Neurovascular Coupling of the Posterior Cerebral Artery in Spinal Cord Injury: A Pilot Study
Abstract
:1. Introduction
2. Methods
| Pre-stimulation | Value at peak-response | Peak percent change CBFv (%) | |
|---|---|---|---|
| PCAv (cm/s) | |||
| AB | |||
| Peak Systolic | 59 ± 10 | 72 ± 13 | 24 ± 7 |
| End Diastolic | 22 ± 10 | 34 ± 5 | 12 ± 6 |
| Mean | 37 ± 6 | 47 ± 7 | 29 ± 12 |
| SCI | |||
| Peak Systolic | 57 ± 16 | 59 ± 14 | 6 ± 7 * |
| End Diastolic | 27 ± 9 | 29 ± 8 | 10 ± 4 * |
| Mean | 37 ± 10 | 38 ± 7 | 6 ± 6 * |
| Cardiorespiratory Metrics | |||
| AB | |||
| PETCO2 (mmHg) | 33.6 ± 3.9 | 32.6 ± 3.7 | −2.9 ± 2.3 |
| MAP (mmHg) | 90 ± 6 | 92 ± 6 | 3 ± 2 |
| HR (beats/min) | 73 ± 10 | 75 ± 11 | 4 ± 3 |
| SCI | |||
| PETCO2 (mmHg) | 33.1 ± 4.1 | 31.3 ± 4.9 | −4.9 ± 5.0 |
| MAP | 72 ± 12 * | 74 ± 11 * | 3 ± 3 |
| HR | 75 ± 23 | 77 ± 24 | 4 ±4 |
2.1. Experimental Protocol
2.2. Data Analysis
3. Results
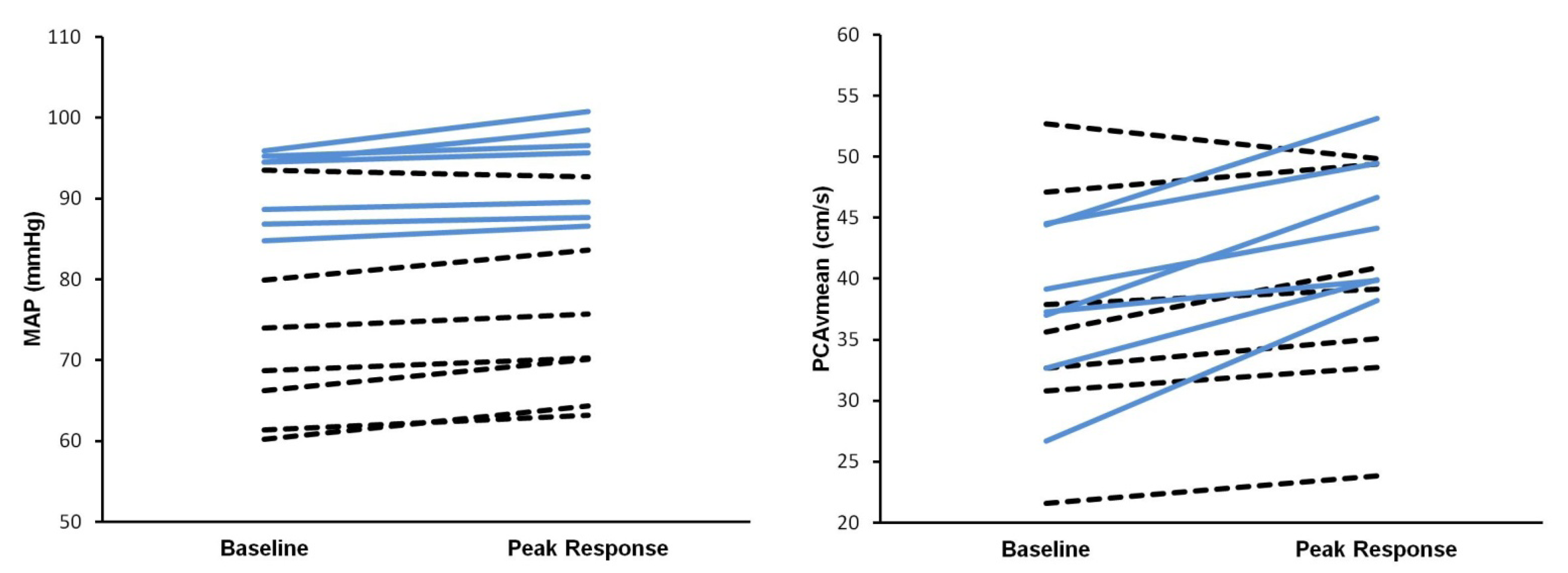
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Warburton, D.E.R.; Sproule, S.; Krassioukov, A.; Eng, J.J. Cardiovascular health and exercise following spinal cord injury. In Spinal Cord Injury Rehabilitation Evidence; Eng, J.J., Teasell, R.W., Miller, W.C., Wolfe, D.L., Townson, A.F., Aubut, J., Abramson, C., Hsieh, J.T.C., Connolly, S., Eds.; International Collaboration on Repair Discoveries: Vancouver, Canada, 2006; pp. 7.1–7.28. [Google Scholar]
- Myers, J.; Lee, M.; Kiratli, J. Cardiovascular disease in spinal cord injury: An overview of prevalence, risk, evaluation, and management. Am. J. Phys. Med. Rehabil. 2007, 86, 142–152. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Eng, J.J.; Krassioukov, A.; Sproule, S.; the SCIRE Research Team. Cardiovascular health and exercise rehabilitation in spinal cord injury. Top. Spinal Cord Inj. Rehabil. 2007, 13, 98–122. [Google Scholar] [CrossRef]
- Phillips, A.A.; Cote, A.T.; Bredin, S.S.; Krassioukov, A.V.; Warburton, D.E. Aortic stiffness increased in spinal cord injury when matched for physical activity. Med. Sci. Sports Exerc. 2012, 44, 2065–2070. [Google Scholar] [CrossRef]
- Myers, J.; Kiratli, J.; Jaramillo, J. The cardiometabolic benefits of routine physical activity in persons living with spinal cord injury. Curr. Cardiovasc. Risk Rep. 2012, 6, 323–330. [Google Scholar] [CrossRef]
- Wu, J.C.; Chen, Y.C.; Liu, L.; Chen, T.J.; Huang, W.C.; Cheng, H.; Tung-Ping, S. Increased risk of stroke after spinal cord injury: A nationwide 4-year follow-up cohort study. Neurology 2012, 78, 1051–1057. [Google Scholar] [CrossRef]
