Acute Confusional Migraine: Distinct Clinical Entity or Spectrum of Migraine Biology?
Abstract
:1. Introduction
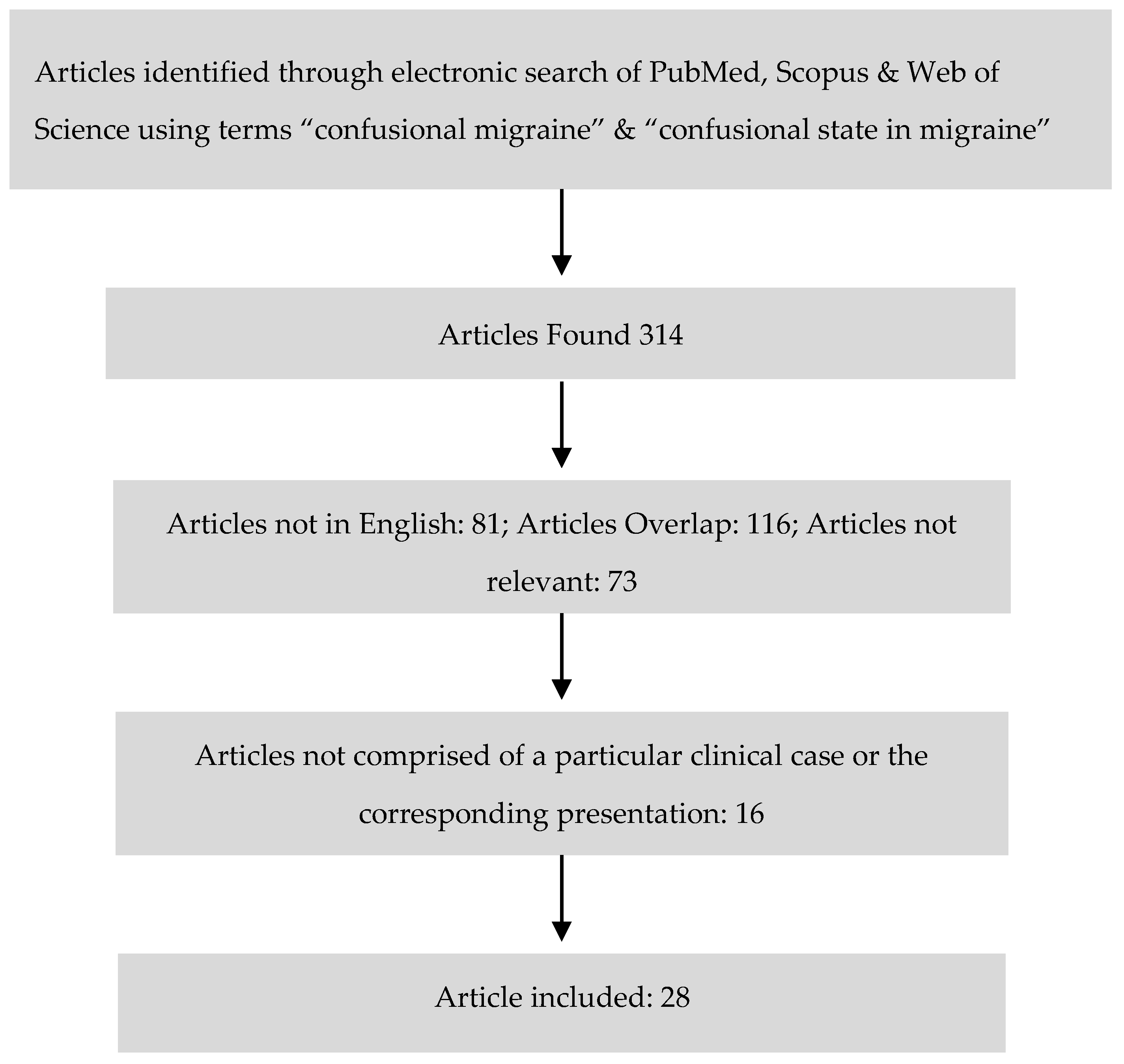
2. Materials and Methods
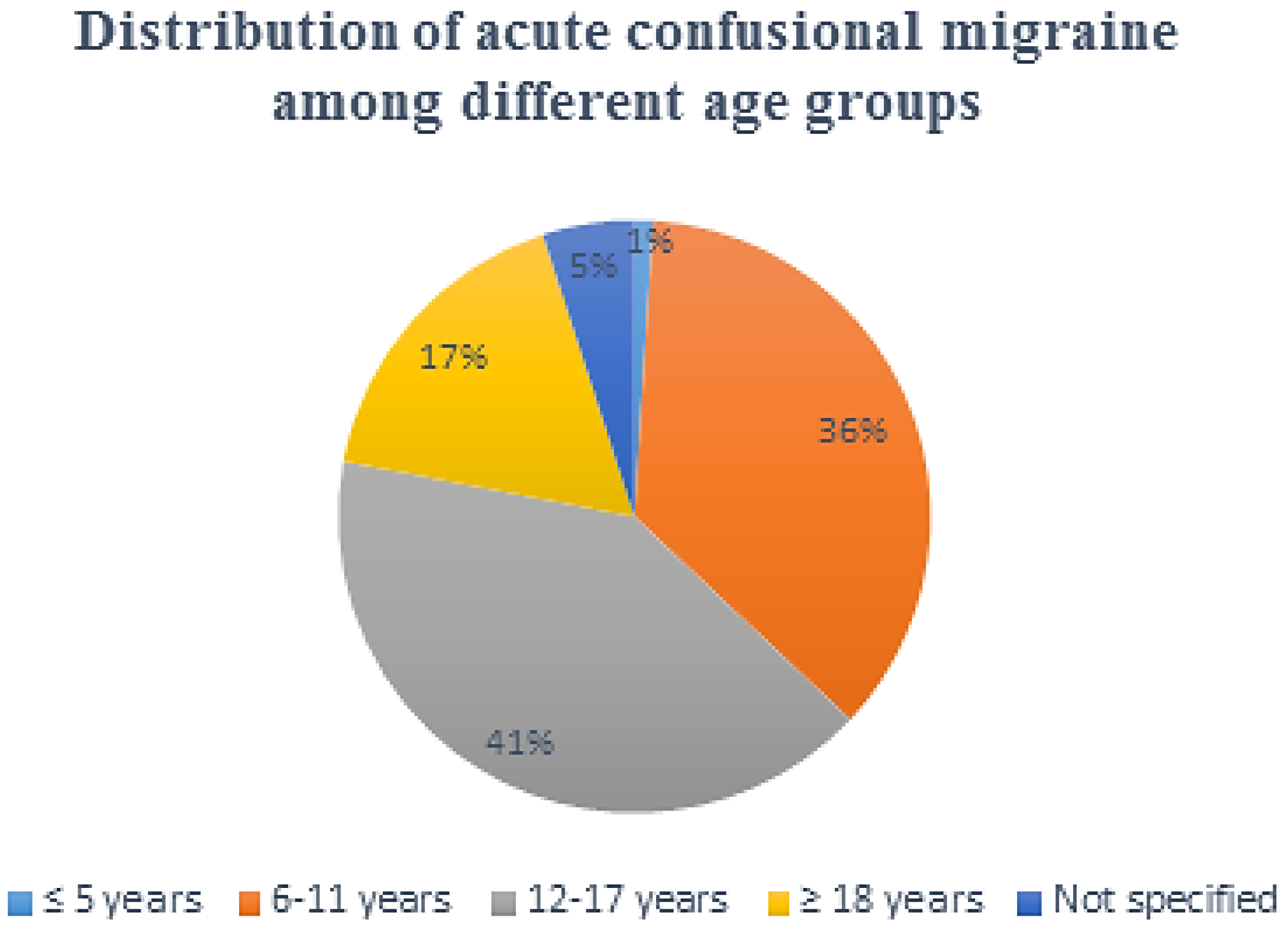
3. Results
Clinical Features
4. Discussion
4.1. Auras
4.2. Premonitory Symptoms
4.3. Headache Phase
4.4. Postdrome Phase
5. Conclusions
Conflicts of Interest
Abbreviations
| ACM | Acute confusional migraine |
| ICHD-3β | International Headache Society’s International Classification of Headache Disoders-3 beta version |
| FHM | Familial hemiplegic migraine |
| EA2 | Episodic ataxia type 2 |
| CADASIL | Cerebral Autosomal-Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy |
| MRA | Magnetic resonance imaging |
| FIRDA | Frontal intermittent rhythmic delta activity |
| CSD | Cortical Spreading Depression |
| EEG | Electroencephalography |
References
- Gascon, G.; Barlow, C. Juvenile migraine, presenting as an acute confusional state. Pediatrics 1970, 45, 628–635. [Google Scholar] [PubMed]
- Ehyai, A.; Fenichel, G.M. The natural history of acute confusional migraine. Arch. Neurol. 1978, 35, 368–369. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.S. Acute confusional state in children with migraine. Pediatrics 1977, 60, 111–114. [Google Scholar]
- Parrino, L.; Pietrini, V.; Spaggiari, M.C.; Terzano, M.G. Acute confusional migraine attacks resolved by sleep: Lack of significant abnormalities in post-ictal polysomnograms. Cephalalgia 1986, 6, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Sacquegna, T.; Cortelli, P.; Baldrati, A.; de Carolis, P.; Tinuper, P.; Lugaresi, E. Impairment of memory and consciousness in migraine: Clinical and EEG study. Funct. Neurol. 1986, 1, 431–436. [Google Scholar] [PubMed]
- Pietrini, V.; D’andrea, G.; Cananzi, A.; Ferro-Milone, F.; Terzano, M.G.; Parrino, L. Acute confusional migraine: Clinical and electroencephalographic aspects. Cephalalgia 1987, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Haan, J.; Ferrari, M.D.; Brouwer, O.F. Acute confusional migraine. Case report and review of literature. Clin. Neurol. Neurosurg. 1988, 90, 275–278. [Google Scholar] [CrossRef]
- Piatella, L.; Tavoni, M.A.; Cardinali, C. Acute Confusional State as a possible manifestation of migraine: A case study. In Juvenile Headache: Etiopathogenesis, Clinical Diagnosis, and Therapy. In Proceedings of the International Juvenile Headache Congress, Rome, Italy, 6–9 March 1991; Excerpta Medica: Amsterdam, The Netherlands; New York, NY, USA; Sole Distributors for the USA and Canada: New York, NY, USA; Elsevier Science Pub. Co.: New York, NY, USA, 1991; pp. 539–542. [Google Scholar]
- D’Cruz, O.F.; Walsh, D.J. Acute confusional migraine: Case series and review of literature. Wis. Med. J. 1992, 91, 130–131. [Google Scholar] [PubMed]
