Gas Permeation Property of Silicon Carbide Membranes Synthesized by Counter-Diffusion Chemical Vapor Deposition
Abstract
1. Introduction
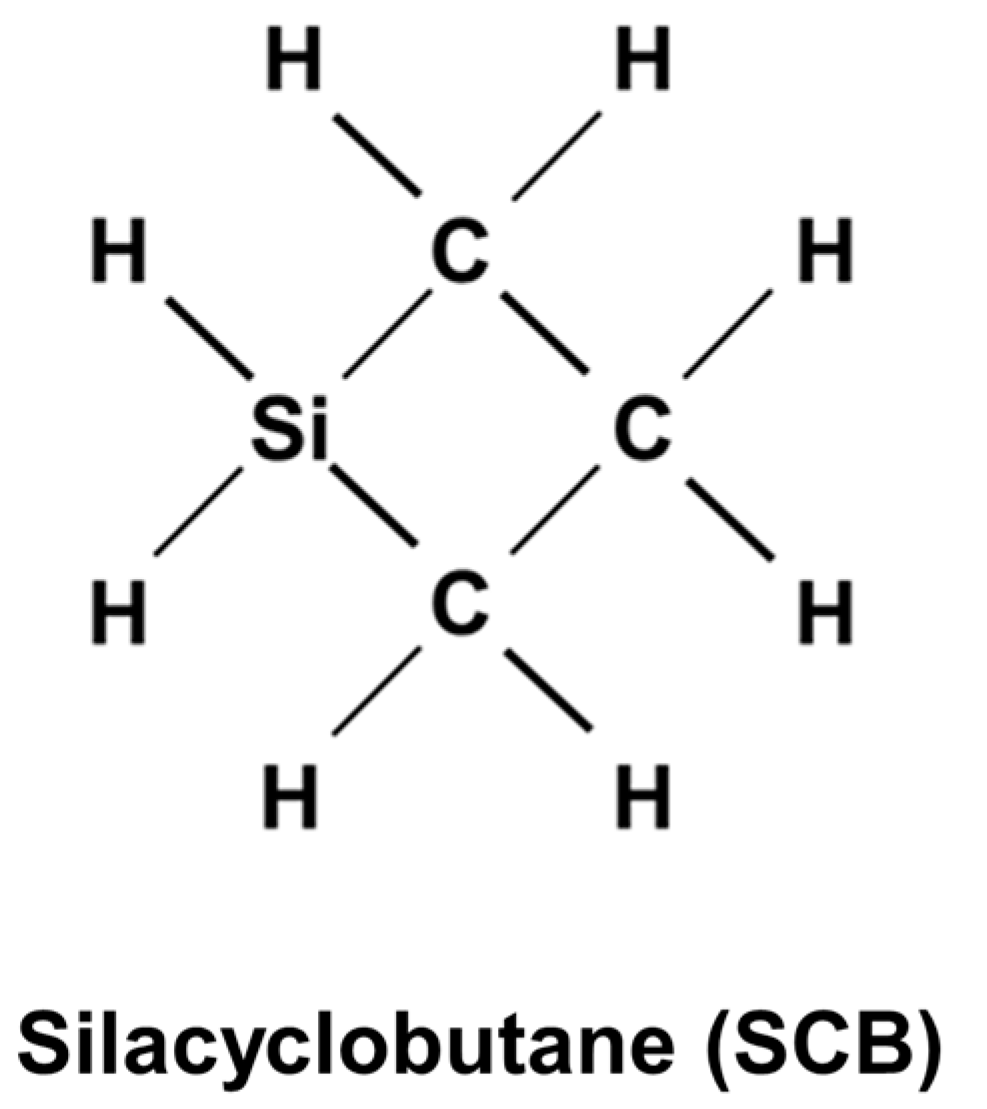
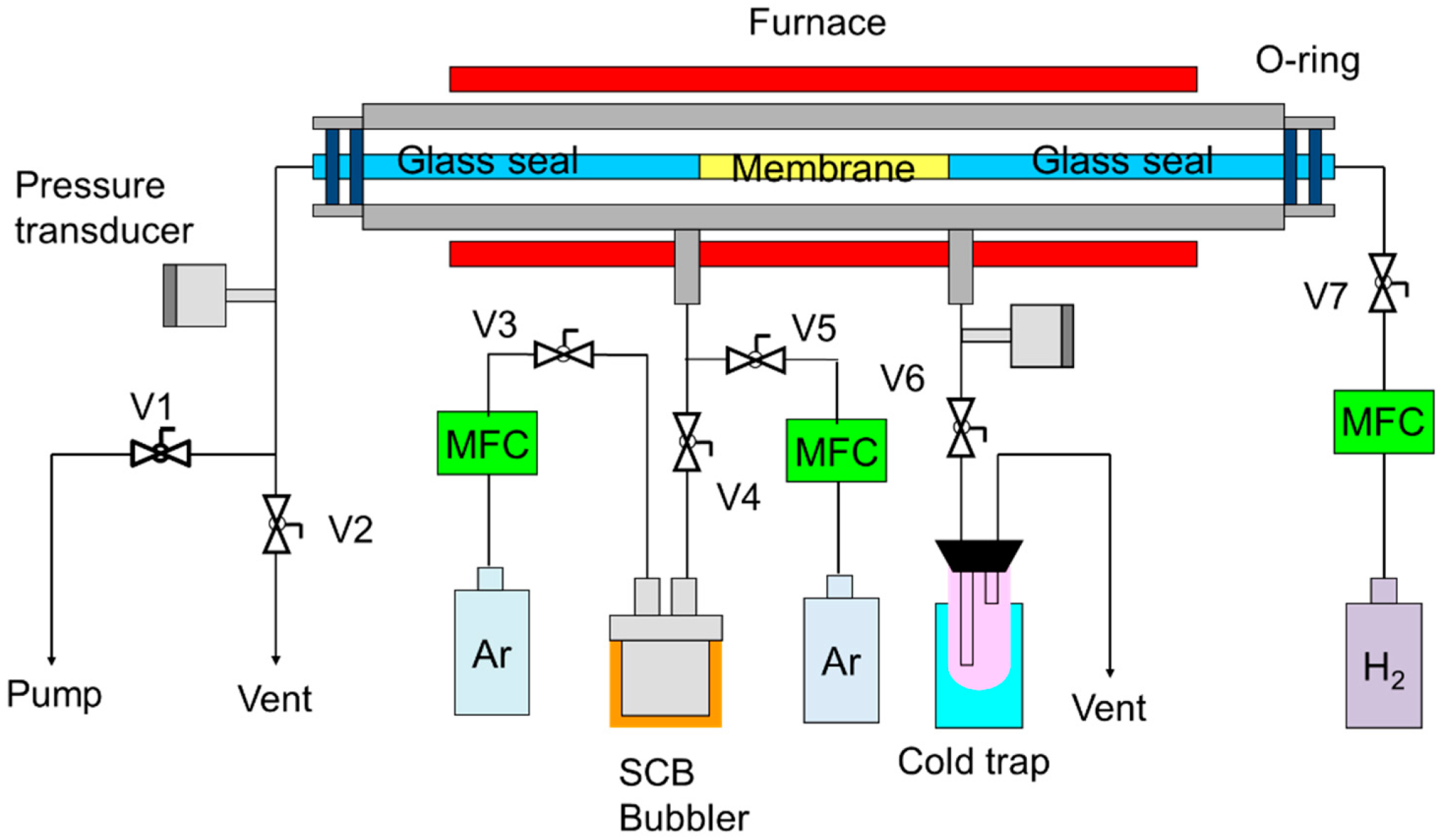
2. Experimental Procedure
3. Results and Discussion
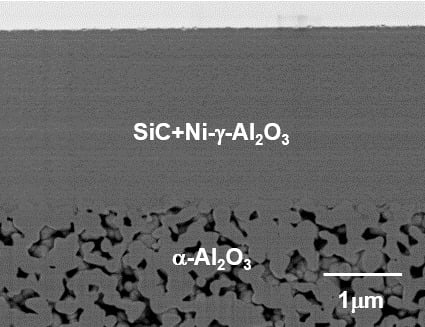
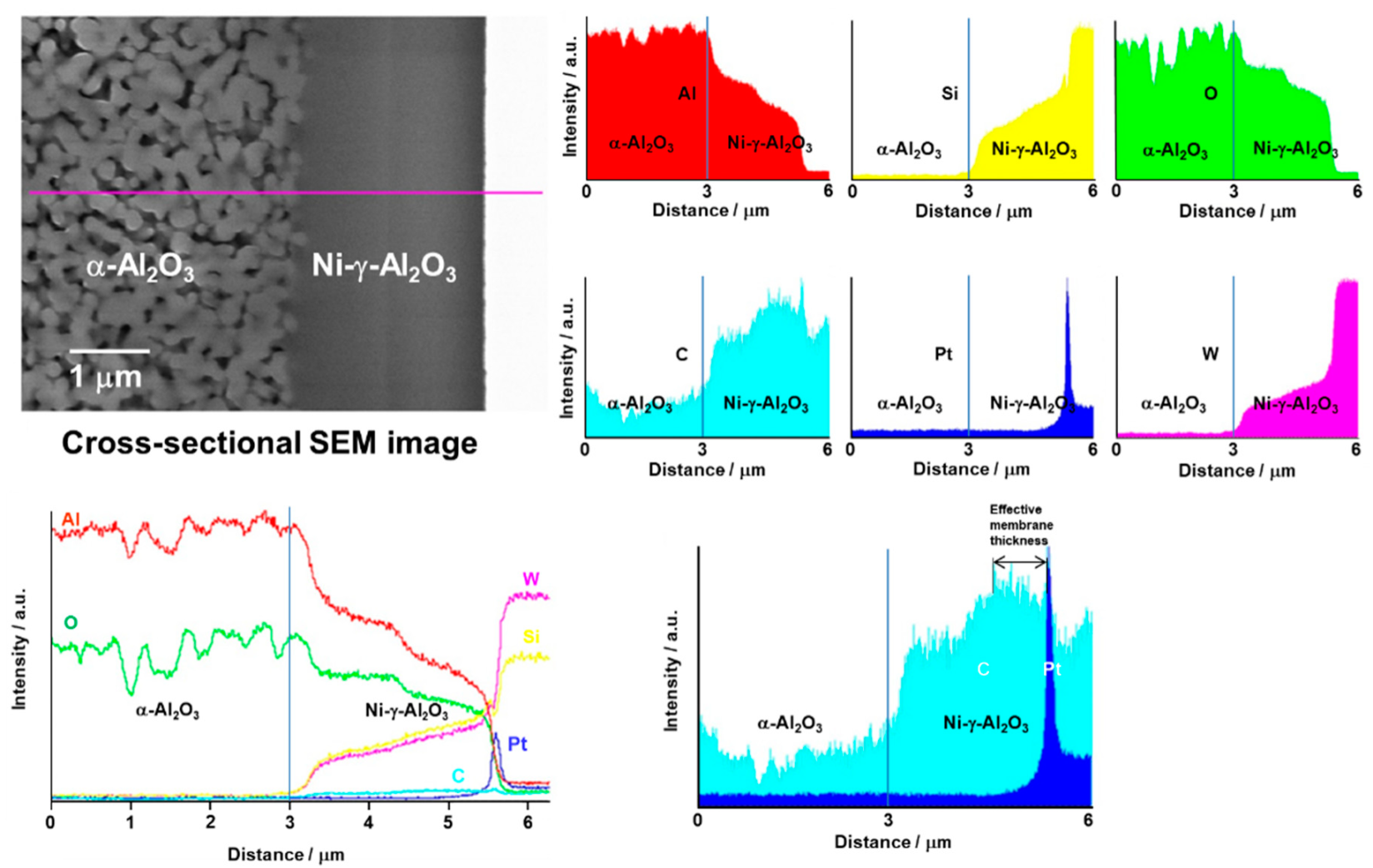
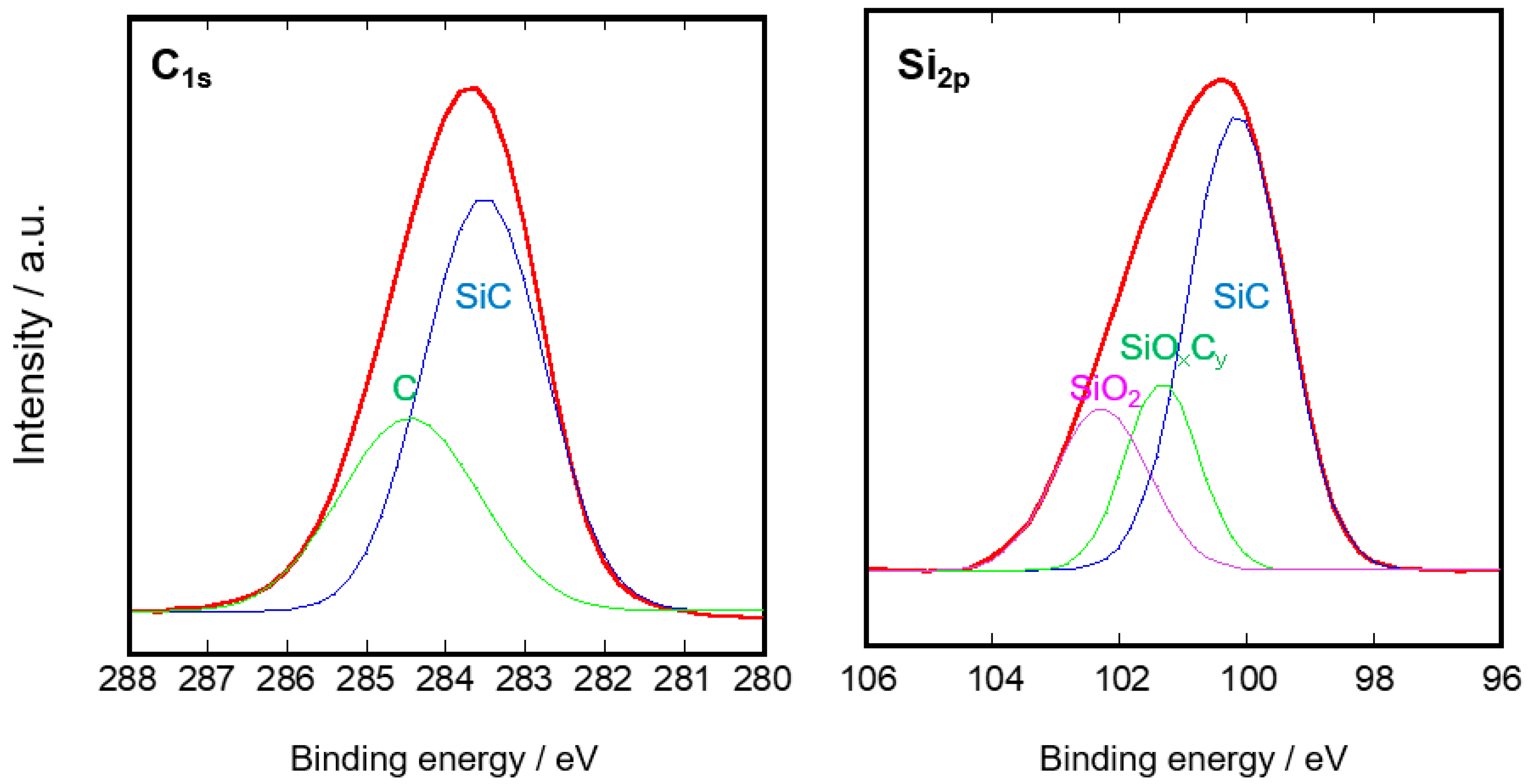
3.1. Microstructure of the SiC Membrane
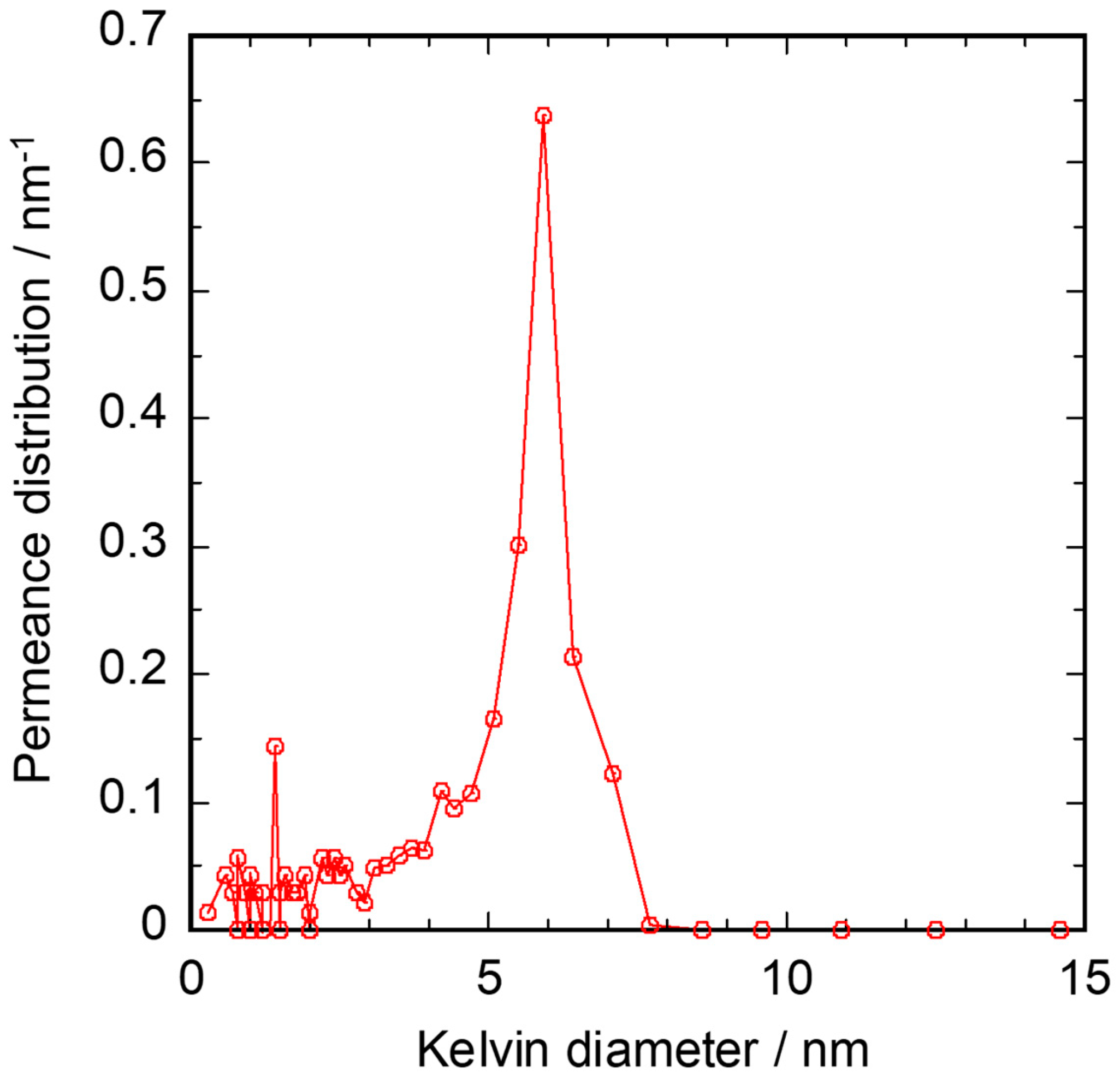
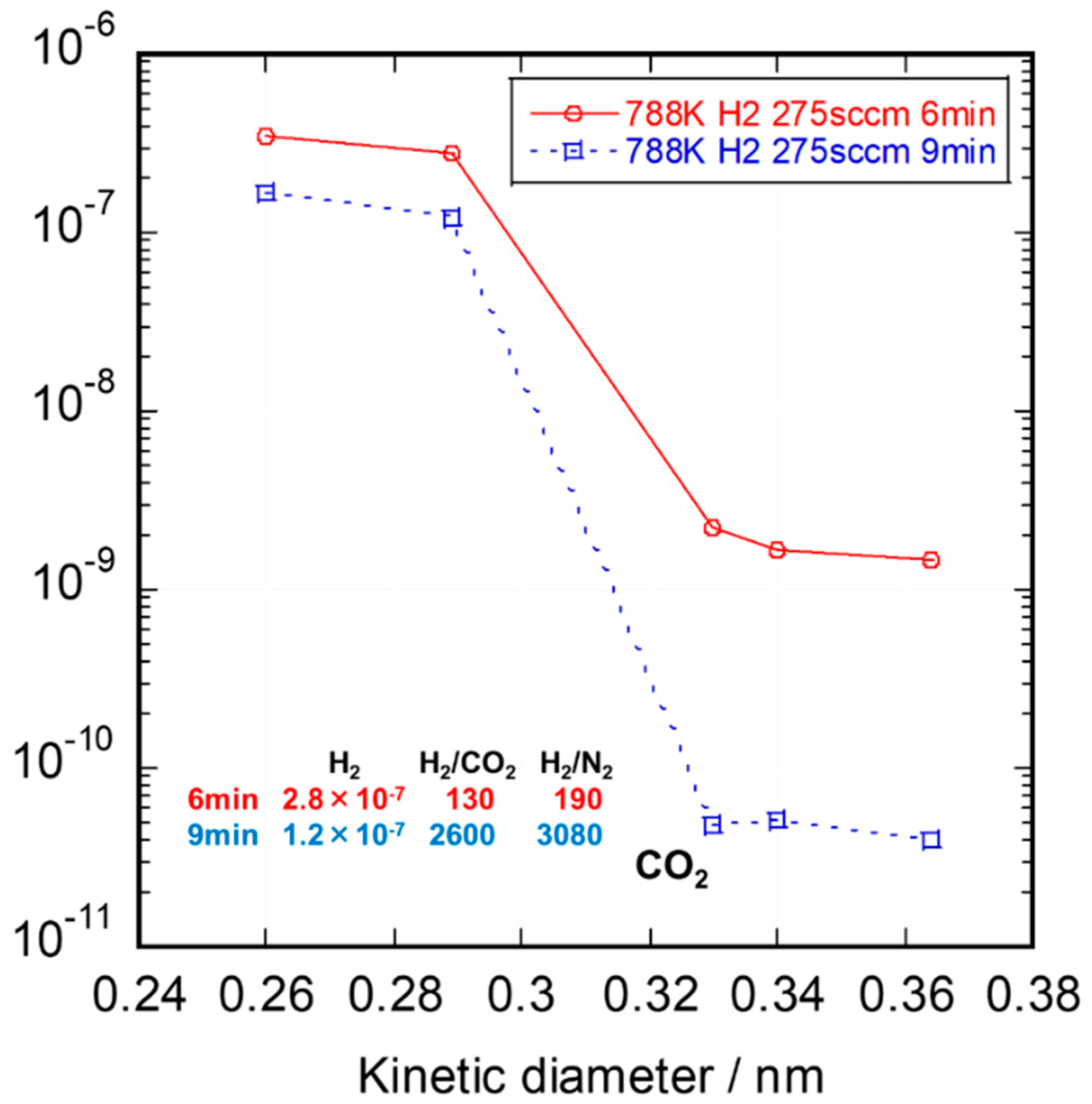
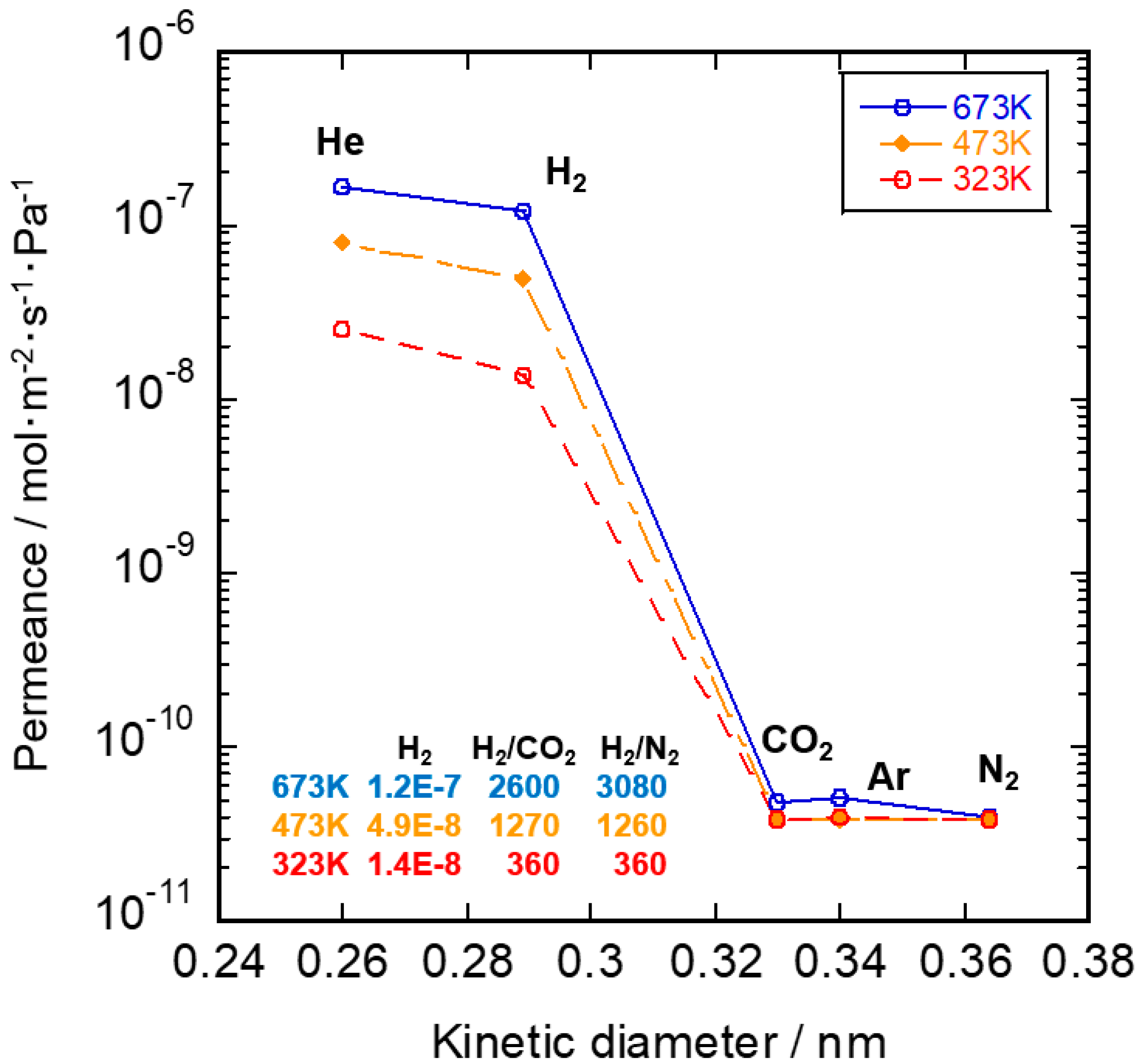
3.2. Gas Permeation Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shelekhin, A.B.; Grosgogeat, E.J.; Hwang, S.-T. Gas separation properties of a new polymer/inorganic composite membrane. J. Membr. Sci. 1991, 66, 129–141. [Google Scholar] [CrossRef]
- Kusakabe, K.; Li, Z.; Maeda, H.; Morooka, S. Preparation of supported composite membrane by pyrolysis of polycarbosilane for gas separation at high temperature. J. Membr. Sci. 1995, 103, 175–180. [Google Scholar] [CrossRef]
