1. Introduction
Morphine is a potent analgesic that is widely used clinically for pain management. However, long-term use of morphine can lead to morphine tolerance and addiction [
1]. In addition, morphine can profoundly and detrimentally affect the body’s immune system, at both the cellular and molecular levels. Morphine suppresses lymphocyte trafficking; decreases natural killer cell activity; inhibits the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) [
2,
3,
4]; and induces atrophy of immune organs, such as the spleen and thymus [
5,
6,
7,
8,
9].
Morphine-induced immunosuppression significantly increases the risk of bacterial infection [
10]. Although the immunosuppressive effects of morphine have been widely studied, the mechanisms involved in the body’s inflammatory response to pathogens during morphine tolerance have not been fully investigated.
Inflammasomes are multi-protein complexes assembled from nucleotide oligomerization domain receptor proteins known as nucleotide-binding oligomerization domain (NOD)-like receptors (NLR) [
11]. They function as important mediators of innate immunity. Inflammasomes play a key role in the regulation of inflammation and immune responses by participating in the production of pro-inflammatory cytokines, including IL-1β and interleukin-18 (IL-18) [
11,
12,
13]. Both IL-1β and IL-18 produce a wide variety of biological effects associated with infection, inflammation, and autoimmune processes.
There have been about 20 NLR inflammasomes identified in humans. The most commonly studied ones include NLR Family Pyrin Domain Containing 1 (NLPR1), NLR family apoptosis inhibitory protein 6 (NAIP2), NLR Family Pyrin Domain Containing 3 (NLRP3), NLR Family Pyrin Domain Containing 5 (NLRP5), NLR Family Pyrin Domain Containing 6 (NLRP6), NLR Family Pyrin Domain Containing 12 (NLRP12), NLR family member X1 (NLRPX1), and NLR Family CARD Domain Containing 4 (NLRC4) [
14]. Inflammasomes have been sub-divided into two groups; pro-inflammatory inflammasomes and anti-inflammatory inflammasomes. The pro-inflammatory inflammasomes include NLRP3 and NLRC4, and their functions have been widely studied. The anti-inflammatory inflammasomes include NLRP12, NLRX1, NLRC3, and NLRC5. They appear to function by limiting or suppressing a pro-inflammatory response; however, this group has not been well studied to date.
There have been a few recent reports characterizing the anti-inflammatory properties of NLRP12 [
15,
16]. NLRP12 can inhibit NF-κB signaling through both the canonical and non-canonical pathways, which are important in the control and regulation of innate immune responses [
15,
16]. NLRP12 inhibits IL-1 receptor-associated kinase 1 (IRAK1), a downstream component of the pathogen activated Toll-like receptor (TLR) pathway, which, in turn, decreases the signaling of the canonical NF-κB pathway [
17]. In the non-canonical NF-κB pathway, NLRP12 interacts with and rapidly degrades NF-κB-inducing kinase (NIK), thereby suppressing NF-κB signaling [
18].
The Library of Integrated Network-Based Cellular Signatures (LINCS) [
19] is a database which implements a biological network-based strategy to make assessments regarding the impact of drugs, genetics, and related biological perturbations (alterations induced by external or internal mechanisms) on cellular states. The library database is based on the philosophy that typical human pathology, biology, and pharmacology are most aptly understood using a systems-level approach. It was constructed to generate a robust approach for perturbing a diversity of cell types, measuring cellular responses, integrating and analyzing data, and visualizing and interrogating the database for a variety of biomedical research applications [
19]. This library allows researchers to access a wide variety of data by using a matrix consisting of cell type by experimental treatment by phenotypic assay. Using LINCS, researchers are able to inquire about information regarding mechanism-based relationships among the effects of different drug responses and their targets (perturbents) as well as associations among responding cellular components, in the format of network interactions and structure-function relationships [
20].
