Heterologous Humoral Response against H5N1, H7N3, and H9N2 Avian Influenza Viruses after Seasonal Vaccination in a European Elderly Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. Avian Influenza Viruses Analyzed
2.3. Composition of Seasonal Influenza Vaccines Administered
2.4. Haemagglutination Inhibition Assay
2.5. Phylogenetic Analysis
2.6. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Presence of Pre- and Post-Vaccine Heterotypic Abs against AIV
3.3. Heterotypic Abs Response Induced by Influenza Seasonal Vaccination
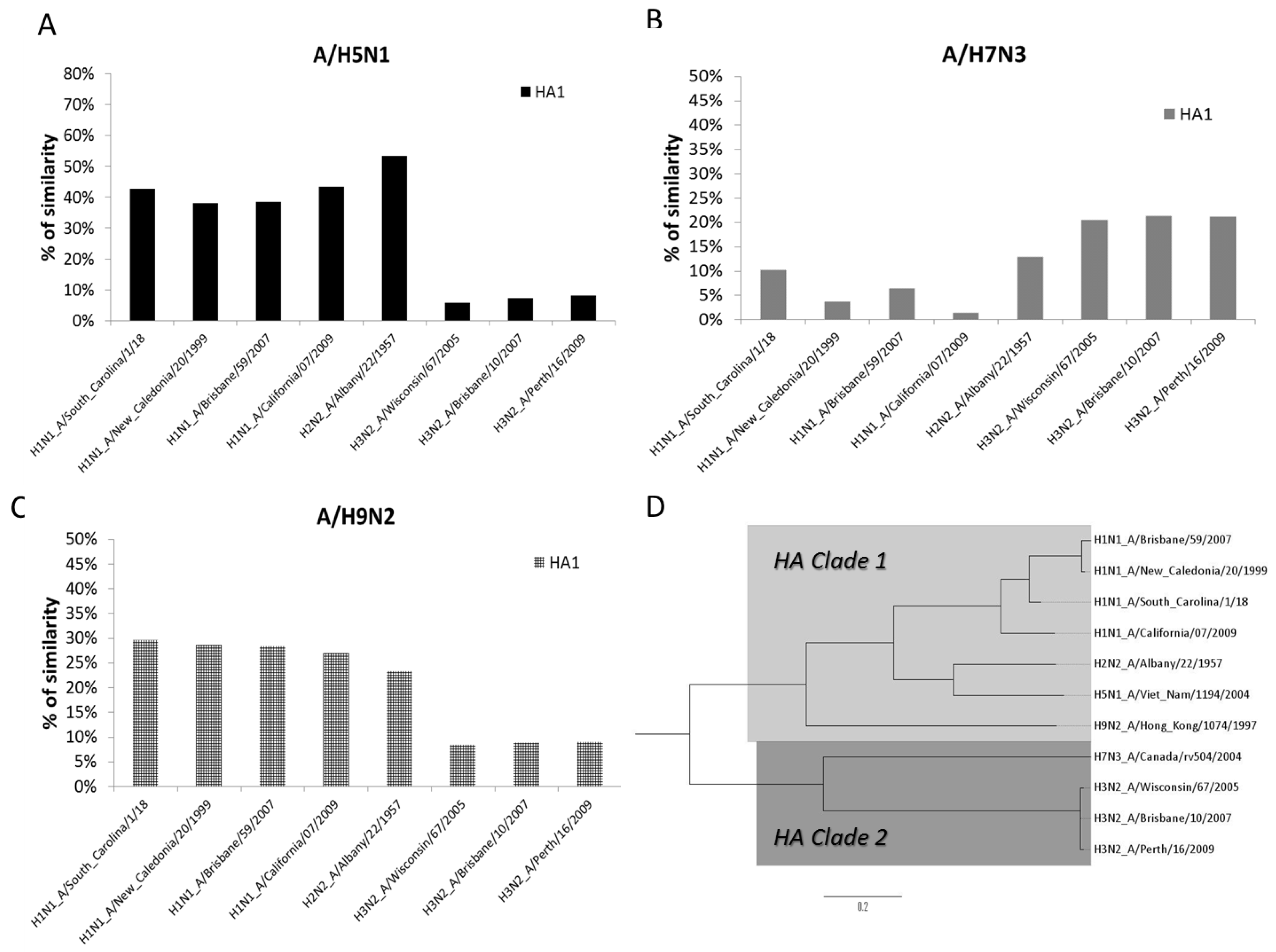
3.4. Phylogenetic Analysis of AIV and SIV
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nachbagauer, R.; Krammer, F. Universal influenza virus vaccines and therapeutic antibodies. Clin. Microbiol. Infect 2017, 23, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. Strategies to induce broadly protective antibody responses to viral glycoproteins. Expert Rev. Vaccines 2017, 16, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Impagliazzo, A.; Milder, F.; Kuipers, H.; Wagner, M.V.; Zhu, X.; Hoffman, R.M.B.; van Meersbergen, R.; Huizingh, J.; Wanningen, P.; Verspuij, J.; et al. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science 2015, 349, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-J.; Ermler, M.E.; Tan, G.S.; Krammer, F.; Palese, P.; Hai, R. Influenza A Viruses Expressing Intra- or Inter-group Chimeric Hemagglutinins. J. Virol. 2016, 90, 3789–3793. [Google Scholar] [CrossRef] [PubMed]
- Ermler, M.E.; Kirkpatrick, E.; Sun, W.; Hai, R.; Amanat, F.; Chromikova, V.; Palese, P.; Krammer, F. Chimeric Hemagglutinin Constructs Induce Broad Protection against Influenza B Virus Challenge in the Mouse Model. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, S.A.; Mallajosyula, V.V.A.; Li, O.T.W.; Chin, A.W.H.; Carnell, G.; Temperton, N.; Varadarajan, R.; Poon, L.L.M. Stalking influenza by vaccination with pre-fusion headless HA mini-stem. Sci. Rep. 2016, 6, 22666. [Google Scholar] [CrossRef] [PubMed]