- Lee, L.L.; Tsai, D.S. A hydrogen-permselective silicon oxycarbide membrane derived from polydimethylsilane. J. Am. Ceram. Soc. 1999, 82, 2796–2800. [Google Scholar] [CrossRef]
- Li, Z.; Kusakabe, K.; Morooka, S. Preparation of thermostable amorphous Si-C-O membrane and its application to gas separation at elevated temperature. J. Membr. Sci. 1996, 118, 159–168. [Google Scholar]
- Li, Z.; Kusakabe, K.; Morooka, S. Pore structure and permeance of amorphous Si-C-O membranes with high durability at elevated temperature. Sep. Sci. Technol. 1997, 32, 1233–1254. [Google Scholar] [CrossRef]
- Suda, H.; Uchimaru, Y.; Fujiwara, I.; Haraya, K. Preparation and gas permeation properties of silicon carbide-based inorganic membranes for hydrogen separation. Desalination 2006, 193, 252–255. [Google Scholar] [CrossRef]
- Suda, H.; Uchimaru, Y.; Fujiwara, I.; Haraya, K. Structural evolution during conversion of polycarbosilane precursor into silicon carbide-based microporous membranes. J. Ceram. Soc. Jpn. 2006, 114, 539–544. [Google Scholar] [CrossRef]
