; Soylemezoglu, F.; Oge, K.; Ozcan, O.E. Transcorneal stimulation of trigeminal nerve afferents to increase cerebral blood flow in rats with cerebral vasospasm: A noninvasive method to activate the trigeminovascular reflex. J. Neurosurg. 2002, 97, 1179–1183. [Google Scholar] [CrossRef]
- Edvinsson, L.; Ekman, R.; Thulin, T. Reduced levels of calcitonin gene-related peptide (CGRP) but not substance P during and after treatment of severe hypertension in man. J. Hum. Hypertens. 1989, 3, 267–270. [Google Scholar]
- McCulloch, J.; Uddman, R.; Kingman, T.A.; Edvinsson, L. Calcitonin gene-related peptide: Functional role in cerebrovascular regulation. Proc. Natl. Acad. Sci. USA 1986, 83, 5731–5735. [Google Scholar] [CrossRef]
- Uddman, R.; Edvinsson, L.; Ekman, R.; Kingman, T.; McCulloch, J. Innervation of the feline cerebral vasculature by nerve fibers containing calcitonin gene-related peptide: Trigeminal origin and co-existence with substance P. Neurosci. Lett. 1985, 62, 131–136. [Google Scholar] [CrossRef]
- Edvinsson, L.; Brodin, E.; Jansen, I.; Uddman, R. Neurokinin A in cerebral vessels: Characterization, localization and effects in vitro. Regul. Pept. 1988, 20, 181–197. [Google Scholar] [CrossRef]
- Edvinsson, L.; Elsas, T.; Suzuki, N.; Shimizu, T.; Lee, T.J. Origin and co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc. Res. Tech. 2001, 53, 221–228. [Google Scholar] [CrossRef]
- Bayliss, W.M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J. Physiol. 1901, 26, 173–209. [Google Scholar]
- Samsam, M.; Covenas, R.; Csillik, B.; Ahangari, R.; Yajeya, J.; Riquelme, R.; Narvaez, J.A.; Tramu, G. Depletion of substance P, neurokinin A and calcitonin gene-related peptide from the contralateral and ipsilateral caudal trigeminal nucleus following unilateral electrical stimulation of the trigeminal ganglion; a possible neurophysiological and neuroanatomical link to generalized head pain. J. Chem. Neuroanat. 2001, 21, 161–169. [Google Scholar] [CrossRef]
- Richardson, J.D.; Vasko, M.R. Cellular mechanisms of neurogenic inflammation. J. Pharmacol. Exp. Ther. 2002, 302, 839–845. [Google Scholar] [CrossRef]
- Saria, A.; Lundberg, J.M. Capsaicin pretreatment inhibits heat-induced oedema in the rat skin. Naunyn. Schmiedebergs Arch. Pharmacol. 1983, 323, 341–342. [Google Scholar] [CrossRef]
- Holzer, P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998, 30, 5–11. [Google Scholar] [CrossRef]
- Saria, A. The tachykinin NK1 receptor in the brain: Pharmacology and putative functions. Eur. J. Pharmacol. 1999, 375, 51–60. [Google Scholar] [CrossRef]
- Fiebich, B.L.; Schleicher, S.; Butcher, R.D.; Craig, A.; Lieb, K. The neuropeptide substance P activates p38 mitogen-activated protein kinase resulting in IL-6 expression independently from NF-kappa B. J. Immunol. 2000, 165, 5606–5611. [Google Scholar]
- Yamaguchi, M.; Kojima, T.; Kanekawa, M.; Aihara, N.; Nogimura, A.; Kasai, K. Neuropeptides stimulate production of interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha in human dental pulp cells. Inflamm. Res. 2004, 53, 199–204. [Google Scholar] [CrossRef]
- Alves, R.V.; Campos, M.M.; Santos, A.R.; Calixto, J.B. Receptor subtypes involved in tachykinin-mediated edema formation. Peptides 1999, 20, 921–927. [Google Scholar] [CrossRef]
- Markowitz, S.; Saito, K.; Moskowitz, M.A. Neurogenically mediated leakage of plasma protein occurs from blood vessels in dura mater but not brain. J. Neurosci. 1987, 7, 4129–4136. [Google Scholar]