- Wyrzucki, A.; Dreyfus, C.; Kohler, I.; Steck, M.; Wilson, I.A.; Hangartner, L. Alternative recognition of the conserved stem epitope in influenza A virus hemagglutinin by a VH3-30-encoded heterosubtypic antibody. J. Virol. 2014, 88, 7083–7092. [Google Scholar] [CrossRef] [PubMed]
- Feery, B.J.; Evered, M.G.; Hayes, K. Homologous and heterologous antibody responses to subunit influenza virus vaccine. J. Hyg. 1978, 81, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Kolpe, A.; Schepens, B.; Fiers, W.; Saelens, X. M2-based influenza vaccines: Recent advances and clinical potential. Expert Rev. Vaccines 2017, 16, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Epstein, S.L.; Price, G.E. Cross-protective immunity to influenza A viruses. Expert Rev. Vaccines 2010, 9, 1325–1341. [Google Scholar] [CrossRef] [PubMed]
- Lara-Ramírez, E.E.; Segura-Cabrera, A.; Salazar, M.I.; Rodríguez-Pérez, M.A.; Guo, X. Large scale genome analysis shows that the epitopes for broadly cross-reactive antibodies are predominant in the pandemic 2009 influenza virus A H1N1 strain. Viruses 2013, 5, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Hancock, K.; Veguilla, V.; Lu, X.; Zhong, W.; Butler, E.N.; Sun, H.; Liu, F.; Dong, L.; DeVos, J.R.; Gargiullo, P.M.; et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 2009, 361, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, N.; Strengell, M.; Kinnunen, L.; Osterlund, P.; Pirhonen, J.; Broman, M.; Davidkin, I.; Ziegler, T.; Julkunen, I. High frequency of cross-reacting antibodies against 2009 pandemic influenza A(H1N1) virus among the elderly in Finland. Euro Surveill. 2010, 15. [Google Scholar] [PubMed]
- WHO Avian Influenza Weekly Update Number 569 2017. Available online: http://www.wpro.who.int/emerging_diseases/ai_weekly_569_wpro_20170127_final.pdf (accessed on 1 May 2017).