- Nagano, T.; Sato, K.; Saitoh, T.; Iwamoto, Y. Gas permeation properties of amorphous SiC membranes synthesized from polycarbosilane without oxygen-curing Process. J. Ceram. Soc. Jpn. 2006, 114, 533–538. [Google Scholar] [CrossRef]
- Takeyama, A.; Sugimoto, M.; Yoshikawa, M. Gas permeation property of SiC membrane using curing of polymer precursor film by electron beam irradiation in helium atmosphere. Mater. Trans. 2011, 52, 1276–1280. [Google Scholar] [CrossRef]
- Takeda, Y.; Shibata, N.; Kubo, Y. SiC coating on porous γ-Al2O3 using alternative-supply CVI method. J. Ceram. Soc. Jpn. 2001, 109, 305–309. [Google Scholar] [CrossRef][Green Version]
- Pages, X.; Rouessac, V.; Cot, D.; Nabias, G.; Durand, J. Gas permeation of PECVD membranes inside alumina substrate tubes. Sep. Purif. Technol. 2001, 25, 399–406. [Google Scholar] [CrossRef]
- Sea, B.-K.; Ando, K.; Kusakabe, K.; Morooka, S. Separation of hydrogen from steam using a SiC-based membrane formed by chemical vapor deposition of triisopropylsilane. J. Membr. Sci. 1998, 146, 73–82. [Google Scholar] [CrossRef]
- Ciora, R.J.; Fayyaz, B.; Liu, P.K.; Suwanmethanond, V.; Mallada, R.; Sahimi, M.; Tsotis, T. Preparation and reactive applications of nanoporous silicon carbide membranes. Chem. Eng. Sci. 2004, 59, 4957–4965. [Google Scholar] [CrossRef]