- Sheth, R.D.; Riggs, J.E.; Bodensteiner, J.B. Acute confusional migraine: Variant of transient global amnesia. Pediatr. Neurol. 1995, 12, 129–131. [Google Scholar] [CrossRef]
- Ferrera, P.C.; Reicho, P.R. Acute confusional migraine and trauma-triggered migraine. Am. J. Emerg. Med. 1996, 14, 276–278. [Google Scholar] [CrossRef]
- Shaabat, A. Confusional migraine in childhood. Pediatr. Neurol. 1996, 15, 23–25. [Google Scholar] [CrossRef]
- Nezu, A.; Kimura, S.; Ohtsuki, N.; Tanaka, M.; Takebayashi, S. Acute confusional migraine and migrainous infarction in childhood. Brain Dev. 1997, 19, 148–151. [Google Scholar] [CrossRef]
- Neinstein, L.; Milgrom, E. Trauma-triggered migraine and acute confusional migraine. J. Adolesc. Health 2000, 27, 119–224. [Google Scholar] [CrossRef]
- Soriani, S.; Cavaliere, B.; Faggioli, R.; Scarpa, P.; Borgna-Pignatti, C. Confusional migraine precipitated by mild head trauma. Arch. Pediatr. Adolesc. Med. 2000, 154, 90–91. [Google Scholar] [PubMed]
- Al-Twaijri, W.A.; Shevell, M.I. Pediatric migraine equivalents: Occurrence and clinical features in practice. Pediatr. Neurol. 2002, 26, 365–368. [Google Scholar] [CrossRef]
- Bechtel, K. Acute mental status change due to acute confusional migraine. Pediatr. Emerg. Care 2004, 20, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Gascon, G.; Coskun, C.; Brown, W. Acute confusional migraine: Case series and brief review. Int. J. Child Neuropsychiatry 2005, 2, 189–194. [Google Scholar]
- Fujita, M.; Fujiwara, J.; Maki, T.; Shigeta, M.; Shibasaki, K.; Takahashi, N.; Takahashi, M. The efficacy of sodium valproate and a MRA finding in confusional migraine. Brain Dev. 2007, 29, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.; Deperalta, E.; Pastores, G.; Kolodny, E.H. Acute confusional migraine may be a presenting feature of Cadasil. Headache 2009, 49, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Khatri, R.; Hershey, A.D.; Wong, B. Prochlorperazine-Treatment for acute confusional migraine. Headache 2009, 49, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Avraham, S.B.; Har-Gil, M.; Watemberg, N. Acute confusional migraine in an adolescent: Response to intravenous valproate. Pediatrics 2010, 125, e956–e959. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, A.R.; Riederer, F.; Mathys, J.; Biethahn, S.; Gossrau, G.; Waldvogel, D.; Sándor, P.S. Confusional migraine is an adult as well as a childhood disease. Cephalalgia 2011, 31, 206–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rota, E.; Morelli, N.; Immovilli, P.; De Mitri, P.; Magnifico, F.; Terlizzi, E.; Mazza, L.; Sala, B.; Biasucci, G.; Guidetti, D. “Possessed”: Acute confusional migraine in an adolescent, prevented by topiramate. Case Rep. Neurol. 