- Cyrino, L.A.; Cardoso, R.C.; Hackl, L.P.; Nicolau, M. Effect of quercetin on plasma extravasation in rat CNS and dura mater by ACE and NEP inhibition. Phytother. Res. 2002, 16, 545–549. [Google Scholar] [CrossRef]
- Stumm, R.; Culmsee, C.; Schafer, M.K.; Krieglstein, J.; Weihe, E. Adaptive plasticity in tachykinin and tachykinin receptor expression after focal cerebral ischemia is differentially linked to gabaergic and glutamatergic cerebrocortical circuits and cerebrovenular endothelium. J. Neurosci. 2001, 21, 798–811. [Google Scholar]
- Donkin, J.J.; Turner, R.J.; Hassan, I.; Vink, R. Substance P in traumatic brain injury. Prog. Brain Res. 2007, 161, 97–109. [Google Scholar] [CrossRef]
- Nimmo, A.J.; Cernak, I.; Heath, D.L.; Hu, X.; Bennett, C.J.; Vink, R. Neurogenic inflammation is associated with development of edema and functional deficits following traumatic brain injury in rats. Neuropeptides 2004, 38, 40–47. [Google Scholar] [CrossRef]
- Vink, R.; Young, A.; Bennett, C.J.; Hu, X.; Connor, C.O.; Cernak, I.; Nimmo, A.J. Neuropeptide release influences brain edema formation after diffuse traumatic brain injury. Acta Neurochir. Suppl. 2003, 86, 257–260. [Google Scholar] [CrossRef]
- Bae, Y.C.; Oh, J.M.; Hwang, S.J.; Shigenaga, Y.; Valtschanoff, J.G. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J. Comp. Neurol. 2004, 478, 62–71. [Google Scholar] [CrossRef]
- Hu, D.E.; Easton, A.S.; Fraser, P.A. TRPV1 activation results in disruption of the blood-brain barrier in the rat. Br. J. Pharmacol. 2005, 146, 576–584. [Google Scholar] [CrossRef]
- Donkin, J.J.; Nimmo, A.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009, 29, 1388–1398. [Google Scholar] [CrossRef]
- Harford-Wright, E.; Thornton, E.; Vink, R. Angiotensin-converting enzyme (ACE) inhibitors exacerbate histological damage and motor deficits after experimental traumatic brain injury. Neurosci. Lett. 2010, 481, 26–29. [Google Scholar] [CrossRef]
- Bertrand, C.; Geppetti, P.; Baker, J.; Petersson, G.; Piedimonte, G.; Nadel, J.A. Role of peptidases and NK1 receptors in vascular extravasation induced by bradykinin in rat nasal mucosa. J. Appl. Physiol. 1993, 74, 2456–2461. [Google Scholar]
- Donkin, J.J.; Cernak, I.; Blumbergs, P.C.; Vink, R. A substance P antagonist reduces axonal injury and improves neurologic outcome when administered up to 12 hours after traumatic brain injury. J. Neurotrauma 2011, 28, 217–224. [Google Scholar] [CrossRef]
- Turner, R.; Wells, A.; Helps, S.; Vink, R. Characterisation of a New Model of Middle Cerebral Artery Occlusion in the Sheep. In Proceedings of 31st Annual Meeting of the Australian Neuroscience Society, Auckland, Australia, 31 January–3 February 2011.
- Turner, R.J.; Blumbergs, P.C.; Sims, N.R.; Helps, S.C.; Rodgers, K.M.; Vink, R. Increased substance P immunoreactivity and edema formation following reversible ischemic stroke. Acta Neurochir. Suppl. 2006, 96, 263–266. [Google Scholar] [CrossRef]
- Turner, R.J.; Helps, S.C.; Thornton, E.; Vink, R. A substance P antagonist improves outcome when administered 4 h after onset of ischaemic stroke. Brain Res. 2011, 1393, 84–90. [Google Scholar] [CrossRef]
- Turner, R.J.; Vink, R. Combined tissue plasminogen activator and an NK1 tachykinin receptor antagonist: An effective treatment for reperfusion injury following acute ischemic stroke in rats. Neuroscience 2012, 220, 1–10. [Google Scholar] [CrossRef]
- Vink, R. The Role of Neuropeptides in BBB Permeability and Increased ICP after Traumatic Brain Innury. In Proceedings of 14th Symposium on Signal Transduction in the Blood Brain Barriers, Istanbul, Turkey, 7–9 September 2011.