- Nagano, T.; Sato, K.; Fujisaki, S.; Saitoh, T.; Iwamoto, Y. Helium-permselective amorphous SiC membrane modified by chemical vapor infiltration. Soft Mater. 2007, 4, 109–122. [Google Scholar] [CrossRef]
- Nagano, T.; Fujisaki, S.; Sato, K.; Hataya, K.; Iwamoto, Y.; Nomura, M.; Nakao, S.-I. Relationship between the mesoporous intermediate layer structure and the gas permeation property of an amorphous silica membrane synthesized by counter diffusion chemical vapor deposition. J. Am. Ceram. Soc. 2007, 91, 71–76. [Google Scholar] [CrossRef]
- Nomura, M.; Suraj, G.; Aida, H.; Sugawara, T.; Nakao, S.-I.; Yamazaki, S.; Iwamoto, Y.; Kojima, R.; Nakao, A. Investigation of a hydrothermal stable hydrogen permselective silica membrane for a large membrane area module. Membrane 2005, 30, 275–281. [Google Scholar] [CrossRef][Green Version]
- Nagano, T.; Sato, K. Degradation mechanism of an H2-permselective amorphous silica membrane. J. Mater. Sci. 2014, 49, 4115–4120. [Google Scholar] [CrossRef]
- Nagano, T.; Sato, K.; Takahashi, S. Hydrothermal stability of mesoporous Ni-doped γ-Al2O3. J. Ceram. Soc. Jpn. 2009, 117, 832–835. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Phuong, H.T.; Jin, F.; Yang, J.F.; Ishizaki, K. Gas permeability and adsorbability of the glass-bonded porous silicon carbide ceramics with controlled pore size. Ceram. Int. 2015, 41, 2279–2285. [Google Scholar] [CrossRef]
- Ding, S.Q.; Zeng, Y.P.; Jiang, D.L. Gas permeability behavior of mullite-bonded porous silicon carbide ceramics. J. Mater. Sci. 2007, 42, 7171–7175. [Google Scholar] [CrossRef]
- Fukushima, M.; Nakata, M.; Ohji, T.; Yoshizawa, Y.I. Fabrication and properties of ultra highly porous silicon carbide by a gelation-freezing method. J. Eur. Ceram. Soc. 2010, 30, 2889–2896. [Google Scholar] [CrossRef]
- Qiao, H.; Feng, S.; Low, Z.; Chen, J.; Zhang, F.; Zhong, Z.; Xing, W. Al-DTPA microfiber assisted formwork construction technology for high-performance SiC membrane preparation. J. Membr. Sci. 2019, 594, 117464. [Google Scholar] [CrossRef]