- Novak, V.; Hajjar, I. The relationship between blood pressure and cognitive function. Nat. Rev. Cardiol. 2010, 7, 686–698. [Google Scholar]
- Willie, C.K.; Cowan, E.C.; Ainslie, P.N.; Taylor, C.E.; Smith, K.J.; Sin, P.Y.; Tzeng, Y.C. Neurovascular coupling and distribution of cerebral blood flow during exercise. J. Neurosci. Methods 2011, 198, 270–273. [Google Scholar] [CrossRef]
- Willie, C.K.; Colino, F.L.; Bailey, D.M.; Tzeng, Y.C.; Binsted, G.; Jones, L.W.; Haykowsky, M.J.; Bellapart, J.; Ogoh, S.; Smith, K.J.; et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J. Neurosci. Methods 2011, 196, 221–237. [Google Scholar] [CrossRef]
- Schuepbach, D.; Goenner, F.; Staikov, I.; Mattle, H.P.; Hell, D.; Brenner, H.D. Temporal modulation of cerebral hemodynamics under prefrontal challenge in schizophrenia: A transcranial Doppler sonography study. Psychiatry Res. 2002, 115, 155–170. [Google Scholar] [CrossRef]
- Girouard, H.; Iadecola, C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006, 100, 328–335. [Google Scholar] [CrossRef]
- Suh, M.; Ma, H.; Zhao, M.; Sharif, S.; Schwartz, T.H. Neurovascular coupling and oximetry during epileptic events. Mol. Neurobiol. 2006, 33, 181–197. [Google Scholar] [CrossRef]
- Boms, N.; Yonai, Y.; Molnar, S.; Rosengarten, B.; Bornstein, N.M.; Csiba, L.; Olah, L. Effect of smoking cessation on visually evoked cerebral blood flow response in healthy volunteers. J. Vasc. Res. 2010, 47, 214–220. [Google Scholar] [CrossRef]
- Yano, Y.; Inokuchi, T.; Hoshide, S.; Kanemaru, Y.; Shimada, K.; Kario, K. Association of poor physical function and cognitive dysfunction with high nocturnal blood pressure level in treated elderly hypertensive patients. Am. J. Hypertens. 2011, 24, 285–291. [Google Scholar] [CrossRef]
- Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004, 5, 347–360. [Google Scholar] [CrossRef]
- Panczel, G.; Pohlmann-Eden, B. Visually evoked blood flow responses and vasoneuronal coupling in partial epilepsy. Acta Neurol. Scand. 2004, 110, 308–312. [Google Scholar] [CrossRef]
- Jennings, J.R.; Muldoon, M.F.; Ryan, C.; Price, J.C.; Greer, P.; Sutton-Tyrrell, K.; van der Veen, F.M.; Meltzer, C.C. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology 2005, 64, 1358–1365. [Google Scholar] [CrossRef]
- Schuepbach, D.; Weber, S.; Kawohl, W.; Hell, D. Impaired rapid modulation of cerebral hemodynamics during a planning task in schizophrenia. Clin. Neurophysiol. 2007, 118, 1449–1459. [Google Scholar] [CrossRef]