In this study, we used an inflammasome PCR array containing 84 inflammasome-related genes to investigate the expression of inflammasomes during an inflammatory response to the bacterial endotoxin, lipopolysaccharide (LPS), in the rat brain during morphine tolerance. LINCS was then used to project possible candidate targets from the mRNA gene expression profiles generated from the PCR array analysis.
2. Materials and Methods
2.1. Animals
Fisher/NHsd 344 (F344) rats were purchased from Harlan Laboratories (Indianapolis, IN, USA). The animals were housed in groups of 3–5 animals in a temperature controlled (21 °C–22 °C) animal holding room, under a 12-h light/12-h dark illumination cycle (lights on at 7:00 a.m.). Food and tap water were provided ad libitum. The Institutional Animal Care and Use Committee (IACUC) at Seton Hall University, South Orange, NJ, USA approved the experimental protocol.
2.2. Morphine and LPS Administration
A 2 + 4 regimen was used to produce morphine tolerance in 7–8 mo old (250–350 g) male F344 rats [
5,
21,
22,
23,
24]. The rats (
n = 16) were randomly assigned into two groups. The morphine-tolerant group received two 75 mg morphine sulfate pellets (NIDA, Rockville, MD, USA) on Day 1 via subcutaneous (s.c.) implantation and four pellets on Day 2, whereas the control group received placebo pellets on both days. On Day 5, the two groups were randomly assigned to receive either LPS (250 µg/kg, Sigma, St. Louis, MO, USA) or saline (vehicle) [
5,
21,
22,
23,
24]. Thus, the four experimental groups were placebo-control + saline, placebo-control + LPS, morphine-tolerant + saline, and morphine-tolerant + LPS. Two hours after the treatment with LPS or saline, the animals were euthanized and the brains were harvested.
2.3. RNA Isolation and Preparation of cDNA
Total RNA was extracted from the brain tissue using TRIZOL (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s protocol. To remove contaminating DNA, the total RNA samples were treated with RNase-free DNase (Qiagen, Valencia, CA, USA), followed by further purification using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The RNA quality and quantity were assessed using a nanodrop spectrophotometer (Thermo Scientific, Waltham, MA, USA). An equal amount of RNA (400 ng) from each sample was then converted into first-strand cDNA using a RT2 First Strand Kit (SABiosciences, Frederick, MD, USA) for a PCR array.
2.4. Real-Time PCR Array
The expression of 84 key genes involved in the function of inflammasomes, general NLR signaling, and cytokine and chemokine genes was quantified using a custom PCR array and RT2 SYBR Green Fluorescein qPCR Master Mix (SABiosciences, Frederick, MD, USA), according to the manufacturer’s protocol. Using an ABI Prism 7900HT Fast Detection System (Applied Biosystems, Foster, CA, USA), real-time PCR was performed by first denaturing the PCR mix at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and at 60 °C for 1 min.
2.5. PCR Array Data Analysis
The expression of each gene was normalized to housekeeping genes and calculated using the ∆∆
Ct method. The threshold and baseline values were set manually, and the resulting threshold cycle values (
Ct) were analyzed using the PCR array data analysis template supplied on the manufacturer’s website [
25]. The mean fold change in mRNA expression from 3 to 5 biological replicates was considered significant at
p < 0.05. The gene profile signatures were created for every two groups compared.
2.6. LINCS Analysis
The differentially expressed genes were input into the Query App (apps.lincscloud.org/query), as described previously [
26,
27]. Based on the LINCS database, LincsCloud utilized gene profile signatures generated from the PCR array to generate a report, including probability outcomes in terms of gene knockdown effects and drug mimics. The scores given in the report evaluated how much a particular set of gene regulation features (named pertubagens) was likely to be connected with the genes listed in the LINCS report. Positive readings in the Consensus Knockdown Connections in the report indicate that knockdown of the genes listed in the LINCS report would match the gene changes input into the Query App, and thus the genes with high scores represent potential target genes for the experimental treatment.
