Brief Report: Using the Internet to Identify Persons with Cognitive Impairment for Participation in Clinical Trials
Abstract
:1. Introduction
2. Materials and Methods
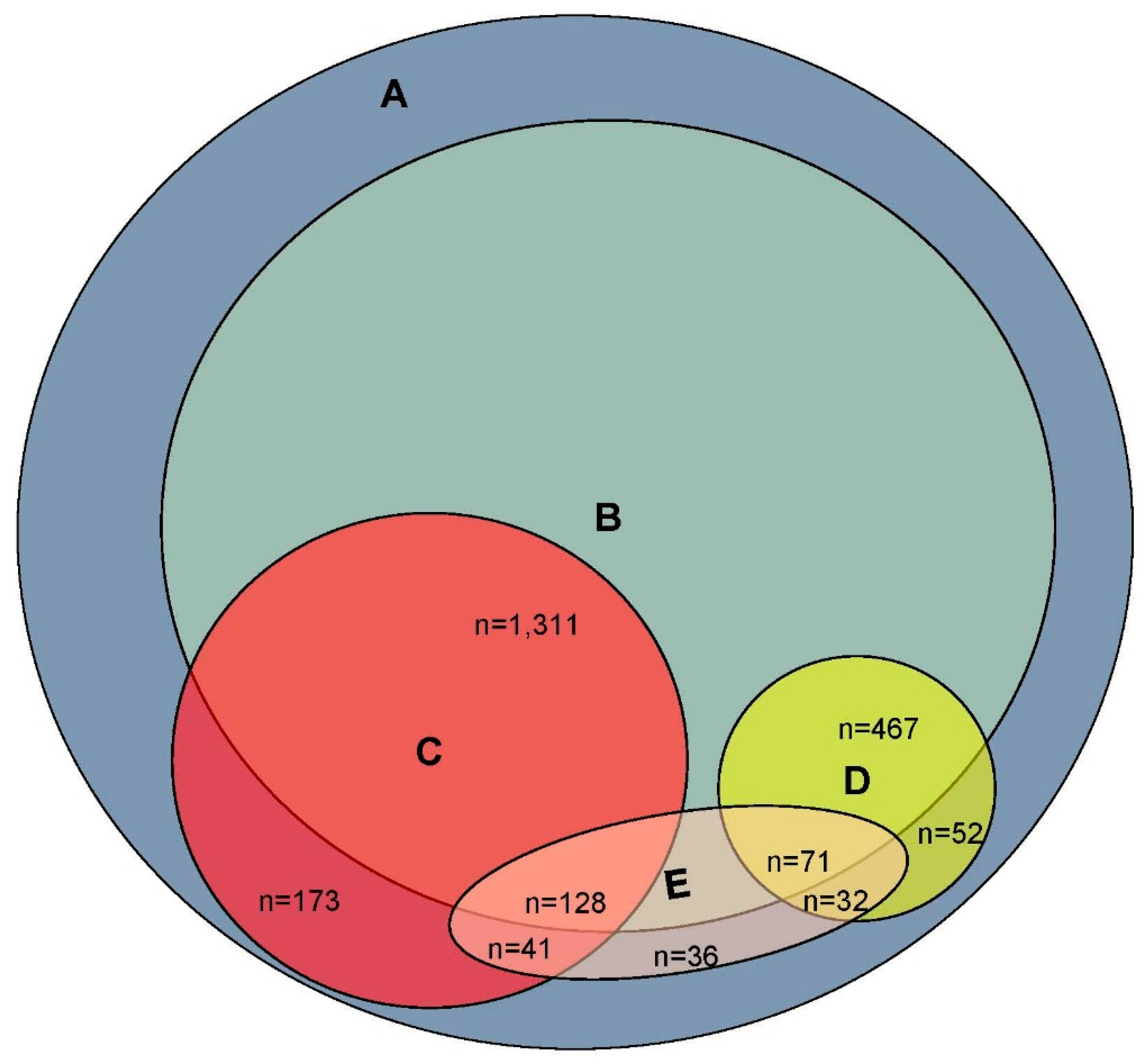
3. Results
4. Discussion
Author Contributions
Conflicts of Interest
References
- Fargo, K.N.; Carrillo, M.C.; Weiner, M.W.; Potter, W.Z.; Khachaturian, Z. The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimers Dement. 2016, 12, 1113–1115. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.W.; Goldstein, F.C.; Kilgo, P.; Brumfield, J.R.; Ravichandran, T.; Danielson, M.L.; Laplaca, M. Use of a novel technology for presenting screening measures to detect mild cognitive impairment in elderly patients. Int. J. Clin. Pract. 2010, 64, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Lovato, L.C.; Hill, K.; Hertert, S.; Hunninghake, D.B.; Probstfield, J.L. Recruitment for controlled clinical trials: Literature summary and annotated bibliography. Control. Clin. Trials 1997, 18, 328–352. [Google Scholar] [CrossRef]
- Cherbuin, N.; Anstey, K.J.; Lipnicki, D.M. Screening for dementia: A review of self- and informant-assessment instruments. Int. Psychogeriatr. 2008, 20, 431–458. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Frank, L.; Bayley, P.J.; Boustani, M.; Dean, M.; Lin, P.J.; McCarten, J.R.; Morris, J.C.; Salmon, D.P.; Schmitt, F.A.; et al. Improving dementia care: The role of screening and detection of cognitive impairment. Alzheimers Dement. 2013, 9, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.E.; Marsiske, M.; McCoy, K.J.M. The use of the Modified Telephone Interview for Cognitive Status (TICS-M) in the detection of amnestic mild cognitive impairment. J. Geriatr. Psychiatry Neurol. 2009, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Lonie, J.A.; Tierney, K.M.; Ebmeier, K.P. Screening for mild cognitive impairment: A systematic review. Int. J. Geriatr. Psychiatry 2009, 24, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Manly, J.J.; Schupf, N.; Stern, Y.; Brickman, A.M.; Tang, M.X.; Mayeux, R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011, 68, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Reckess, G.Z.; Brandt, J.; Luis, C.A.; Zandi, P.; Martin, B.; Breitner, J.C. Screening by telephone in the Alzheimer’s Disease Anti-inflammatory Prevention Trial. J. Alzheimers Dis. 2013, 36, 433–443. [Google Scholar] [PubMed]