- Phillips, A.A.; Krassioukov, A.V.; Ainslie, P.; Warburton, D.E.R. Baroreflex function following spinal cord injury. J. Neurotrauma 2012, 29, 2431–2445. [Google Scholar] [CrossRef]
- Krassioukov, A.V.; Furlan, J.C.; Fehlings, M.G. Autonomic dysreflexia in acute spinal cord injury: An under-recognized clinical entity. J. Neurotrauma 2003, 20, 707–716. [Google Scholar] [CrossRef]
- McDonald, D.A.; Potter, J.M. The distribution of blood to the brain. J. Physiol. 1951, 114, 356–371. [Google Scholar]
- Krassioukov, A.; Claydon, V.E. The clinical problems in cardiovascular control following spinal cord injury: An overview. Prog. Brain Res. 2006, 152, 223–229. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef]
- Metea, M.R.; Newman, E.A. Glial cells dilate and constrict blood vessels: A mechanism of neurovascular coupling. J. Neurosci. 2006, 26, 2862–2870. [Google Scholar] [CrossRef]
- Duschek, S.; Schandry, R. Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology 2004, 41, 905–913. [Google Scholar] [CrossRef]
- Krassioukov, A.; Weaver, L.C. Anatomy of the autonomic nervous system. Phys. Med. Rehabil. 1996, 10, 1–14. [Google Scholar]
- Azevedo, E.; Castro, P.; Santos, R.; Freitas, J.; Coelho, T.; Rosengarten, B.; Panerai, R. Autonomic dysfunction affects cerebral neurovascular coupling. Clin. Auton. Res. 2011, 21, 395–403. [Google Scholar] [CrossRef]
- Serrador, J.M.; Picot, P.A.; Rutt, B.K.; Shoemaker, J.K.; Bondar, R.L. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 2000, 31, 1672–1678. [Google Scholar] [CrossRef]
- Peebles, K.C.; Richards, A.M.; Celi, L.; McGrattan, K.; Murrell, C.J.; Ainslie, P.N. Human cerebral arteriovenous vasoactive exchange during alterations in arterial blood gases. J. Appl. Physiol. 2008, 105, 1060–1068. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Phillips, A.A.; Krassioukov, A.V.; Zheng, M.M.Z.; Warburton, D.E.R. Neurovascular Coupling of the Posterior Cerebral Artery in Spinal Cord Injury: A Pilot Study. Brain Sci. 2013, 3, 781-789. https://doi.org/10.3390/brainsci3020781
Phillips AA, Krassioukov AV, Zheng MMZ, Warburton DER. Neurovascular Coupling of the Posterior Cerebral Artery in Spinal Cord Injury: A Pilot Study. Brain Sciences. 2013; 3(2):781-789. https://doi.org/10.3390/brainsci3020781
Chicago/Turabian StylePhillips, Aaron A., Andrei V. Krassioukov, Mei Mu Zi Zheng, and Darren E. R. Warburton. 2013. "Neurovascular Coupling of the Posterior Cerebral Artery in Spinal Cord Injury: A Pilot Study" Brain Sciences 3, no. 2: 781-789. https://doi.org/10.3390/brainsci3020781




