Coagulopathy in the Setting of Mild Traumatic Brain Injury: Truths and Consequences
Abstract
:1. Introduction
2. Definition of Mild Traumatic Brain Injury
3. Coagulopathy in Traumatic Brain Injury
4. Proposed Mechanisms
5. Clinical Tests for Coagulopathy
6. Incidence
7. Consequence of Coagulopathy in Mild TBI
8. Future Directions
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cassidy, J.D.; Carroll, L.J.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Kraus, J.; Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, (Suppl. 43), 28–60. [Google Scholar] [CrossRef]
- Eme, R. Neurobehavioral Outcomes of Mild Traumatic Brain Injury: A Mini Review. Brain Sci. 2017, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild Traumatic Brain Injury in U.S. Soldiers Returning from Iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Carroll, L.J.; Cassidy, J.D.; Peloso, P.M.; Borg, J.; von Holst, H.; Holm, L.; Paniak, C.; Pépin, M.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Prognosis for mild traumatic brain injury: Results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, (Suppl. 43), 84–105. [Google Scholar] [CrossRef]
- Carroll, L.J.; Cassidy, J.D.; Holm, L.; Kraus, J.; Coronado, V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Methodological issues and research recommendations for mild traumatic brain injury: The WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 2004, (Suppl. 43), 113–125. [Google Scholar] [CrossRef]
- Rapp, P.E.; Curley, K.C. Is a diagnosis of “mild traumatic brain injury” a category mistake? J. Trauma Acute Care Surg. 2012, 73, S13–S23. [Google Scholar] [CrossRef] [PubMed]
- Harmon, K.G.; Drezner, J.A.; Gammons, M.; Guskiewicz, K.M.; Halstead, M.; Herring, S.A.; Kutcher, J.S.; Pana, A.; Putukian, M.; Roberts, W.O. American Medical Society for Sports Medicine position statement: Concussion in sport. Br. J. Sports Med. 2013, 47, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Management of Concussion/mTBI Working Group. VA/DoD Clinical Practice Guideline for Management of Concussion/Mild Traumatic Brain Injury. J. Rehabil. Res. Dev. 2009, 46, CP1–CP68. [Google Scholar]
- Hunt, H.; Stanworth, S.; Curry, N.; Woolley, T.; Cooper, C.; Ukoumunne, O.; Zhelev, Z.; Hyde, C. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst. Rev. 2015, CD010438. [Google Scholar] [CrossRef]
- Leo, P.; McCrea, M. Epidemiology. In Translational Research in Traumatic Brain Injury; Laskowitz, D., Grant, G., Eds.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016; Chapter 1. [Google Scholar]
- Helmick, K.M.; Spells, C.A.; Malik, S.Z.; Davies, C.A.; Marion, D.W.; Hinds, S.R. Traumatic brain injury in the US military: Epidemiology and key clinical and research programs. Brain Imaging Behav. 2015, 9, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Department of Defense. DoD Worldwide Numbers for TBI. Available online: http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi (accessed on 25 June 2017).
- Chang, R.; Cardenas, J.C.; Wade, C.E.; Holcomb, J.B. Advances in the understanding of trauma-induced coagulopathy. Blood 2016, 128, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Owen, B.A.; Ballinger, B.A.; Sarr, M.G.; Schiller, H.J.; Zietlow, S.P.; Jenkins, D.H.; Ereth, M.H.; Owen, W.G.; Heit, J.A. Quantification of hypercoagulable state after blunt trauma: Microparticle and thrombin generation are increased relative to injury severity, while standard markers are not. Surgery 2012, 151, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.; Shreve, J.; Thomas, S.; Moore, E.; Moore, H.; Hake, D.; Pohlman, T.; Davis, P.; Ploplis, V.; Piscoya, A.; et al. Fibrinolysis in Trauma: “Myth”, “Reality,” or “Something in Between”. Semin. Thromb. Hemost. 2017, 43, 200–212. [Google Scholar] [CrossRef] [PubMed]
- CRASH-2 Trial Collaborators; Shakur, H.; Roberts, I.; Bautista, R.; Caballero, J.; Coats, T.; Dewan, Y.; El-Sayed, H.; Gogichaishvili, T.; Gupta, S.; Herrera, J.; et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar] [CrossRef]