- Pu, J.; Wang, S.; Yin, Y.; Zhang, G.; Carter, R.A.; Wang, J.; Xu, G.; Sun, H.; Wang, M.; Wen, C.; et al. Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus. Proc. Natl. Acad. Sci. USA 2015, 112, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Peiris, J.S.M.; de Jong, M.D.; Guan, Y. Avian Influenza Virus (H5N1): A Threat to Human Health. Clin. Microbiol. Rev. 2007, 20, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Perez, A.M.; Sánchez-Vizcaíno, J.M.; Muñoz, M.J.; Martínez, M.; de la Torre, A. Reproductive ratio for the local spread of highly pathogenic avian influenza in wild bird populations of Europe, 2005–2008. Epidemiol. Infect. 2011, 139, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Martínez, M.; Muñoz, M.J.; de la Torre, A.; Sánchez-Vizcaíno, J.M. First case of highly pathogenic avian influenza in poultry in Spain. Transbound. Emerg. Dis. 2010, 57, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, U.; Harris, K.; Stroud, D.; Guberti, V.; Busani, L.; Pittman, M.; Piazza, V.; Cook, A.; Brown, I. Avian influenza surveillance in wild birds in the European Union in 2006. Influenza Other Respir. Viruses 2009, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Call for vigilance as H5N8 avian influenza confirmed in Lincolnshire. Vet. Rec. 2016, 179, 638. [CrossRef]
- Globig, A.; Starick, E.; Homeier, T.; Pohlmann, A.; Grund, C.; Wolf, P.; Zimmermann, A.; Wolf, C.; Heim, D.; Schlößer, H.; et al. Epidemiological and Molecular Analysis of an Outbreak of Highly Pathogenic Avian Influenza H5N8 clade 2.3.4.4 in a German Zoo: Effective Disease Control with Minimal Culling. Transbound. Emerg. Dis. 2016. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.; Voas, S.; Glossop, C.; Huey, R. Heightened risk of H5N8 highly pathogenic avian influenza. Vet. Rec. 2016, 179, 577. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Brown, I.H.; Angelova, S.G.; Bálint, Á.; Bouwstra, R.; Buda, S.; Castrucci, M.R.; Dabrera, G.; Dán, Á.; Grund, C.; et al. Highly pathogenic avian influenza A(H5N8) outbreaks: Protection and management of exposed people in Europe, 2014/15 and 2016. Eurosurveillance. 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Sharshov, K.; Swayne, D. E.; Kurskaya, O.; Sobolev, I.; Kabilov, M.; Alekseev, A.; Irza, V.; Shestopalov, A. Novel Reassortant Clade 2.3.4.4 Avian Influenza A(H5N8) Virus in Wild Aquatic Birds, Russia, 2016. Emerg. Infect. Dis. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- FAO. H5N8 HPAI Global situation update [Internet]. 2017. Available online: http://www.fao.org/AG/AGAINFO/PROGRAMMES/EN/empres/H5N8/situation_update.htmal# (accessed on 30 January 2017).
- WHO. Assessment of risk associated with influenza A(H5N8) virus [Internet]. 2016. Available online: http://who.int/influenza/human_animal_interface/avian_influenza/riskassessment_AH5N8_201611/en/ (accessed on 30 January 2017).
- WHO. Avian Influenza Weekly Update Number 509 [Internet]. 2015. Available online: http://www.wpro.who.int/emerging_diseases/AvianInfluenza/en/ (accessed on 3 March 2017).
- Su, S.; Bi, Y.; Wong, G.; Gray, G.C.; Gao, G.F.; Li, S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J. Virol. 2015, 89, 8671–8676. [Google Scholar] [CrossRef] [PubMed]
- WHO. Recommendations for Influenza Vaccine Composition: Northern Hemisphere: 2006–2007 [Internet]. 2006. Available online: http://www.who.int/entity/influenza/vaccines/2007northreport.pdf (accessed on 18 November 2016).
- WHO. Recommended Composition of Influenza Virus Vaccines for Use in the 2008–2009 Influenza Season [Internet]. 2008. Available online: http://www.who.int/entity/influenza/vaccines/recommended_compositionFeb08FullReport.pdf (accessed on 20 January 2017).