- Vink, R.; Donkin, J.J.; Cruz, M.I.; Nimmo, A.J.; Cernak, I. A substance P antagonist increases brain intracellular free magnesium concentration after diffuse traumatic brain injury in rats. J. Am. Coll. Nutr. 2004, 23, 538S–540S. [Google Scholar]
- Malcangio, M.; Ramer, M.S.; Jones, M.G.; McMahon, S.B. Abnormal substance P release from the spinal cord following injury to primary sensory neurons. Eur. J. Neurosci. 2000, 12, 397–399. [Google Scholar] [CrossRef]
- Sharma, H.S.; Nyberg, F.; Olsson, Y.; Dey, P.K. Alteration of substance P after trauma to the spinal cord: An experimental study in the rat. Neuroscience 1990, 38, 205–212. [Google Scholar] [CrossRef]
- Bruno, G.; Tega, F.; Bruno, A.; Graf, U.; Corelli, F.; Molfetta, R.; Barucco, M. The role of substance P in cerebral ischemia. Int. J. Immunopathol. Pharmacol. 2003, 16, 67–72. [Google Scholar]
- Yu, Z.; Cheng, G.; Huang, X.; Li, K.; Cao, X. Neurokinin-1 receptor antagonist SR140333: A novel type of drug to treat cerebral ischemia. Neuroreport 1997, 8, 2117–2119. [Google Scholar] [CrossRef]
- Kim, D.K.; Oh, E.K.; Summers, B.A.; Prabhakar, N.R.; Kumar, G.K. Release of substance P by low oxygen in the rabbit carotid body: Evidence for the involvement of calcium channels. Brain Res. 2001, 892, 359–369. [Google Scholar]
- Khatibi, N.H.; Jadhav, V.; Charles, S.; Chiu, J.; Buchholz, J.; Tang, J.; Zhang, J.H. Capsaicin pre-treatment provides neurovascular protection against neonatal hypoxic-ischemic brain injury in rats. Acta Neurochir. Suppl. 2011, 111, 225–230. [Google Scholar] [CrossRef]
- Gauden, V.; Hu, D.E.; Kurokawa, T.; Sarker, M.H.; Fraser, P.A. Novel technique for estimating cerebrovascular permeability demonstrates capsazepine protection following ischemia-reperfusion. Microcirculation 2007, 14, 767–778. [Google Scholar] [CrossRef]
- Beggs, S.; Liu, X.J.; Kwan, C.; Salter, M.W. Peripheral nerve injury and TRPV1-expressing primary afferent c-fibers cause opening of the blood-brain barrier. Mol. Pain 2010, 6, 74. [Google Scholar] [CrossRef]
- Bondy, B.; Baghai, T.C.; Minov, C.; Schule, C.; Schwarz, M.J.; Zwanzger, P.; Rupprecht, R.; Moller, H.J. Substance P serum levels are increased in major depression: Preliminary results. Biol. Psychiatry 2003, 53, 538–542. [Google Scholar] [CrossRef]
- Zacest, A.C.; Vink, R.; Manavis, J.; Sarvestani, G.T.; Blumbergs, P.C. Substance P immunoreactivity increases following human traumatic brain injury. Acta Neurochir. Suppl. 2010, 106, 211–216. [Google Scholar] [CrossRef]
- Preston, E.; Sutherland, G.; Finsten, A. Three openings of the blood-brain barrier produced by forebrain ischemia in the rat. Neurosci. Lett. 1993, 149, 75–78. [Google Scholar] [CrossRef]
- Kramer, J.H.; Phillips, T.M.; Weglicki, W.B. Magnesium-deficiency-enhanced post-ischemic myocardial injury is reduced by substance p receptor blockade. J. Mol. Cell. Cardiol. 1997, 29, 97–110. [Google Scholar] [CrossRef]
- Vishwanath, R.; Mukherjee, R. Substance P promotes lymphocyte-endothelial cell adhesion preferentially via LFA-1/ICAM-1 interactions. J. Neuroimmunol. 1996, 71, 163–171. [Google Scholar] [CrossRef]
- Guo, C.J.; Douglas, S.D.; Gao, Z.; Wolf, B.A.; Grinspan, J.; Lai, J.P.; Riedel, E.; Ho, W.Z. Interleukin-1beta upregulates functional expression of neurokinin-1 receptor (NK-1R) via NF-kappaB in astrocytes. Glia 2004, 48, 259–266. [Google Scholar] [CrossRef]
- Marriott, D.R.; Wilkin, G.P.; Wood, J.N. Substance P-induced release of prostaglandins from astrocytes: Regional specialisation and correlation with phosphoinositol metabolism. J. Neurochem. 1991, 56, 259–265. [Google Scholar] [CrossRef]
- Palma, C.; Minghetti, L.; Astolfi, M.; Ambrosini, E.; Silberstein, F.C.; Manzini, S.; Levi, G.; Aloisi, F. Functional characterization of substance P receptors on cultured human spinal cord astrocytes: Synergism of substance P with cytokines in inducing interleukin-6 and prostaglandin E2 production. Glia 1997, 21, 183–193. [Google Scholar] [CrossRef]
- De Giorgio, R.; Tazzari, P.L.; Barbara, G.; Stanghellini, V.; Corinaldesi, R. Detection of substance P immunoreactivity in human peripheral leukocytes. J. Neuroimmunol. 1998, 82, 175–181. [Google Scholar] [CrossRef]