- Han, F.; Zhong, Z.; Zhang, F.; Xing, W.; Fan, Y. Preparation and characterization of SiC whisker-reinforced SiC porous ceramics for hot gas filtration. Ind. Eng. Chem. Res. 2015, 54, 226–232. [Google Scholar] [CrossRef]
- Han, F.; Zhong, Z.; Yang, Y.; Wei, W.; Zhang, F.; Xing, W.; Fan, Y. High gas permeability of SiC porous ceramics reinforced by mullite fibers. J. Eur. Ceram. Soc. 2016, 36, 3909–3917. [Google Scholar] [CrossRef]
- Dabirs, S.; Deng, W.; Sahimi, M.; Tsotsis, T. Fabrication of silicon carbide membranes on highly permeable supports. J. Membr. Sci. 2017, 537, 239–247. [Google Scholar] [CrossRef]
- Barrer, R.M. Permeation, diffusion and solution of gases in organic polymers. Trans. Faraday Soc. 1934, 35, 628–643. [Google Scholar] [CrossRef]
- Knudsen, M. The law of the molecular flow and viscosity of gases moving through tubes. Ann. Phys. 1909, 28, 75–130. [Google Scholar] [CrossRef]
- Yoshioka, T.; Tsuru, T.; Asaeda, M. Molecular dynamics studies on gas permeation through amorphous silica network and inter-particle pores on microporous silica membranes. Mol. Phys. 2004, 102, 191–202. [Google Scholar] [CrossRef]