4. Discussion
Inflammasomes recognize a variety of pathogen-associated molecular patterns (PAMPs), including endotoxins such as LPS. Depending on the NLR proteins that constitute inflammasomes, an inflamasome can be pro-inflammatory or anti-inflammatory in nature [
28]. For pro-inflammatory inflammasomes such as NLRP3, in vitro studies have shown that the activation and release of pro-inflammatory cytokines requires two signals. The first signal, triggered by PAMPs, leads to the activation of inflammasomes, which then provide the second signal. The activated inflammasomes, through caspase 1 activation, promote the production of the pro-inflammatory cytokines, IL-1β and IL-18. However, the signaling pathways during infection or inflammation in vivo are not yet completely defined [
29], and the characteristics of anti-inflammatory inflammasomes such as NLRP12 have not yet been extensively investigated. To our knowledge, our study is one of the first to report the modulation of NLRP12 expression in response to LPS and morphine in vivo.
Recently, NLRP12 was designated as an anti-inflammatory NLR inflammasome protein. It is believed to be a negative regulator of the NF-κB signaling pathway by inhibiting downstream signaling of TLRs, particularly IRAK-1 [
28,
30]. Our results showed that NLRP12 expression decreased in the brains of both the control (placebo-control + LPS) and morphine-tolerant (morphine-tolerant + LPS) rats in response to an LPS challenge, indicating that one of the mechanisms by which LPS induces an inflammatory response is by inhibiting the expression of the anti-inflammatory NLRP12 inflammasome.
Although NLRP12 expression was decreased in both groups given LPS, the decrease was significantly greater in the control rats than in the morphine-tolerant rats, which suggests that the LPS-induced NLRP12 decrease is countered during morphine tolerance. Hence morphine may also modulate NLRP12 activity, directly or indirectly, thereby exerting its immunosuppressive effects and opposing the LPS-induced decrease in NLRP12 in the presence of morphine tolerance.
Birc3, a downstream regulator of inflammasome signaling, is essential for controlling the synthesis of cytokines and chemokines in the inflammatory Mapk and NF-κB pathways. It is also required for inflammasome activation, subsequent caspase 1 activity, and IL-1β formation [
31]. In our study, Birc3 expression was significantly increased in both the placebo-control and morphine-tolerant rats in response to LPS, indicating that LPS is able to induce an inflammatory response through Birc3 activity, following inhibition of the anti-inflammatory NLRP12. However, in response to LPS, Birc3 expression in the morphine-tolerant rats did not change in comparison to the placebo-control rats. This indicates that morphine may not be able to modulate Birc3 expression, and therefore there is no change, increase or decrease, in its expression in the morphine tolerant state.
NF-κB is important in the activation of inflammatory mediators such as cytokines and chemokines [
16]. Previous studies have reported that NLRP12 inhibits both canonical and non-canonical NF-κB activation [
16,
28] and that Nfkbia, a downstream regulator of inflammasomes, inhibits the activity of dimeric NF-κB/Rel complexes [
32]. In our study, Nfkbia was significantly increased in response to LPS in both the placebo-control and morphine-tolerant rats. During an inflammatory response, one would expect the expression and activity of a positive regulator of inflammation to be increased, whereas that of a negative regulator would be decreased. However, from a physiological standpoint, there is a constant effort to balance pro- and anti-inflammatory activity [
33,
34]. This quest to balance the pro- and anti-inflammatory responses could be one of the reasons for an increase in Nfkbia, which is known to inhibit the activity of the pro-inflammatory dimeric NF-κB/REL, thus reducing the production of pro-inflammatory mediators.
As expected, we found that the expression of pro-inflammatory cytokines (IL-1β and IL-6) and chemokines (Ccl2, Ccl7, Cxcl1, and Cxcl3) was increased in response to LPS in the placebo-control rats [
35,
36,
37]. In the morphine-tolerant rats, however, the LPS-induced cytokine and chemokine expression levels were lower, suggesting that NLRP12 inhibition in response to LPS may be opposed or subdued in the morphine tolerant state.