2012, 4, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Pacheva, I.; Ivanov, I. Acute confusional migraine: Is it a distinct form of migraine? Int. J. Clin. Pract. 2013, 67, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Pacheva, I.H.; Ivanov, I.S. Migraine variants-Occurrence in pediatric neurology practice. Clin. Neurol. Neurosurg. 2013, 115, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Sahu, R.; Jaiswal, A.; Kumar, N. Acute confusional migraine: A variant not to be missed. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Shin, J.H.; Kim, Y.H.; Eom, T.H.; Kim, S.H.; Kim, J.M. Source localization of intermittent rhythmic delta activity in a patient with acute confusional migraine: Cross-spectral analysis using standardized low-resolution brain electromagnetic tomography (sLORETA). Neurol. Sci. 2016, 37, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hida, A.; Arai, N.; Takeuchi, S. Low-dose intravenous propofol as a possible therapeutic option for acute confusional migraine. Am. J. Emerg. Med. 2017, 35, 195.e5–195.e6. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J. The International Classification of Headache Disorders, 3rd edition. Cephalagia 2013, 33, 629–808. [Google Scholar] [CrossRef] [Green Version]
- Fisher, C.M. Late-life migraine accompaniments as a cause of unexplained transient ischemic attacks. Can. J. Neurol. Sci. 1980, 7, 9–17. [Google Scholar] [PubMed]
- Gowers, W.R. The Border-Land of Epilepsy: Faints, Vagal Attacks, Vertigo, Migraine, Sleep Symptoms and Their Treatment; Churchill: London, UK, 1907. [Google Scholar]
- Mendez, M.F.; Kremen, S.A.; Daroff, R.B.; Fenichel, G.M.; Jankovic, J.; Mazziotta, J.C.; Delirium. Bradley’s Neurology in Clinical Practice, Volume 1: Principles of Diagnosis and Management, 6th ed.; Daroff, R.B., Fenichel, G.M., Jankovic, J., Mazziotta, J.C., Eds.; Saunders: Philadelphia, PA, USA, 2012; pp. 26–36. ISBN 9996085309, 978-1437704341. [Google Scholar]
- American Psychiatric Association. Neurocognitive Disorders. In Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 978–0890425558. [Google Scholar]
- Gil-Gouveia, R.; Oliveira, A.G.; Martins, I.P. Assessment of cognitive dysfunction during migraine attacks: A systematic review. J. Neurol. 2015, 262, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Merwick, A.; Fernandez, D.; McNamara, B.; Harrington, H. Acute encephalopathy in familial hemiplegic migraine with ATP1A2 mutation. BMJ Case Rep. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Spranger, M.; Spranger, S.; Schwab, S.; Benninger, C.; Dichgans, M. Familial hemiplegic migraine with cerebellar ataxia and paroxysmal psychosis. Eur. Neurol. 1999, 41, 150–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feely, M.P.; O’Hare, J.; Veale, D.; Callaghan, N. Episodes of acute confusion or psychosis in familial hemiplegic migraine. Acta Neurol. Scand. 1982, 65, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.Y.Y.; Markus, H.S. CADASIL: Migraine, encephalopathy, stroke and their inter-relationships. PLoS ONE 2016, 11, e0157613. [Google Scholar] [CrossRef] [PubMed]