- WHO. Recommended Composition of Influenza Virus Vaccines for Use in the 2009–2010 Influenza Sason [Internet]. 2009. Available online: http://www.who.int/entity/influenza/vaccines/200902_recommendation.pdf (accessed on 20 February 2017).
- WHO. Recommended Viruses for Influenza Vaccines for Use in the 2010–2011 Northern Hemisphere Influenza Season [Internet]. 2010. Available online: http://www.who.int/entity/influenza/vaccines/virus/recommendations/201002_Recommendation.pdf (accessed on 20 February 2017).
- WHO. Recommendations and Laboratory Procedures for Detection of Avian Influenza A(H5N1) Virus in Specimens from Suspected Human Cases [Internet]. 2007. Available online: http://www.who.int/influenza/resources/documents/RecAIlabtestsAug07.pdf (accessed on 24 May 2017).
- WHO Global Influenza, Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza 2011. Available online: http://apps.who.int/iris/bitstream/10665/44518/1/9789241548090_eng.pdf (accessed on 1 May 2017).
- He, W.; Mullarkey, C.E.; Miller, M.S. Measuring the neutralization potency of influenza A virus hemagglutinin stalk/stem-binding antibodies in polyclonal preparations by microneutralization assay. Methods San Diego Calif. 2015, 90, 95–100. [Google Scholar] [CrossRef] [PubMed]
- EMA. Note for Guiadance on Harmonisation of Requirements for Influenza Vaccines (CPMP/BWP/214/96) [Internet]. 1997. Available online: http://www.ema-europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf (accessed on 21 April 2016).
- Zacour, M.; Ward, B.J.; Brewer, A.; Tang, P.; Boivin, G.; Li, Y.; Warhuus, M.; McNeil, S.A.; LeBlanc, J.J.; Hatchette, T.F. Standardization of Hemagglutination Inhibition Assay for Influenza Serology Allows for High Reproducibility between Laboratories. Clin. Vaccine Immunol. CVI 2016, 23, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C. M.; Perini, D.; Mather, S.; Temperton, N.; Montomoli, E. Overview of Serological Techniques for Influenza Vaccine Evaluation: Past, Present and Future. Vaccines 2014, 2, 707–734. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.; Bosman, A.; van de Kamp, E.E.H.M.; Wilbrink, B.; Du Ry van Beest Holle, M.; Koopmans, M. Measurement of antibodies to avian influenza virus A(H7N7) in humans by hemagglutination inhibition test. J. Virol. Methods 2006, 132, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.C.; McCarthy, T.; Capuano, A.W.; Setterquist, S.F.; Alavanja, M.C.; Lynch, C.F. Evidence for avian influenza A infections among Iowa’s agricultural workers. Influenza Other Respir. Viruses 2008, 2, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Coman, A.; Maftei, D.N.; Krueger, W.S.; Heil, G.L.; Friary, J.A.; Chereches, R.M.; Sirlincan, E.; Bria, P.; Dragnea, C.; Kasler, I.; et al. Serological evidence for avian H9N2 influenza virus infections among Romanian agriculture workers. J. Infect. Public Health 2013, 6, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Dung, T.C.; Dinh, P.N.; Nam, V.S.; Tan, L.M.; Hang, N.L.K.; Thanh, L.T.; Mai, L.Q. Seroprevalence survey of avian influenza A(H5N1) among live poultry market workers in northern Viet Nam, 2011. Western Pac. Surveill. Response J. 2014, 5, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, A.-R.; Liu, Z.-H.; Liang, W.; Li, X.-X.; Tang, Y.-J.; Miao, Z.-M.; Chai, T.-J. Seroprevalence of avian influenza H9N2 among poultry workers in Shandong Province, China. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fang, S.; Lu, X.; Xu, C.; Cowling, B.J.; Tang, X.; Peng, B.; Wu, W.; He, J.; Tang, Y.; et al. Seroprevalence to avian influenza A(H7N9) virus among poultry workers and the general population in southern China: A longitudinal study. Clin. Infect. Dis. 2014, 59, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhu, W.; Gu, H.; Fu, X.; Wang, L.; Zheng, Y.; He, S.; Ke, C.; Wang, H.; Yuan, Z.; et al. Avian influenza H9N2 seroprevalence among swine farm residents in China. J. Med. Virol. 2014, 86, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Detection of H5N8 virus in wild birds “no surprise”, says Defra. Vet. Rec. 2017, 180, 3. [CrossRef]
- ECDC Priority risk groups for Influenza vaccination. Available online: http://ecdc.europa.eu/en/healthtopics/seasonal_influenza/vaccines/Pages/influenza_vaccination.aspx#riskgroups (accessed on 2 February 2017).