In a previous study, we observed that, in peripheral immune organs such as the spleen, NLRP3 expression, but not NLRP12 expression, is altered in response to LPS, with and without morphine tolerance [
38], suggesting that the mechanism(s) of inflammasome activation in response to pathogens may be different in peripheral immune organs, compared to the central nervous system. During morphine tolerance, the LPS-induced expression of NLRP3, as well as that of cytokines and chemokines, is reduced in comparison to the placebo-control rats given LPS [
38]. These observations are consistent with previous studies showing that immune activation, including an inflammatory response, is diminished during morphine tolerance [
39]. Therefore, the data from the present study, as well as from our previous report [
38], collectively indicate that morphine may exert its effects through both pro- and anti-inflammatory inflammasomes.
LINCS analysis is able to predict potential target genes based on a certain treatment and the gene profile signatures in its database. In our study, LINCS was able to generate a report of potential targets with a p value of <0.05 from the list of genes with altered expression in response to LPS in control rats (placebo-control + saline versus placebo-control + LPS) but not from the other two comparisons (placebo-control + saline versus morphine-tolerant + saline, morphine-tolerant + saline versus morphine-tolerant + LPS), because there were not enough significant gene features in those two groups. When enlarging the set of gene features for the comparison of placebo-control + saline versus placebo-control + LPS to a p-value of <0.1, LINCS generated a report with similar potential targets. Thus, the gene features were then studied with a p-value of <0.1 on all three sets of comparisons. VPS28, PROCR, and CHMP2A were the top three with the highest scores in LINCS report generated based on placebo-control + saline versus placebo-control + LPS gene alternations, suggesting that Vps28, Procr and Chmp2a were