- Cleves, C.; Parikh, S.; Rothner, A.D.; Tepper, S.J. Link between confusional migraine, hemiplegic migraine and episodic ataxia type 2: Hypothesis, family genealogy, gene typing and classification. Cephalalgia 2010, 30, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Lapkin, M.L.; French, J.H.; Golden, G.S.; Rowan, A.J. The electroencephalogram in childhood basilar artery migraine. Neurology 1977, 27, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Ganji, S. Basilar artery migraine: EEG and evoked potential patterns during acute stage. Headache 1986, 26, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Frequin, S.T.; Linssen, W.H.; Pasman, J.W.; Hommes, O.R.; Merx, H.L. Recurrent prolonged coma due to basilar artery migraine. A case report. Headache 1991, 31, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Nakajima, S.; Nishioka, R.; Nakamura, H. Basilar artery migraine with transient MRI and EEG abnormalities. Rinsho Shinkeigaku 1993, 33, 61–67. [Google Scholar] [PubMed]
- Ganji, S.; Hellman, S.; Stagg, S.; Furlow, J. Episodic coma due to acute basilar artery migraine: Correlation of EEG and brainstem auditory evoked potential patterns. Clin. Electroencephalogr. 1993, 24, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Muellbacher, W.; Mamoli, B. Prolonged impaired consciousness in basilar artery migraine. Headache 1994, 34, 282–285. [Google Scholar] [CrossRef] [PubMed]
- La Spina, I.; Vignati, A.; Porazzi, D. Basilar artery migraine: Transcranial doppler EEG and SPECT from the aura phase to the end. Headache 1997, 37, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Ramelli, G.P.; Sturzenegger, M.; Donati, F.; Karbowski, K. EEG findings during basilar migraine attacks in children. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 374–378. [Google Scholar] [CrossRef]
- Pisani, F.; Fusco, C. Ictal and interictal EEG findings in children with migraine. J. Headache Pain. 2004, 5, 23–29. [Google Scholar] [CrossRef]