- Holcomb, J.B.; del Junco, D.J.; Fox, E.E.; Wade, C.E.; Cohen, M.J.; Schreiber, M.A.; Alarcon, L.H.; Bai, Y.; Brasel, K.J.; Bulger, E.M.; et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: Comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013, 148, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Armand, R.; Hess, J.R. Treating coagulopathy in trauma patients. Transfus. Med. Rev. 2003, 17, 223–231. [Google Scholar] [CrossRef]
- Mackman, N. The role of tissue factor and factor VIIa in hemostasis. Anesth. Analg. 2009, 108, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Cohen, M.J.; Ganter, M.T.; Schultz, M.J.; Levi, M.; Mackersie, R.C.; Pittet, J.F. Acute coagulopathy of trauma: Hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J. Trauma 2008, 64, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Cohen, M.J.; Davenport, R.A. Acute coagulopathy of trauma: Mechanism, identification and effect. Curr. Opin. Crit. Care 2007, 13, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, D.B. A clinical review of bleeding dilemmas in trauma. Semin. Hematol. 2004, 41, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Martini, W.Z.; Dubick, M.A.; Salinas, J.; Butenas, S.; Kheirabadi, B.S.; Pusateri, A.E.; Vos, J.A.; Guymon, C.H.; Wolf, S.E.; et al. Thromboelastography as a better indicator of hypercoagulable state after injury than prothrombin time or activated partial thromboplastin time. J. Trauma 2009, 67, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Penick, G.D.; McLendon, W.W. Disorders of the hemostatic mechanism. Int. Rec. Med. 1960, 173, 491–496. [Google Scholar] [PubMed]
- Zhao, Z.; Zhou, Y.; Tian, Y.; Li, M.; Dong, J.F.; Zhang, J. Cellular microparticles and pathophysiology of traumatic brain injury. Protein Cell 2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, R.; Liu, L.; Watkins, T.; Zhang, F.; Dong, J. Traumatic Brain Injury-Associated Coagulopathy. J. Neurotrauma 2012, 29, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Talving, P.; Benfield, R.; Hadjizacharia, P.; Inaba, K.; Chan, L.S.; Demetriades, D. Coagulopathy in severe traumatic brain injury: A prospective study. J. Trauma 2009, 66, 55–61. [Google Scholar] [CrossRef] [PubMed]
- McCully, S.P.; Schreiber, M.A. Traumatic brain injury and its effect on coagulopathy. Semin. Thromb. Hemost. 2013, 39, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.J.; Brohi, K.; Ganter, M.T.; Manley, G.T.; Mackersie, R.C.; Pittet, J.F. Early coagulopathy after traumatic brain injury: The role of hypoperfusion and the protein C pathway. J. Trauma 2007, 63, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.C.; Smith, D.H. Coagulopathy in traumatic brain injury. Neurocrit. Care 2004, 1, 479–488. [Google Scholar] [CrossRef]
- Hulka, F.; Mullins, R.J.; Frank, E.H. Blunt brain injury activates the coagulation process. Arch. Surg. 1996, 131, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Harhangi, B.S.; Kompanje, E.J.; Leebeek, F.W.; Maas, A.I. Coagulation disorders after traumatic brain injury. Acta. Neurochir. (Wien.) 2008, 150, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Cap, A.P.; Spinella, P.C. Severity of head injury is associated with increased risk of coagulopathy in combat casualties. J. Trauma 2011, 71, S78–S81. [Google Scholar] [CrossRef] [PubMed]
- Castellino, F.J.; Chapman, M.P.; Donahue, D.L.; Thomas, S.; Moore, E.E.; Wohlauer, M.V.; Fritz, B.; Yount, R.; Ploplis, V.; Davis, P.; et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J. Trauma Acute Care Surg. 2014, 76, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Bazzazi, A.M.; Porhomayon, J.; Nader, N.D. Correlation between coagulopathy and outcome in severe head trauma in neurointensive care and trauma units. J. Crit. Care 2011, 26, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, J.; Wu, X.; Xi, C.; Gai, Y.; Liu, H.; Yuan, Q.; Wang, E.; Gao, L.; Hu, J.; Zhou, L. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury—analysis of 242 cases. Br. J. Neurosurg. 2011, 25, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Wafaisade, A.; Lefering, R.; Tjardes, T.; Wutzler, S.; Simanski, C.; Paffrath, T.; Fischer, P.; Bouillon, B.; Maegele, M.; Trauma Registry of DGU. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit. Care 2010, 12, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, N.; Abu Fanne, R.; Abramovitch, R.; Yarovoi, S.; Higazi, M.; Abdeen, S.; Basheer, M.; Maraga, E.; Cines, D.B.; Higazi, A.A. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood 2015, 125, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.P.; Moore, E.E.; Moore, H.B.; Gonzalez, E.; Gamboni, F.; Chandler, J.G.; Mitra, S.; Ghasabyan, A.; Chin, T.L.; Sauaia, A.; et al. Overwhelming tPA release, not PAI-1 degradation, is responsible for hyperfibrinolysis in severely injured trauma patients. J. Trauma Acute Care Surg. 2016, 80, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Burnier, L.; Fontana, P.; Kwak, B.R.; Angelillo-Scherrer, A. Cell-derived microparticles in haemostasis and vascular medicine. Thromb. Haemost. 2009, 101, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Pravettoni, E.; Colombo, A.; Schenk, U.; Möller, T.; Matteoli, M.; Verderio, C. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J. Immunol. 2005, 174, 7268–7277. [Google Scholar] [CrossRef] [PubMed]
- Falati, S.; Liu, Q.; Gross, P.; Merrill-Skoloff, G.; Chou, J.; Vandendries, E.; Celi, A.; Croce, K.; Furie, B.C.; Furie, B. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J. Exp. Med. 2003, 197, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Curry, N.; Raja, A.; Beavis, J.; Stanworth, S.; Harrison, P. Levels of procoagulant microvesicles are elevated after traumatic injury and platelet microvesicles are negatively correlated with mortality. J. Extracell. Vesicles 2014, 3, 25625. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Xue, A.; Spears, G.M.; Halling, T.M.; Ferrara, M.J.; Kuntz, M.M.; Dhillon, S.K.; Jenkins, D.H.; Harmsen, W.S.; Ballman, K.V.; et al. Thrombin generation and procoagulant microparticle profiles after acute trauma: A prospective cohort study. J. Trauma Acute Care Surg. 2015, 79, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Nekludov, M.; Mobarrez, F.; Gryth, D.; Bellander, B.M.; Wallen, H. Formation of microparticles in the injured brain of patients with severe isolated traumatic brain injury. J. Neurotrauma. 2014, 31, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Morel, N.; Morel, O.; Petit, L.; Hugel, B.; Cochard, J.F.; Freyssinet, J.M.; Sztark, F.; Dabadie, P. Generation of procoagulant microparticles in cerebrospinal fluid and peripheral blood after traumatic brain injury. J. Trauma 2008, 64, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Salsbery, B.; Wang, M.; Yuan, H.; Yang, J.; Zhao, Z.; Wu, X.; Zhang, Y.; Konkle, B.A.; Thiagarajan, P.; et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 2015, 125, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Dzik, W.H. Predicting hemorrhage using preoperative coagulation screening assays. Curr. Hematol. Rep. 2004, 3, 324–330. [Google Scholar] [PubMed]
- Da Luz, L.T.; Nascimento, B.; Shankarakutty, A.K.; Rizoli, S.; Adhikari, N.K. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: Descriptive systematic review. Crit. Care. 2014, 18, 518. [Google Scholar] [CrossRef] [PubMed]
- Donahue, S.M.; Otto, C.M. Thromboelastography: A tool for measuring hypercoagulability, hypocoagulability, and fibrinolysis. J. Vet. Emerg. Crit. Care 2005, 15, 9–16. [Google Scholar] [CrossRef]
- Bochsen, L.; Wiinberg, B.; Kjelgaard-Hansen, M.; Steinbrüchel, D.A.; Johansson, P.I. Evaluation of the TEG platelet mapping assay in blood donors. Thromb. J. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Cortiana, M.; Zagara, G.; Fava, S.; Seveso, M. Coagulation abnormalities in patients with head injury. J. Neurosurg. Sci. 1986, 30, 133–138. [Google Scholar] [PubMed]
- Gómez, P.A.; Lobato, R.D.; Ortega, J.M.; De La Cruz, J. Mild head injury: Differences in prognosis among patients with a Glasgow Coma Scale score of 13 to 15 and analysis of factors associated with abnormal CT findings. Br. J. Neurosurg. 1996, 10, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Herbert, J.P.; Guillotte, A.R.; Division of Neurological Surgery, University of Missouri School of Medicine, Columbia, Missouri, USA. Unpublished work. 2017.