- Russell, C.J. Stalking influenza diversity with a universal antibody. N. Engl. J. Med. 2011, 365, 1541–1542. [Google Scholar] [CrossRef] [PubMed]
- Corti, D.; Voss, J.; Gamblin, S.J.; Codoni, G.; Macagno, A.; Jarrossay, D.; Vachieri, S.G.; Pinna, D.; Minola, A.; Vanzetta, F.; et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 2011, 333, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: the mother of all pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Nachbagauer, R.; Wohlbold, T.J.; Hirsh, A.; Hai, R.; Sjursen, H.; Palese, P.; Cox, R.J.; Krammer, F. Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans. J. Virol. 2014, 88, 13260–13268. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.; Lowen, A.C.; Wang, T T.; Yondola, M.; Gao, Q.; Haye, K.; García-Sastre, A.; Palese, P. Influenza virus vaccine based on the conserved hemagglutinin stalk domain. mBio 2010, 1. [Google Scholar] [CrossRef] [PubMed]
- Air, G.M. Sequence relationships among the hemagglutinin genes of 12 subtypes of influenza A virus. Proc. Natl. Acad. Sci. USA 1981, 78, 7639–7643. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Kawashita, N.; Kubota-Koketsu, R.; Inoue, Y.; Watanabe, Y.; Ibrahim, M.S.; Ideno, S.; Yunoki, M.; Okuno, Y.; Takagi, T.; et al. Highly conserved sequences for human neutralization epitope on hemagglutinin of influenza A viruses H3N2, H1N1 and H5N1: Implication for human monoclonal antibody recognition. Biochem. Biophys. Res. Commun. 2010, 393, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Sui, J.; Hwang, W.C.; Perez, S.; Wei, G.; Aird, D.; Chen, L.; Santelli, E.; Stec, B.; Cadwell, G.; Ali, M.; Wan, H.; et al. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, Y.A.; Lipatov, A.S.; Gitelman, A.K.; Okuno, Y.; Van Beek, R.; Osterhaus, A.D.; Claas, E.C. An epitope shared by the hemagglutinins of H1, H2, H5, and H6 subtypes of influenza A virus. Acta Virol. 1999, 43, 237–244. [Google Scholar] [PubMed]
- Stephenson, I.; Bugarini, R.; Nicholson, K.G.; Podda, A.; Wood, J.M.; Zambon, M.C.; Katz, J.M. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J. Infect. Dis. 2005, 191, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Forrest, H.L.; Khalenkov, A.M.; Govorkova, E.A.; Kim, J.-K.; Giudice, G.D.; Webster, R.G. Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. Vaccine 2009, 27, 4187–4195. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Byun, Y.H.; Lee, Y.J.; Lee, Y.H.; Lee, K.-H.; Seong, B.L. Cold-adapted pandemic 2009 H1N1 influenza virus live vaccine elicits cross-reactive immune responses against seasonal and H5 influenza A viruses. J. Virol. 2012, 86, 5953–5958. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Nakamura, K.; Mase, M.; Tsukamoto, K.; Imada, T.; Yamaguchi, S. Partial protection against challenge with the highly pathogenic H5N1 influenza virus isolated in Japan in chickens infected with the H9N2 influenza virus. Arch. Virol. 