- Hooshmand, H. The clinical significance of frontal intermittent rhythmic delta activity (FIRDA). Clin. Electroencephalogr. 1983, 14, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Accolla, E.A.; Kaplan, P.W.; Maeder-Ingvar, M.; Jukopila, S.; Rossetti, A.O. Clinical correlates of frontal intermittent rhythmic delta activity (FIRDA). Clin. Neurophysiol. 2011, 122, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.D.; Toczek, M.T.; Mitchell, W.G. Frontal intermittent rhythmic delta activity (FIRDA): Is there a clinical significance in children and adolescents? Eur. J. Paediatr. Neurol. 2012, 16, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.C.; Lourie, H. Trauma-triggered migraine: An explanation for common neurological attacks after mild head injury. Review of the literature. J. Neurosurg. 1988, 68, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Karsan, N.; Prabhakar, P.; Goadsby, P.J. Characterising the premonitory stage of migraine in children: A clinic-based study of 100 patients in a specialist headache service. J. Headache Pain 2016, 17, 94. [Google Scholar] [CrossRef] [PubMed]
- Giffin, N.J.; Ruggiero, L.; Lipton, R.B.; Silberstein, S.D.; Tvedskov, J.F.; Olesen, J.; Altman, J.; Goadsby, P.J.; Macrae, A. Premonitory symptoms in migraine—An electronic diary study. Neurology 2003, 60, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Kelman, L. The premonitory symptoms (Prodrome): A tertiary care study of 893 migraineurs. Headache 2004, 44, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Kelman, L. The postdrome of the acute migraine attack. Cephalalgia 2006, 26, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Russo, A.; Trojano, L.; Falco, F.; Marcuccio, L.; Siciliano, M.; Conte, F.; Garramone, F.; Tessitore, A.; Tedeschi, G. Cognitive dysfunctions and psychological symptoms in migraine without aura: A cross-sectional study. J. Headache Pain 2016, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Ayata, C.; Jin, H.; Kudo, C.; Dalkara, T.; Moskowitz, M.A. Suppression of cortical spreading depression in migraine prophylaxis. Ann. Neurol. 2006, 59, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.P.; Shields, K.G.; Goadsby, P.J. GABA and valproate modulate trigeminovascular nociceptive transmission in the thalamus. Neurobiol. Dis. 2010, 37, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.P.; Goadsby, P.J. Topiramate in the treatment of migraine: A kainate (glutamate) receptor antagonist within the trigeminothalamic pathway. Cephalalgia 2011, 31, 1343–1358. [Google Scholar] [CrossRef] [PubMed]
- Schipper, S.; Riederer, F.; Sándor, P.S.; Gantenbein, A.R. Acute confusional migraine: Our knowledge to date. Expert Rev. Neurother. 2012, 12, 307–314. [Google Scholar] [CrossRef] [PubMed]
| Case Report (Reference Number) | No. of Patients (n) | Gender Ratio (M:F) | Age Range (Years) | Mean Age (Mean ± SD) | Clinical Presentation α | Headache Onset Pre/Post-Confusion | Duration of Confusion (h) | Treatment | Recurrence | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Gascon G. and Barlow C. [1] | 4 | 3:1 | 8–16 | 12.3 ± 3.5 | Disorientation (100%), Agitation (100%), Speech (100%), somatosensory (25%) and memory disturbances (50%) | Pre-confusion (4) | 4–24 h | Ergotamine and Phenobarbital | None | Two patients had multiple episodes of headache afterwards |