- Chen, L.; Bracey, A.W.; Radovancevic, R.; Cooper, J.R.; Collard, C.D.; Vaughn, W.K.; Nussmeier, N.A. Clopidogrel and bleeding in patients undergoing elective coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2004, 128, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.N.; Johnson, C.; Lewis, J.; Clevenger, J.W.; Barnes, S.L.; Hammer, R.D.; Ahmad, S. Platelet adenosine diphosphate inhibition in trauma patients by thromboelastography correlates with paradoxical increase in platelet dense granule content by flow cytometry. Surgery 2016, 160, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Hammer, R.D.; Department of Pathology and Anatomical Sciences, University of Missouri School of Medicine, Columbia, Missouri, USA. Unpublished work. 2017.
- Reilly, P.L.; Graham, D.I.; Adams, J.H.; Jennett, B. Patients with head injury who talk and die. Lancet 1975, 2, 375–377. [Google Scholar] [CrossRef]
- Kurland, D.; Hong, C.; Aarabi, B.; Gerzanich, V.; Simard, J.M. Hemorrhagic progression of a contusion after traumatic brain injury: A review. J. Neurotrauma 2012, 29, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.P.; Kene, M.; Vilke, G.M.; Sise, M.J.; Kennedy, F.; Eastman, A.B.; Velky, T.; Hoyt, D.B. Head-injured patients who “talk and die”: The San Diego perspective. J. Trauma 2007, 62, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, O.J.; Prestigiacomo, C.J.; Gala, N.; Slasky, S.; Sifri, Z.C. Delayed neurological deterioration after mild head injury: Cause, temporal course, and outcomes. Neurosurgery 2013, 73, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, R.; Litofsky, N.S. Which traumatic brain injury patients should be treated with anticoagulants and when? Expert. Rev. Neurother. 2014, 14, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Valadka, A.B.; Robertson, C.S. Surgery of cerebral trauma and associated critical care. Neurosurgery 2007, 61, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Hadjizacharia, P.; Aziz, H.; Kulvatunyou, N.; Tang, A.; Pandit, V.; Wynne, J.; O’Keeffe, T.; Friese, R.S.; Rhee, P. Prothrombin complex concentrate: An effective therapy in reversing the coagulopathy of traumatic brain injury. J. Trauma Acute. Care. Surg. 2013, 74, 248–253. [Google Scholar] [CrossRef] [PubMed]