- Smith, M.M.; Tremont, G.; Ott, B.R. A review of telephone-administered screening tests for dementia diagnosis. Am. J. Alzheimers Dis. 2009, 24, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Spencer, M.; Folstein, M. The Telephone Interview for Cognitive Status. Neuropsychiatry Neuropsychol Behav. Neurol. 1988, 1, 111–117. [Google Scholar]
- Gatz, M.; Reynolds, C.A.; John, R.; Johansson, B.; Mortimer, J.A.; Pedersen, N.L. Telephone screening to identify potential dementia cases in a population-based sample of older adults. Int. Psychogeriatr. 2002, 14, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Cherbuin, N.; Anstey, K.J. DIY dementia screening and online assessment tools. Int. Psychogeriatr. 2008, 20, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Trustram Eve, C.; de Jager, C.A. Piloting and validation of a novel self-administered online cognitive screening tool in normal older persons: the Cognitive Function Test. Int. J. Geriatr. Psychiatry 2014, 29, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Rogerson, M. Preliminary findings from an internet-based dementia risk assessment. Alzheimers Dement. 2011, 7, e94–e100. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Sullivan, C.; Burrell, L.E., 2nd; Rogerson, M.; Anderson, A. Internet-based screening for dementia risk. PLoS ONE. 2013, 8, e57476. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Blehar, J.; Anderson, A.; Gross, A.L. Further validation of the Internet-based Dementia Risk Assessment. J. Alzheimers Dis. 2014, 41, 937–945. [Google Scholar] [PubMed]
- Internet World Stats. Available online: http://www.internetworldstats.com/ (accessed on 5 April 2017).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morra, L.F.; Brandt, J. Brief Report: Using the Internet to Identify Persons with Cognitive Impairment for Participation in Clinical Trials. Brain Sci. 2017, 7, 36. https://doi.org/10.3390/brainsci7040036
Morra LF, Brandt J. Brief Report: Using the Internet to Identify Persons with Cognitive Impairment for Participation in Clinical Trials. Brain Sciences. 2017; 7(4):36. https://doi.org/10.3390/brainsci7040036
Chicago/Turabian StyleMorra, Lindsay F., and Jason Brandt. 2017. "Brief Report: Using the Internet to Identify Persons with Cognitive Impairment for Participation in Clinical Trials" Brain Sciences 7, no. 4: 36. https://doi.org/10.3390/brainsci7040036