| Emery III et al. [3] | 4 | 3:1 | 5–14 | 10 ± 4.2 | Confusion (100%), agitation (100%), visual (50%), somatosensory (50%) speech (25%), and memory disturbances (75%) | Pre-confusion (4) | 1.5–9 h | NA | Two patients reported similar episodes in the past | Three patients had intermittent episodes of headache |
| Ehayi A. and Fenichel G. M. [2] | 5 | 3:2 | 9–14 | 11 ± 2 | Confusion and disorientation (100%), Agitation (100%), visual (80%), somatosensory (20%), speech (40%), motor (20%) and memory disturbances (80%) | Pre- (4), post-confusion (1) | 0.5–24 h | Ergotamine, methysergide | Over brief period of time all patients had recurrent ACM episodes | Migraine |
| Parrino L. et al. [4] | 2 | 2:0 | 14–15 | 14.5 ± 0.7 | Confusion and disorientation (100%), agitation (100%), Photophobia (50%), visual (100%), somatosensory (50%), speech (100%), and memory disturbances (100%) | Pre-confusion (2) | 24 h | NA | One patient reported similar episode in the past | None |
| Sacquegna T. et al. 1 [5] | 1 | 0:1 | 17 | 17 ± 0 | Confusion and disorientation (100%), visual (100%), somatosensory (100%), and memory disturbances (100%) | Pre-confusion (1) | 2 h | NA | Several episodes marked by less confusion | NA |
| Pietrini V. et al. [6] | 12 | 6:6 | 8–60 | 19.4 ± 13.4 | Confusion (100%), agitation (100%), visual (42%), somatosensory (42%), speech (25%), and motor symptoms (17%) | Pre-confusion (10) | 1–12 h | NA | NA | NA |
| Haan J. et al. [7] | 1 | 0:1 | 13 | 13 ± 0 | Confusion and disorientation (100%), agitation (100%), memory disturbance (100%) | Pre-confusion (1) | 12 h | NA | One similar episode in the past | NA |
| Piatella L. et al. [8] | 5 | 4:1 | 10–16 | 12.6 ± 2.3 | Confusion and disorientation (%100), agitation (20%), speech (80%), somatosensory disturbances (20%) | Pre-confusion (4) 2 | 15 min–24 h | NA | NA | Three patients developed migraine |
| D’Cruz O. and Walsh D. J. [9] | 3 | 0:3 | 11 | 11 ± 0 | Confusion and disorientation (100%), speech (67%), visual (67%), memory disturbances (100%) | Pre-confusion (3) | 6 h | NA | NA | NA |
| Sheth R. D. et al. [10] | 6 | 1:5 | 7.5–17 | 11.8 ± 3.5 | Confusion and disorientation (100%), agitation (100%), photophobia (50%), visual (50%), memory disturbances (100%) | NA 2 | 1–12 h | Propranolol | Two patients had recurrent ACM episodes | NA |
| Ferrera P. and Reicho P [11] | 2 | 1:1 | 6–9 | 7.5 ± 2.1 | Confusion (100%), agitation (50%), visual (50%), somatosensory (100%), speech (50%), and motor disturbances (50%) | Pre-confusion (1) 2 | NA | Sodium Valproate | Both patients had episodes of confusion in past | One patient had 2 episodes of headache |