- Takaba, H.; Matsuda, E.; Nakao, S.-I. Correlation of temperature dependence of gas permeability with pore size in molecular sieving membranes: A grand canonical ensemble molecular dynamics study. J. Phys. Chem. B 2004, 108, 14142–14147. [Google Scholar] [CrossRef]
- Hwang, C.-J.; Onuki, K.; Shimizu, S. Studies on Hydrogen Separation Membranes for IS Process, Membrane Preparation with Porous α-Alumina Tube; Japan Atomic Energy Research Inst.: Tokyo, Japan, 1998. [Google Scholar]
- Lee, D.; Zhang, L.; Niu, S.; Saraf, R.F. Synthesis, characterization and gas permeation properties of a hydrogen permeable silica membrane supported on porous alumina. J. Membr. Sci. 2004, 231, 117–126. [Google Scholar] [CrossRef]





| Membrane | Permeate Temp. (K) | Activation Energy (kJ/mol) | Ref. | |
|---|---|---|---|---|
| He | H2 | |||
| SiOC | 283–773 | 7–13 | 7–15 | [4] |
| SiOC | 350–473 | 9.8–15.4 | 16.3–16.7 | [3] |
| SiC | 773–873 | 9.4 | 6.0 | [8] |
| 573–773 | 2.7 | 1.2 | [8] | |
| 573–873 | 1.2–1.9 | 0.4–1.0 | [8] | |
| (PCS+CVI) | 573–773 | 0.6 | 0.09 | [14] |
| 773–873 | 1.2 | 0.3 | [14] | |