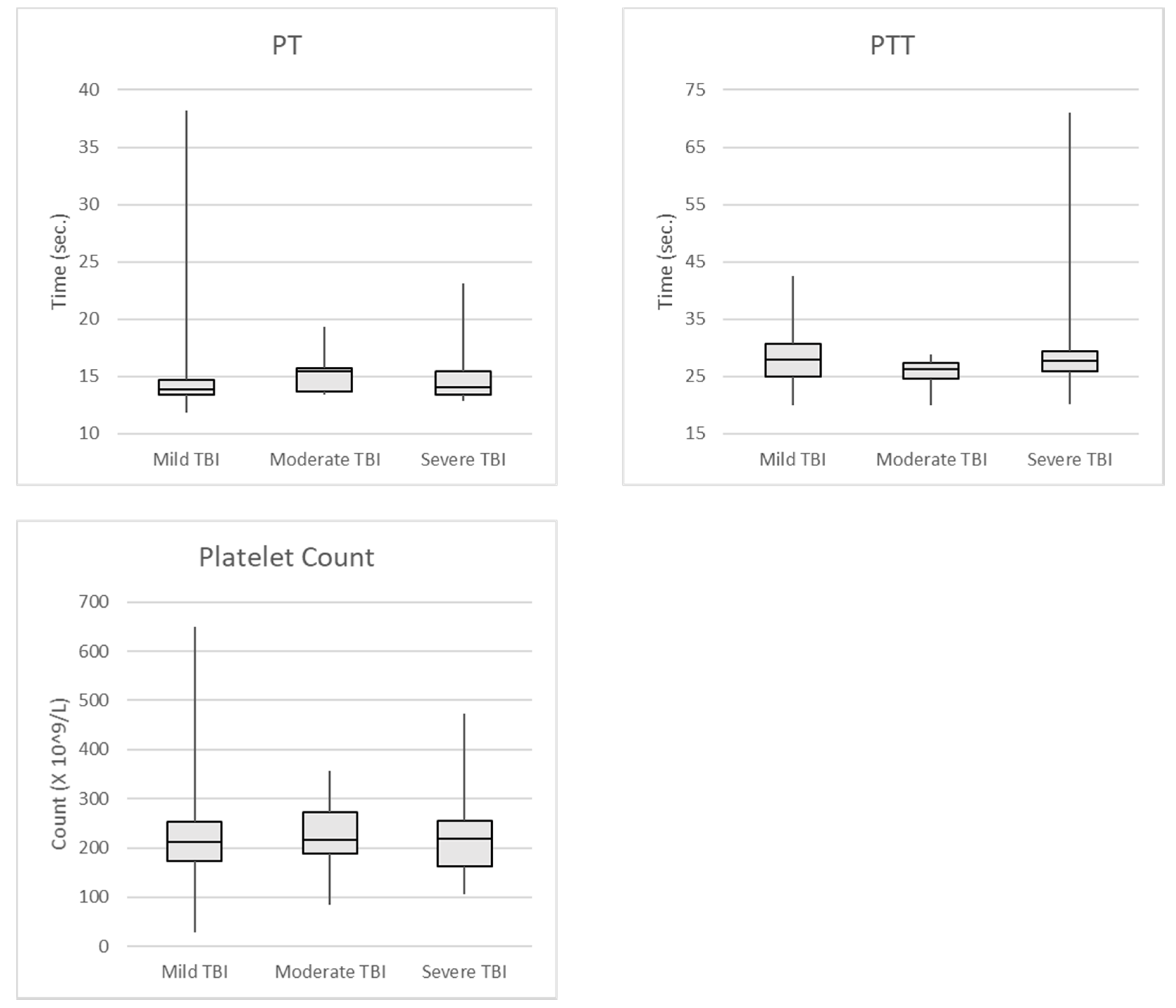
| All TBI Patients N (%) | Isolated TBI N (%) | Non-Isolated TBI N (%) | |
|---|---|---|---|
| TBI patients included in analysis | 97 | 43 | 54 |
| TBI patients with TEG/PM assays | 80 | 36 | 44 |
| Patients with mild TBI | 54 (67.5%) | 29 (80.6%) | 25 (56.8%) |
| mTBI with adenosine diphosphate (ADP) inhibition ≥60% | 17 (31.5%) | 7 (24.1%) | 10 (40.0%) |
| N | Mean | Standard Deviation | 95% CI | |
|---|---|---|---|---|
| Control [57] | 8 | 7.4 | 8.3 | ±6.9 |
| Isolated mTBI 1 | 29 | 42.3 | 27.9 | ±10.6 |
| N | Prolonged PT | Prolonged PTT | Thrombocytopenic | Coagulopathic by at Least One Parameter | |
|---|---|---|---|---|---|
| Mild TBI | 102 | 16 (15.7%) | 4 (3.9%) | 20 (19.6%) | 33 (32.4%) |
| Moderate TBI | 11 | 6 (54.5%) | 0 | 2 (18.2%) | 6 (54.5%) |
| Severe TBI | 29 | 11 (37.9%) | 2 (6.9%) | 0 | 14 (48.3%) |
| N | Prolonged PT | Prolonged PTT | Thrombocytopenic | |
|---|---|---|---|---|
| Control | 40 | 2 (5%) | 1 (2.5%) | - |
| Mild TBI | 32 | 0 | 0 | 2 (6.3%) |
| Moderate TBI | 3 | 1 (33.3%) | 0 | 1 (33.3%) |
| Severe TBI | 4 | 0 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbert, J.P.; Guillotte, A.R.; Hammer, R.D.; Litofsky, N.S. Coagulopathy in the Setting of Mild Traumatic Brain Injury: Truths and Consequences. Brain Sci. 2017, 7, 92. https://doi.org/10.3390/brainsci7070092
Herbert JP, Guillotte AR, Hammer RD, Litofsky NS. Coagulopathy in the Setting of Mild Traumatic Brain Injury: Truths and Consequences. Brain Sciences. 2017; 7(7):92. https://doi.org/10.3390/brainsci7070092
Chicago/Turabian StyleHerbert, Joseph P., Andrew R. Guillotte, Richard D. Hammer, and N. Scott Litofsky. 2017. "Coagulopathy in the Setting of Mild Traumatic Brain Injury: Truths and Consequences" Brain Sciences 7, no. 7: 92. https://doi.org/10.3390/brainsci7070092