potential targets of LPS. In the report for placebo-control + saline versus morphine-tolerant + saline, SMARCE1, AHRR, and GPX7 were the most likely targets altered in morphine tolerance predicted by LINCS. In the morphine-tolerant + saline versus morphine-tolerant + LPS report, AHR, UBE2L6, and PAFAH1B3 were the top three candidates that potentially responsible for the LPS-induced immune responses during morphine tolerance in rats. In the LINCS reports, the listed potential targets had different rankings using the different sets of gene features. This confirms that the response to LPS by those inflammosome-related genes could be affected by morphine tolerance.
Moreover, among the targets listed above, while VPS28 was No. 1 in the control rats in response to LPS, it was No. 4 in morphine-tolerant rats (
Table 4). The VPS28 protein functions in transporting and sorting proteins into sub-cellular vesicles. In our study, LINCS analysis suggests that the actions of VPS28 in response to LPS could be dampened during morphine tolerance.
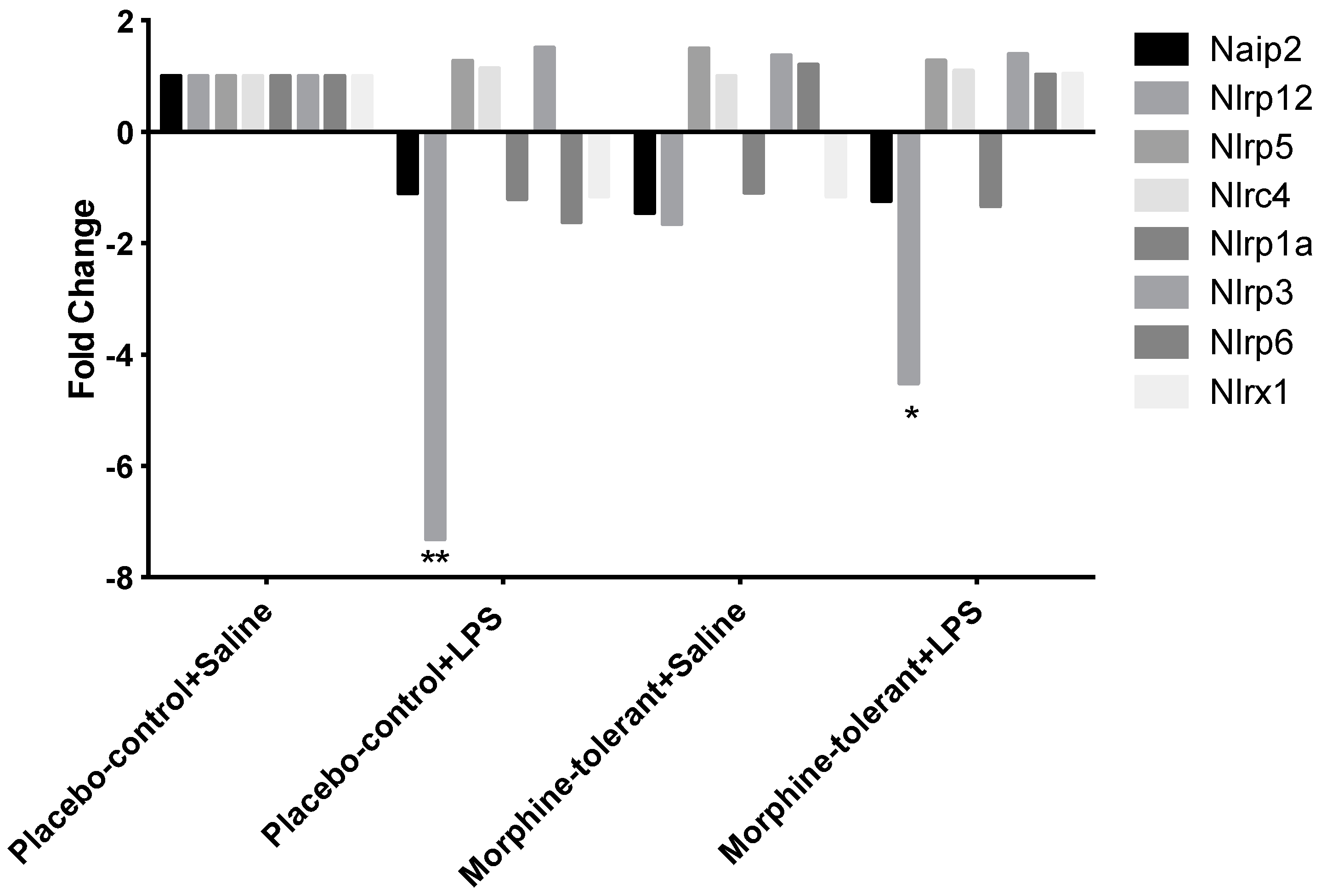

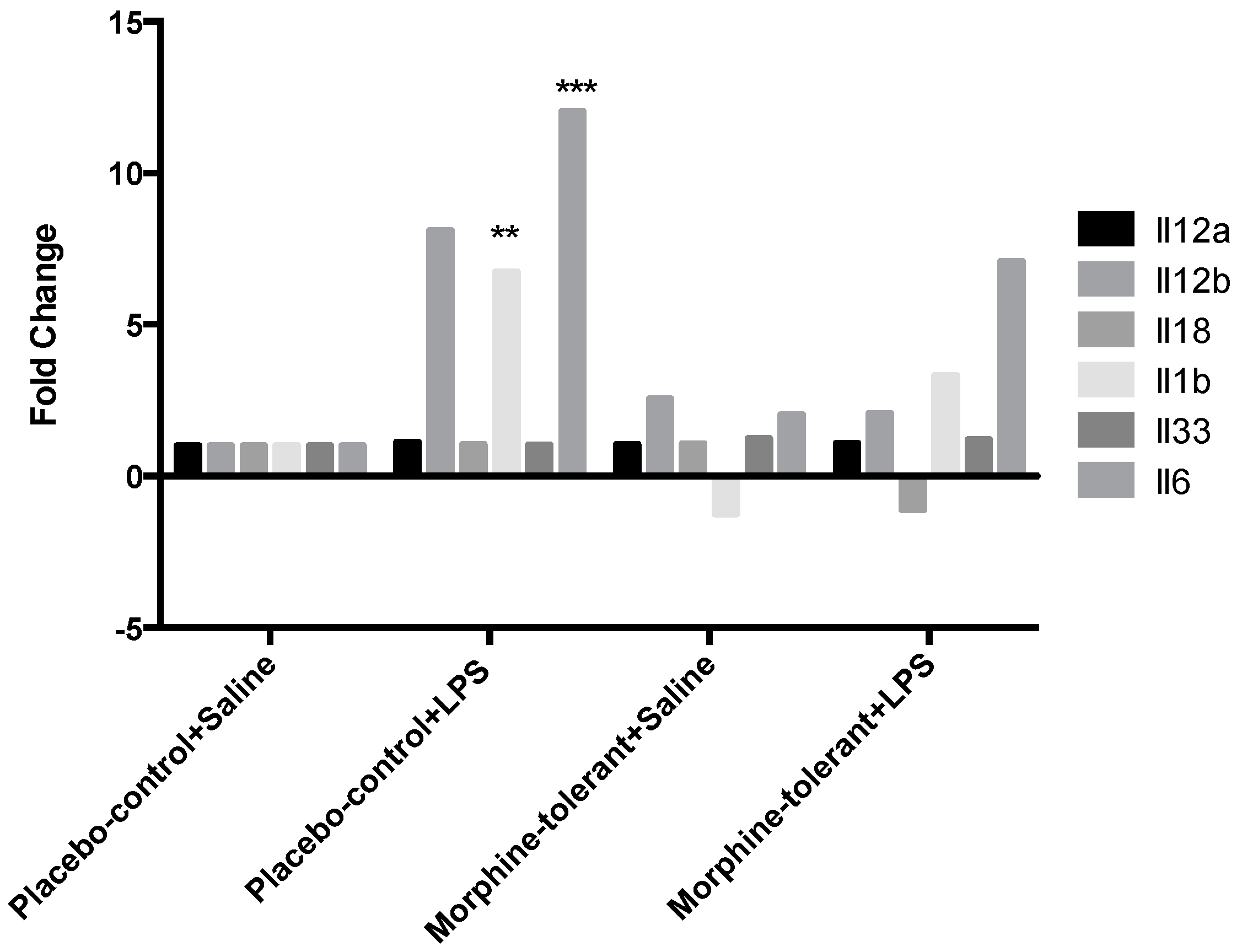
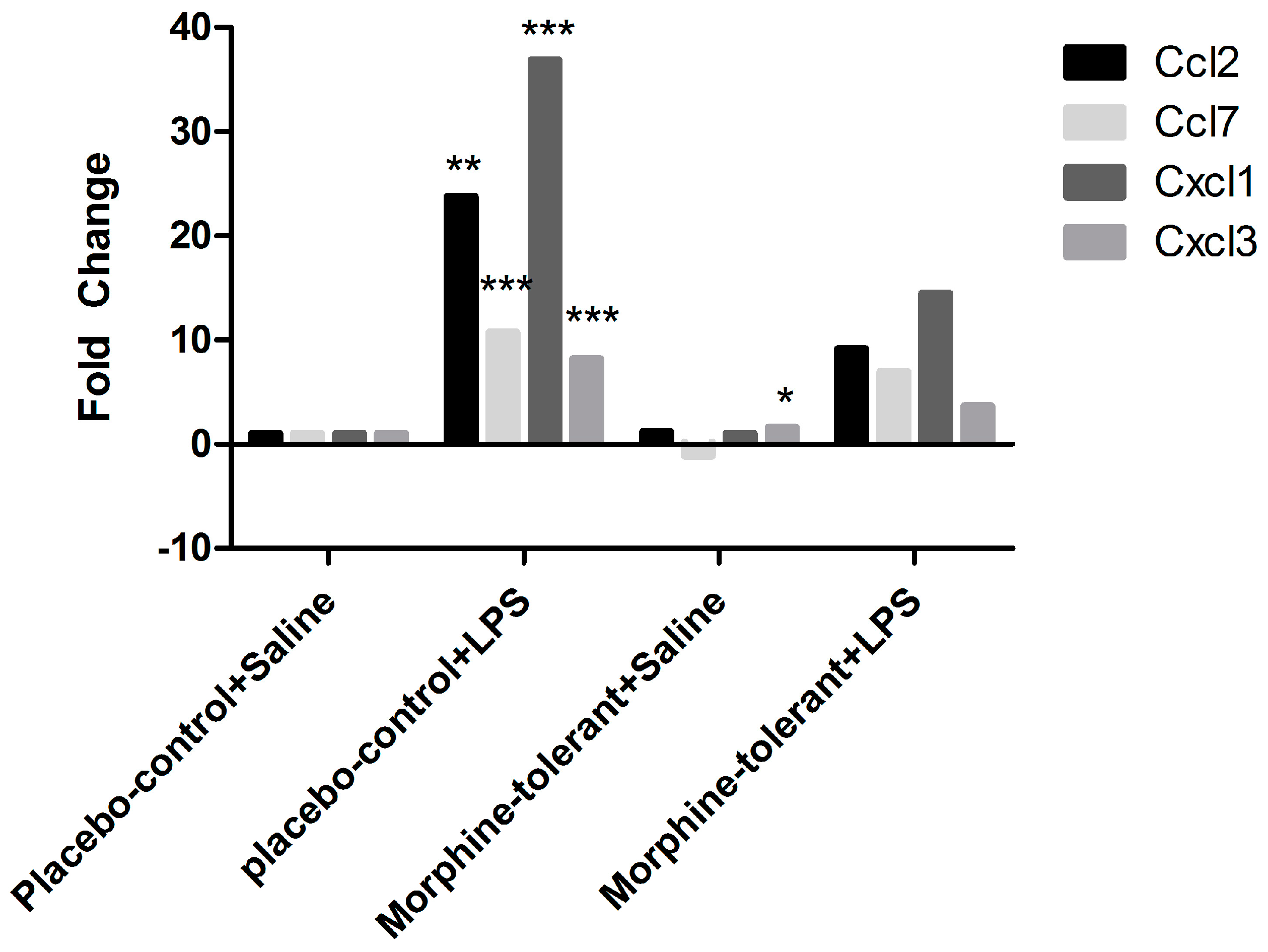
 : 6–10 fold decrease;
: 6–10 fold decrease;  : 2–5 fold decrease;
: 2–5 fold decrease;  : <2 fold;
: <2 fold;  : 2–5 fold increase;
: 2–5 fold increase;  : 6–10 fold increase;
: 6–10 fold increase;  : 11–30 fold increase;
: 11–30 fold increase;  : 31–50 fold increase.
: 31–50 fold increase. : 6–10 fold decrease;
: 6–10 fold decrease;  : 2–5 fold decrease;
: 2–5 fold decrease;  : <2 fold;
: <2 fold;  : 2–5 fold increase;
: 2–5 fold increase;  : 6–10 fold increase;
: 6–10 fold increase;  : 11–30 fold increase;
: 11–30 fold increase;  : 31–50 fold increase.
: 31–50 fold increase. : 6–10 fold decrease;
: 6–10 fold decrease;  : 2–5 fold decrease;
: 2–5 fold decrease;  : <2 fold;
: <2 fold;  : 2–5 fold increase;
: 2–5 fold increase;  : 6–10 fold increase;
: 6–10 fold increase;  : 11–30 fold increase;
: 11–30 fold increase;  : 31–50 fold increase.
: 31–50 fold increase.