2007, 152, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, D.C.; Friesen, R.H.E.; Bhabha, G.; Kwaks, T.; Jongeneelen, M.; Yu, W.; Ophorst, C.; Cox, F.; Korse, H.J.W.M.; Brandenburg, B.; Vogels, R.; et al. A Highly Conserved Neutralizing Epitope on Group 2 Influenza A Viruses. Science 2011, 333, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, E.D. Influenza pandemics of the 20th century. Emerg. Infect. Dis. 2006, 12, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, J.V.; Gorbulev, V.G.; Kurmanova, A.G.; Bayev, A.A.; Shilov, A.A.; Zhdanov, V.M. On the origin of the H1N1 (A/USSR/90/77) influenza virus. J. Gen. Virol. 1981, 56, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Peeters, B.; Reemers, S.; Dortmans, J.; de Vries, E.; de Jong, M.; van de Zande, S.; Rottier, P.J.M.; de Haan, C.A.M. Genetic versus antigenic differences among highly pathogenic H5N1 avian influenza A viruses: Consequences for vaccine strain selection. Virology 2017, 503, 83–93. [Google Scholar] [CrossRef] [PubMed]
| Type | Subtype | Influenza Vaccine Campaigns | |||
|---|---|---|---|---|---|
| 2006–2007 | 2008–2009 | 2009–2010 | 2010–2011 | ||
| A | H1N1 | A/New Caledonia/20/99 | A/Brisbane/59/2007 | A/Brisbane/59/2007 | Not included |
| H3N2 | A/Wisconsin/67/2005 | A/Brisbane/10/2007 | A/Brisbane/10/2007 | A/Victoria/201/2009 | |
| H1N1pdm09 | Not included | Not included | Not included | A/California/07/2009 | |
| B | Yamagata | Not included | B/Florida/4/2006 | Not included | Not included |
| Victoria | B/Malaysia/2506/2004 | Not included | B/Brisbane/60/2008 | B/Brisbane/60/2008 | |
| Vaccinated Cohorts | A/H5N1 | A/H7N3 | A/H9N2 | |||
|---|---|---|---|---|---|---|
| SCn 1 | SCR 2 | SCn | SCR | SCn | SCR | |
| 2006–2007 | 9 | 20.0 | 1 | 2.2 | 11 | 24.4 |
| 2008–2009 | 1 | 2.3 | 0 | 0.0 | 3 | 7.0 |
| 2009–2010 | 3 | 7.0 | 0 | 0.0 | 2 | 4.7 |
| 2010–2011 | 12 | 27.9 | 0 | 0.0 | 3 | 7.0 |
| AIV 1 | Input | Vaccinated Cohorts | |||
|---|---|---|---|---|---|
| 2006–2007 | 2008–2009 | 2009–2010 | 2010–2011 | ||
| A/H5N1 | |||||
| Pre-vaccine GMT2 (CI 95%) | 5.3 (4.9–6.0) | 4.9 (4.9–4.9) | 5.2 (4.9–5.8) | 5.3 (4.9–5.6) | |
| Post-vaccine GMT (CI 95%) | 11.3 (8.4–15.9) | 6.5 (5.7–7.6) | 8.5 (7.0–10.5) | 14.0 (10.8–18.4) | |
| A/H7N3 | |||||
| Pre-vaccine GMT (CI 95%) | 5.3 (4.9–6.0) | 4.9 (4.9–4.9) | 5.2 (4.9–5.5) | 4.9 (4.9–4.9) | |
| Post-vaccine GMT (CI 95%) | 5.3 (4.9–6.0) | 4.9 (4.9–4.9) | 5.2 (4.9–5.5) | 5.3 (4.9–5.9) | |
| A/H9N2 | |||||
| Pre-vaccine GMT (CI 95%) | 6.1 (5.5–6.9) | 6.0 (5.4–6.6) | 6.7 (5.9–7.7) | 5.9 (5.4–6.4) | |
| Post-vaccine GMT (CI 95%) | 19.7 (14.0–29.4) | 9.2 (7.6–11.2) | 8.9 (7.5–10.8) | 8.2 (6.9–10.0) | |