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241, 1218–1221. [Google Scholar]
- Brain, S.D. Sensory neuropeptides: Their role in inflammation and wound healing. Immunopharmacology 1997, 37, 133–152. [Google Scholar] [CrossRef]
- Ruff, M.R.; Wahl, S.M.; Pert, C.B. Substance P receptor-mediated chemotaxis of human monocytes. Peptides 1985, 6, 107–111. [Google Scholar]
- Braun, J.S.; Jander, S.; Schroeter, M.; Witte, O.W.; Stoll, G. Spatiotemporal relationship of apoptotic cell death to lymphomonocytic infiltration in photochemically induced focal ischemia of the rat cerebral cortex. Acta Neuropathol. 1996, 92, 255–263. [Google Scholar] [CrossRef]
- Bar-Shavit, Z.; Goldman, R.; Stabinsky, Y.; Gottlieb, P.; Fridkin, M.; Teichberg, V.I.; Blumberg, S. Enhancement of phagocytosis—A newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem. Biophys. Res. Commun. 1980, 94, 1445–1451. [Google Scholar] [CrossRef]
- Dianzani, C.; Collino, M.; Lombardi, G.; Garbarino, G.; Fantozzi, R. Substance P increases neutrophil adhesion to human umbilical vein endothelial cells. Br. J. Pharmacol. 2003, 139, 1103–1110. [Google Scholar] [CrossRef]
- Cioni, C.; Renzi, D.; Calabro, A.; Annunziata, P. Enhanced secretion of substance P by cytokine-stimulated rat brain endothelium cultures. J. Neuroimmunol. 1998, 84, 76–85. [Google Scholar] [CrossRef]
- Annunziata, P.; Cioni, C.; Santonini, R.; Paccagnini, E. Substance P antagonist blocks leakage and reduces activation of cytokine-stimulated rat brain endothelium. J. Neuroimmunol. 2002, 131, 41–49. [Google Scholar] [CrossRef]
- Nessler, S.; Stadelmann, C.; Bittner, A.; Schlegel, K.; Gronen, F.; Brueck, W.; Hemmer, B.; Sommer, N. Suppression of autoimmune encephalomyelitis by a neurokinin-1 receptor antagonist—A putative role for substance p in CNS inflammation. J. Neuroimmunol. 2006, 179, 1–8. [Google Scholar] [CrossRef]
- Persson, M.G.; Hedqvist, P.; Gustafsson, L.E. Nerve-induced tachykinin-mediated vasodilation in skeletal muscle is dependent on nitric oxide formation. Eur. J. Pharmacol. 1991, 205, 295–301. [Google Scholar] [CrossRef]
- Hafstrom, I.; Gyllenhammar, H.; Palmblad, J.; Ringertz, B. Substance P activates and modulates neutrophil oxidative metabolism and aggregation. J. Rheumatol. 1989, 16, 1033–1037. [Google Scholar]
- Castro-Obregon, S.; del Rio, G.; Chen, S.F.; Swanson, R.A.; Frankowski, H.; Rao, R.V.; Stoka, V.; Vesce, S.; Nicholls, D.G.; Bredesen, D.E. A ligand-receptor pair that triggers a non-apoptotic form of programmed cell death. Cell Death Differ. 2002, 9, 807–817. [Google Scholar] [CrossRef]
- Gibbins, J.M. Tweaking the gain on platelet regulation: The tachykinin connection. Atherosclerosis 2009, 206, 1–7. [Google Scholar] [CrossRef]
- Jones, S.; Gibbins, J.M. The neurokinin 1 receptor: A potential new target for anti-platelet therapy? Curr. Opin. Pharmacol. 2008, 8, 114–119. [Google Scholar] [CrossRef]
- Jones, S.; Tucker, K.L.; Sage, T.; Kaiser, W.J.; Barrett, N.E.; Lowry, P.J.; Zimmer, A.; Hunt, S.P.; Emerson, M.; Gibbins, J.M. Peripheral tachykinins and the neurokinin receptor NK1 are required for platelet thrombus formation. Blood 2008, 111, 605–612. [Google Scholar] [CrossRef]
- Bot, I.; de Jager, S.C.; Bot, M.; van Heiningen, S.H.; de Groot, P.; Veldhuizen, R.W.; van Berkel, T.J.; von der Thusen, J.H.; Biessen, E.A. The neuropeptide substance P mediates adventitial mast cell activation and induces intraplaque hemorrhage in advanced atherosclerosis. Circ. Res. 2010, 106, 89–92. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Turner, R.J.; Vink, R. The Role of Substance P in Ischaemic Brain Injury. Brain Sci. 2013, 3, 123-142. https://doi.org/10.3390/brainsci3010123
Turner RJ, Vink R. The Role of Substance P in Ischaemic Brain Injury. Brain Sciences. 2013; 3(1):123-142. https://doi.org/10.3390/brainsci3010123
Chicago/Turabian StyleTurner, Renée J., and Robert Vink. 2013. "The Role of Substance P in Ischaemic Brain Injury" Brain Sciences 3, no. 1: 123-142. https://doi.org/10.3390/brainsci3010123