| Shaabat A. et al. [12] | 13 | 11:2 | 6–15 | 10.8 ± 2.9 | Confusion (100%), agitation (62%) | Pre-confusion (13) | 1.5–24 h | NA | Four patients had recurrent ACM episodes | NA |
| Nezu A. et al. [13] | 2 | 1:1 | 7–12 | 9.5 ± 3.5 | Confusion (100%), Photophobia (50%), visual (50%), somatosensory (50%), speech (50%), motor (50%) and memory disturbances (100%) | Post-confusion (2) | 6–12 h | Dihydergot | NA | NA |
| Neinstein L. and Milgrom E. [14] | 1 | 1:0 | 14 | 14 ± 0 | Confusion (100%), anisocoria (100%), and ataxic gait (100%) | Pre-confusion (1) | NA | High-dose Oxygen and Sumatriptan | One similar episode | NA |
| Soriani S. et al. [15] | 11 | 8:3 | 6–14 | 9 ± 3 | Confusion (100%), agitation (45%), somnolence (55%), visual (27%), speech disturbances (9%) | Six patients had headache 3 | 1–12 h | NA | NA | Four patients developed migraine with aura & one w/o aura |
| Al-Twaijri W. and Shevell M. [16] | 5 | 2:3 | 6.5–15 | 10.9 | Confused, agitated and memory disturbances 4 | NA 2 | NA | NA | NA | NA |
| Bechtel K. et al. [17] | 2 | 1:1 | 11–14 | 12.5 ± 2.1 | Confusion (100%), speech (100%), visual (50%), somatosensory (50%) and memory disturbances (50%) | Pre- (1), post confusion (1) | Several hours | Acetaminophen | None | One patient had several episodes of headache |
| Gascon G. G. et al. [18] | 13 | 6:7 | 6–16 | 12.3 ± 3.8 | Confusion (69%), speech (46%), somatosensory (7.7%) and memory disturbances (8%) | Pre- (7), post confusion (4) 3 | NA | NA | Two patients had recurrent episodes of ACM | None |
| Fujita M. et al. [19] | 1 | 0:1 | 10 | 10 ± 0 | Confusion and disorientation (100%) visual disturbances (100%) | Pre-confusion (1) | 5–10 h | Sodium Valproate | Recurrent ACM episodes | Attacks were controlled after increasing the dose of sodium valproate |
| Sathe S. et al. [20] | 7 | 5:2 | 42–58 | 51.9 ± 7.3 | Confusion (100%), agitation (100%), visual (100%), somatosensory (57%), speech (57%), motor (14%) and memory disturbances (100%) | NA 2 | NA | NA | Recurrent ACM episodes | CADASIL |
| Khatri et al. [21] | 2 | 1:1 | 11–16 | 13.5 ± 3.5 | Confusion (100%), speech (50%) and memory disturbances (50%) | Pre-confusion (2) | 0.5–72 h | Prochlorperazine | Recurrent ACM episodes | Prochlorperazine was effective in acute management |
| Avraham S. B. et al. [22] | 1 | 1:0 | 12 | 12 ± 0 | Confusion (100%), speech (100%), visual (100%) and somatosensory symptoms (100%) | Pre- (3), during (5), post-confusion (1) | NA | Sodium Valproate | None | None |
| Gantebein A. et al. [23] | 10 | 6:4 | 16–62 | 30.5 ± 14.7 | Confusion (100%), agitation (20%), photophobia (10%), visual (40%), somatosensory (10%), motor (10%) speech (40%), and memory disturbances (60%) | Pre- (4), post-confusion (3) 2 | 1–6 h | NA | Seven patients had recurrent confusional episodes | NA |