| AIV 1 | Input | Vaccinated Cohorts | |||
|---|---|---|---|---|---|
| 2006–2007 | 2008–2009 | 2009–2010 | 2010–2011 | ||
| A/H5N1 | |||||
| SPR 2 | 20.0 | 2.3 | 9.3 | 27.9 | |
| SCR 3 | 20.0 | 2.3 | 7.0 | 27.9 | |
| GMT increase | 2.1 | 1.3 | 1.6 | 2.7 | |
| A/H7N3 | |||||
| SPR | 2.2 | 0.0 | 0.0 | 0.0 | |
| SCR | 0.0 | 0.0 | 0.0 | 0.0 | |
| GMT increase | 1.0 | 1.0 | 1.0 | 1.1 | |
| A/H9N2 | |||||
| SPR | 28.9 | 7.0 | 7.0 | 7.0 | |
| SCR | 24.4 | 7.0 | 4.7 | 7.0 | |
| GMT increase | 3.2 | 1.5 | 1.3 | 1.4 | |
| Influenza A Subtypes and Strains | A/H1N1 (A/South_Carolina/1/18) | A/H1N1pdm09 (A/California/07/2009) | A/H2N2 (A/Albany/22/1957) | A/H5N1 (A/VietNam/1194/2004) | A/H7N3 (A/Canada/rv504/2004) | A/H9N2 (A/Hong Kong/1074/1997) | A/H3N2 (A/Brisbane/10/2007) | A/H1N1 (A/Brisbane/59/2007) | A/H1N1 (A/New_Caledonia/20/1999) | A/H3N2 (A/Perth/16/2009) | A/H3N2 (A/Wisconsin/67/2005) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A/H1N1 (A/South_Carolina/1/18) | 1.000 | ||||||||||
| A/H1N1pdm09 (A/California/07/2009) | 0.785 | 1.000 | |||||||||
| A/H2N2 (A/Albany/22/1957) | 0.439 | 0.416 | 1.000 | ||||||||
| A/H5N1 (A/VietNam/1194/2004) | 0.428 | 0.434 | 0.532 | 1.000 | |||||||
| A/H7N3 (A/Canada/rv504/2004) | 0.101 | 0.013 | 0.128 | 0.133 | 1.000 | ||||||
| A/H9N2 (A/Hong Kong/1074/1997) | 0.296 | 0.270 | 0.233 | 0.265 | 0.059 | 1.000 | |||||
| A/H3N2 (A/Brisbane/10/2007) | 0.089 | 0.088 | 0.090 | 0.073 | 0.212 | 0.087 | 1.000 | ||||
| A/H1N1 (A/Brisbane/59/2007) | 0.802 | 0.680 | 0.395 | 0.385 | 0.063 | 0.284 | 0.013 | 1.000 | |||
| A/H1N1 (A/New Caledonia/20/1999) | 0.815 | 0.689 | 0.399 | 0.380 | 0.037 | 0.285 | 0.013 | 0.969 | 1.000 | ||
| A/H3N2 (A/Perth/16/2009) | 0.082 | 0.076 | 0.082 | 0.082 | 0.211 | 0.089 | 0.988 | 0.000 | 0.006 | 1.000 | |
| A/H3N2 (A/Wisconsin/67/2005) | 0.082 | 0.087 | 0.082 | 0.058 | 0.204 | 0.083 | 0.989 | 0.010 | 0.011 | 0.983 | 1.000 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz, I.; Rojo, S.; Tamames, S.; Eiros, J.M.; Ortiz de Lejarazu, R. Heterologous Humoral Response against H5N1, H7N3, and H9N2 Avian Influenza Viruses after Seasonal Vaccination in a European Elderly Population. Vaccines 2017, 5, 17. https://doi.org/10.3390/vaccines5030017
Sanz I, Rojo S, Tamames S, Eiros JM, Ortiz de Lejarazu R. Heterologous Humoral Response against H5N1, H7N3, and H9N2 Avian Influenza Viruses after Seasonal Vaccination in a European Elderly Population. Vaccines. 2017; 5(3):17. https://doi.org/10.3390/vaccines5030017
Chicago/Turabian StyleSanz, Ivan, Silvia Rojo, Sonia Tamames, José María Eiros, and Raúl Ortiz de Lejarazu. 2017. "Heterologous Humoral Response against H5N1, H7N3, and H9N2 Avian Influenza Viruses after Seasonal Vaccination in a European Elderly Population" Vaccines 5, no. 3: 17. https://doi.org/10.3390/vaccines5030017