| SiO2 | 573–873 | 14 | 15.3 | [30] |
| SiO2 | 373–873 | 9.8 | 14.8 | [31] |
| SiO2 | 373–873 | 8.1 | 16.8 | [15] |
| This study | 323–673 | 9.8 | 11.2 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagano, T.; Sato, K.; Kawahara, K. Gas Permeation Property of Silicon Carbide Membranes Synthesized by Counter-Diffusion Chemical Vapor Deposition. Membranes 2020, 10, 11. https://doi.org/10.3390/membranes10010011
Nagano T, Sato K, Kawahara K. Gas Permeation Property of Silicon Carbide Membranes Synthesized by Counter-Diffusion Chemical Vapor Deposition. Membranes. 2020; 10(1):11. https://doi.org/10.3390/membranes10010011
Chicago/Turabian StyleNagano, Takayuki, Koji Sato, and Koichi Kawahara. 2020. "Gas Permeation Property of Silicon Carbide Membranes Synthesized by Counter-Diffusion Chemical Vapor Deposition" Membranes 10, no. 1: 11. https://doi.org/10.3390/membranes10010011
APA StyleNagano, T., Sato, K., & Kawahara, K. (2020). Gas Permeation Property of Silicon Carbide Membranes Synthesized by Counter-Diffusion Chemical Vapor Deposition. Membranes, 10(1), 11. https://doi.org/10.3390/membranes10010011