| Rota E. et al [24] | 1 | 0:1 | 12 | 12 ± 0 | Confusion (100%), agitation (100%) | Not specified 2 | 4 h | Topiramate prophylaxis | Previous attack characterized by less agitation | No further episodes of confusion and headache after topiramate |
| Pacheva I. and Ivanov I. [25,26] | 3 | 1:2 | 12–14 | 12.7 ± 1.2 | Confusion (100%), agitation (67%), visual (33%), somatosensory (33%), motor (33%), speech (100%) and memory disturbances (67%) | Pre-confusion (3) | 8–10 h | Diazepam and phenobarbital | None | Two patients had 1–2 episodes of migraine without aura per month |
| Verma R. et al. [27] | 1 | 0:1 | 29 | 29 ± 0 | Confusion (100%), Agitation (100%), and memory disturbances (100%) | Pre-confusion (1) | NA | Sodium Valproate | None | NA |
| Kim D. et al. [28] | 1 | 0:1 | 9 | 9 ± 0 | Confusion (100%), agitation (100%), speech (100%) and memory disturbance (100%) | NA 2 | 2 h | Propranolol & Flunarizine | Similar episode in the past | Migraine |
| Sato K. et al [29] | 1 | 1:0 | 24 | 24 ± 0 | Confusion (100%), agitation (100%), visual (100%) and speech disturbance (100%) | Pre-confusion (1) | NA | Propofol | None | NA |
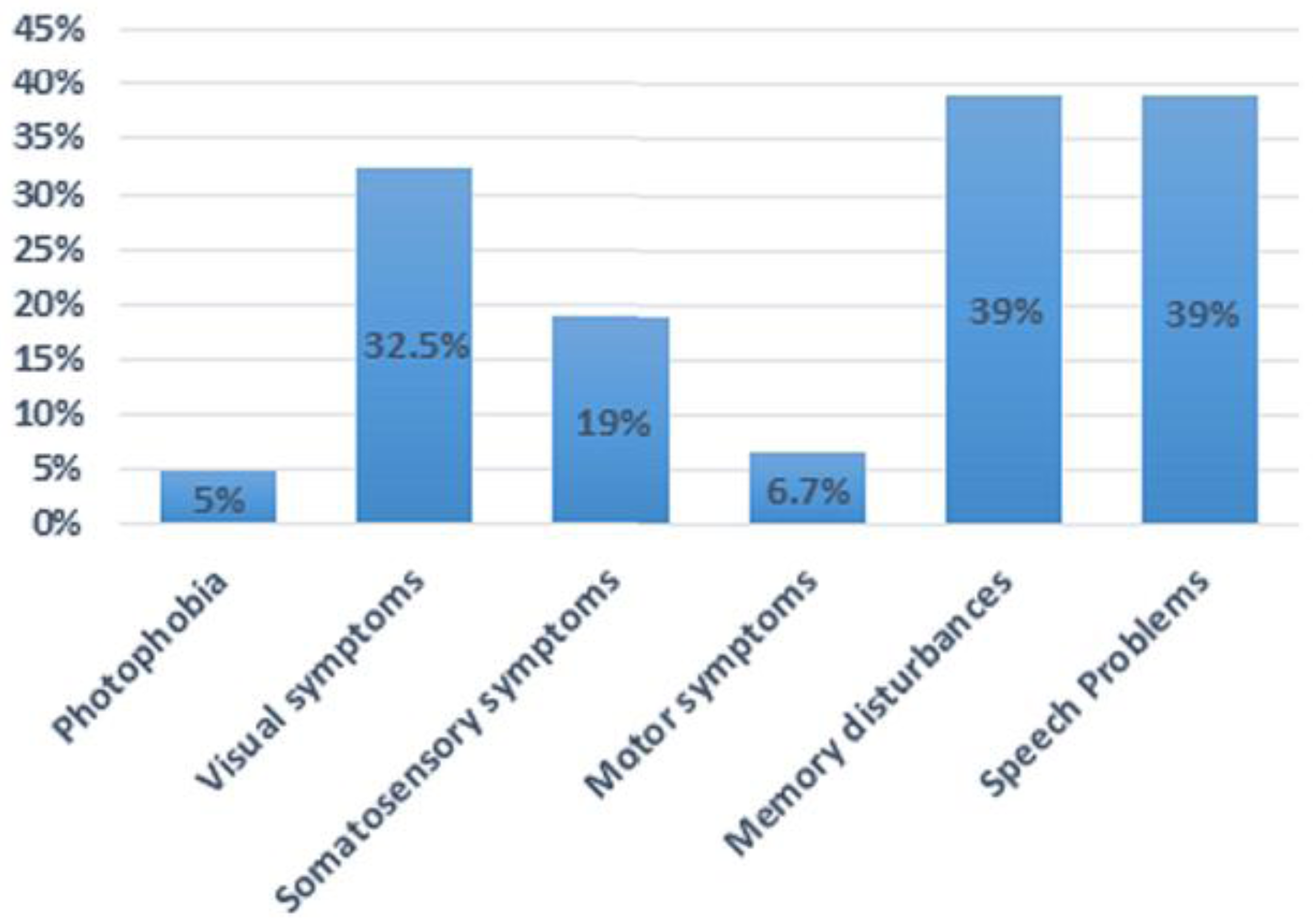
| Total (n = 120) | 68:52 | 5–62 years | Confusion and disorientation (100%), agitation (53%), photophobia (5%), visual (33%), somatosensory (19%), motor (6.7%), speech (39%), memory disturbances (39%) | Pre- (69), post-confusion (11) | 15 min–72 h | ---------- | ---------- | ----------- |
| (A) At least one attack, fulfilling criteria B to G, not attributed to other medical disorder and/or drug intoxication: |
| (B) At least one of the following: |
|
| (C) At least one of the following: |
|
| (D) Complete resolution within 24 h or after sleep with partial or complete amnesia of event |
| (E) Normal neurological or no persistent neurologic deficit examination following the attack |
| (F) At least one of the following: |
|
| (G) Not attributed to another disorder |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqi, A.M.; Padilla, J.M.; Monteith, T.S. Acute Confusional Migraine: Distinct Clinical Entity or Spectrum of Migraine Biology? Brain Sci. 2018, 8, 29. https://doi.org/10.3390/brainsci8020029
Farooqi AM, Padilla JM, Monteith TS. Acute Confusional Migraine: Distinct Clinical Entity or Spectrum of Migraine Biology? Brain Sciences. 2018; 8(2):29. https://doi.org/10.3390/brainsci8020029
Chicago/Turabian StyleFarooqi, Ashar M., Jennifer M. Padilla, and Teshamae S. Monteith. 2018. "Acute Confusional Migraine: Distinct Clinical Entity or Spectrum of Migraine Biology?" Brain Sciences 8, no. 2: 29. https://doi.org/10.3390/brainsci8020029




