Control of Sequential MTO Reactions through an MFI-Type Zeolite Membrane Contactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the MFI-Type Zeolite Membrane
2.2. Characterization
2.3. The MTO Reaction
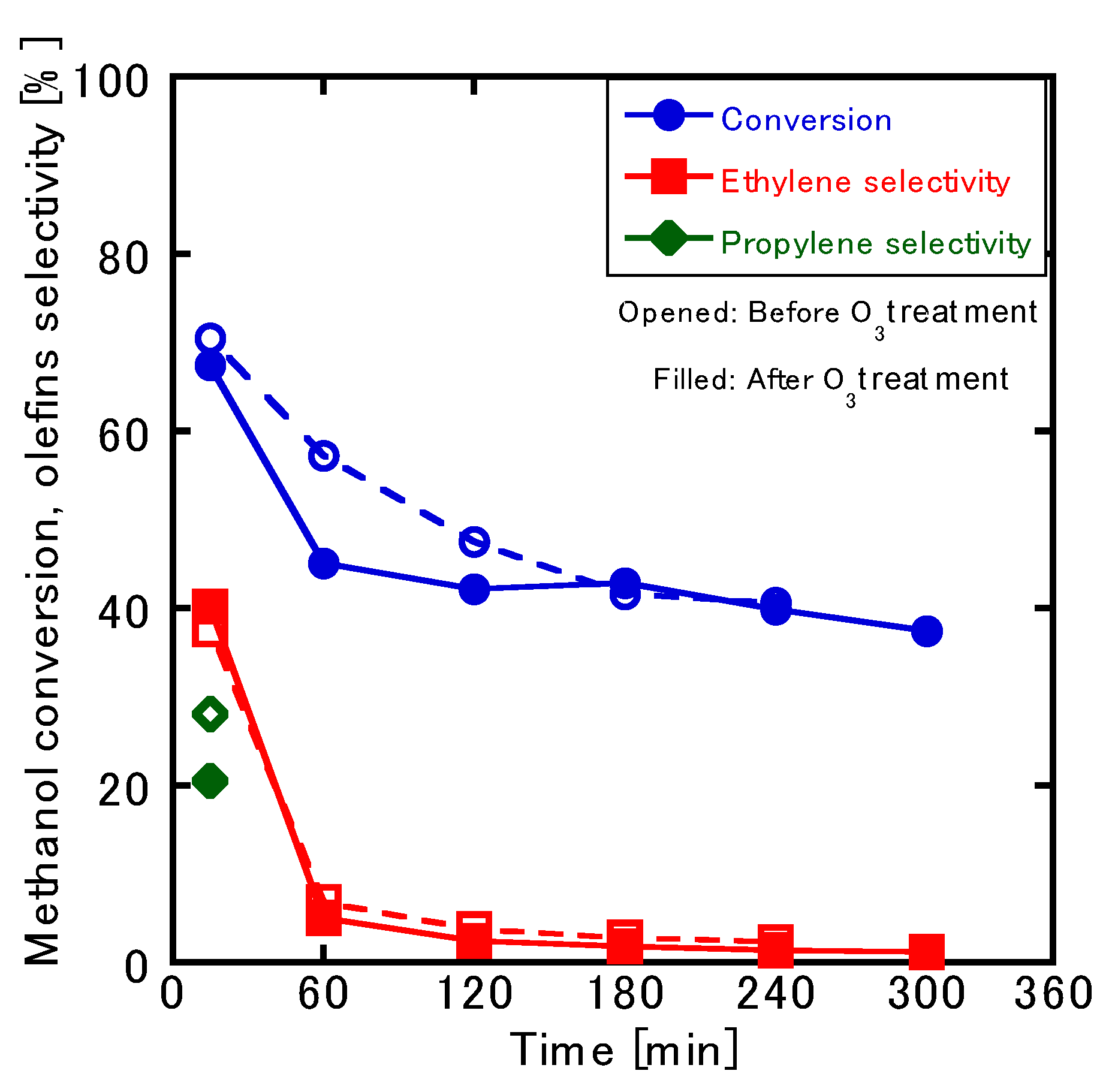
2.4. Regeneration of the Catalyst via O3 Treatment
3. Results and Discussion
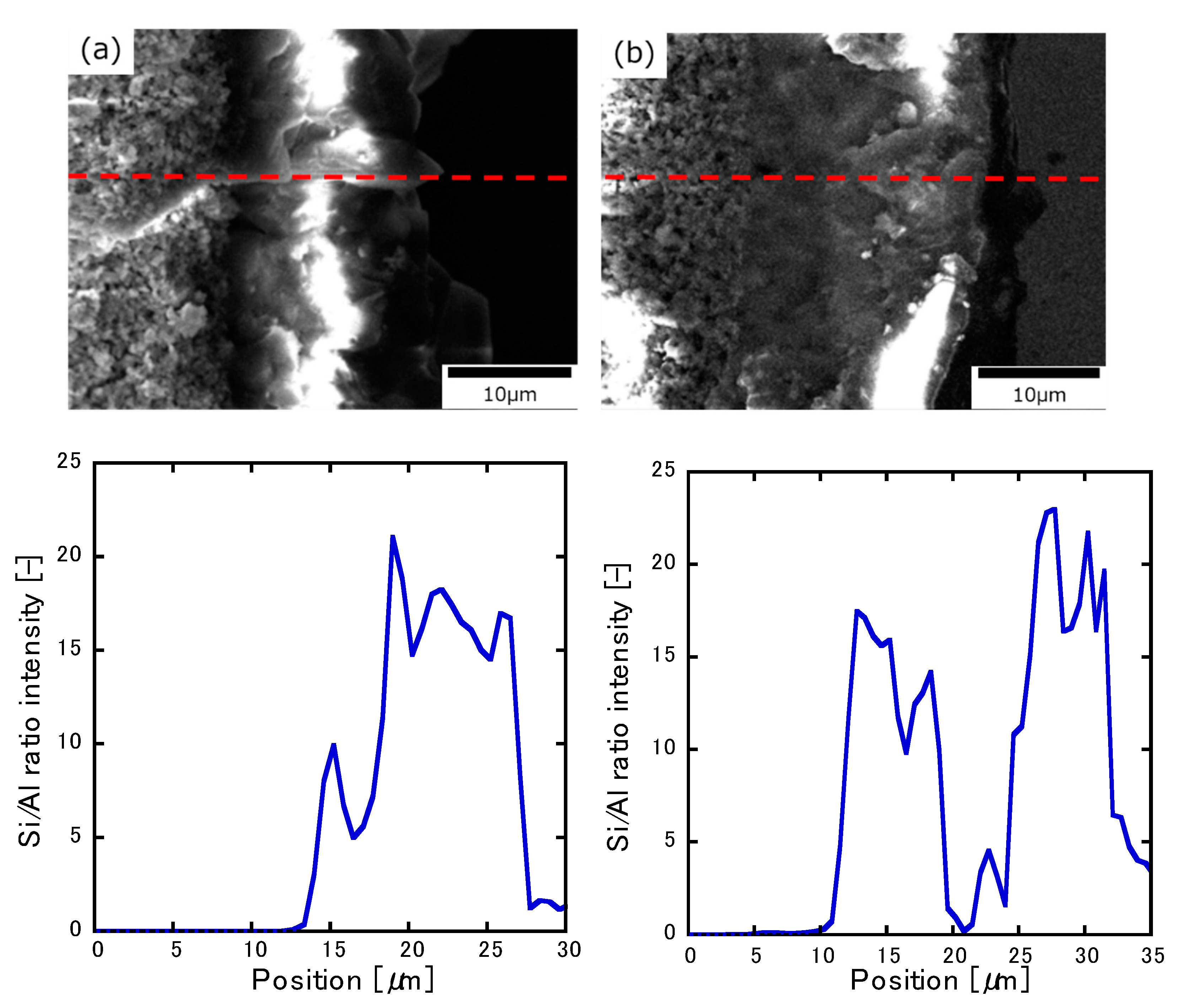
3.1. Characterization
3.2. Gas Permeation Tests
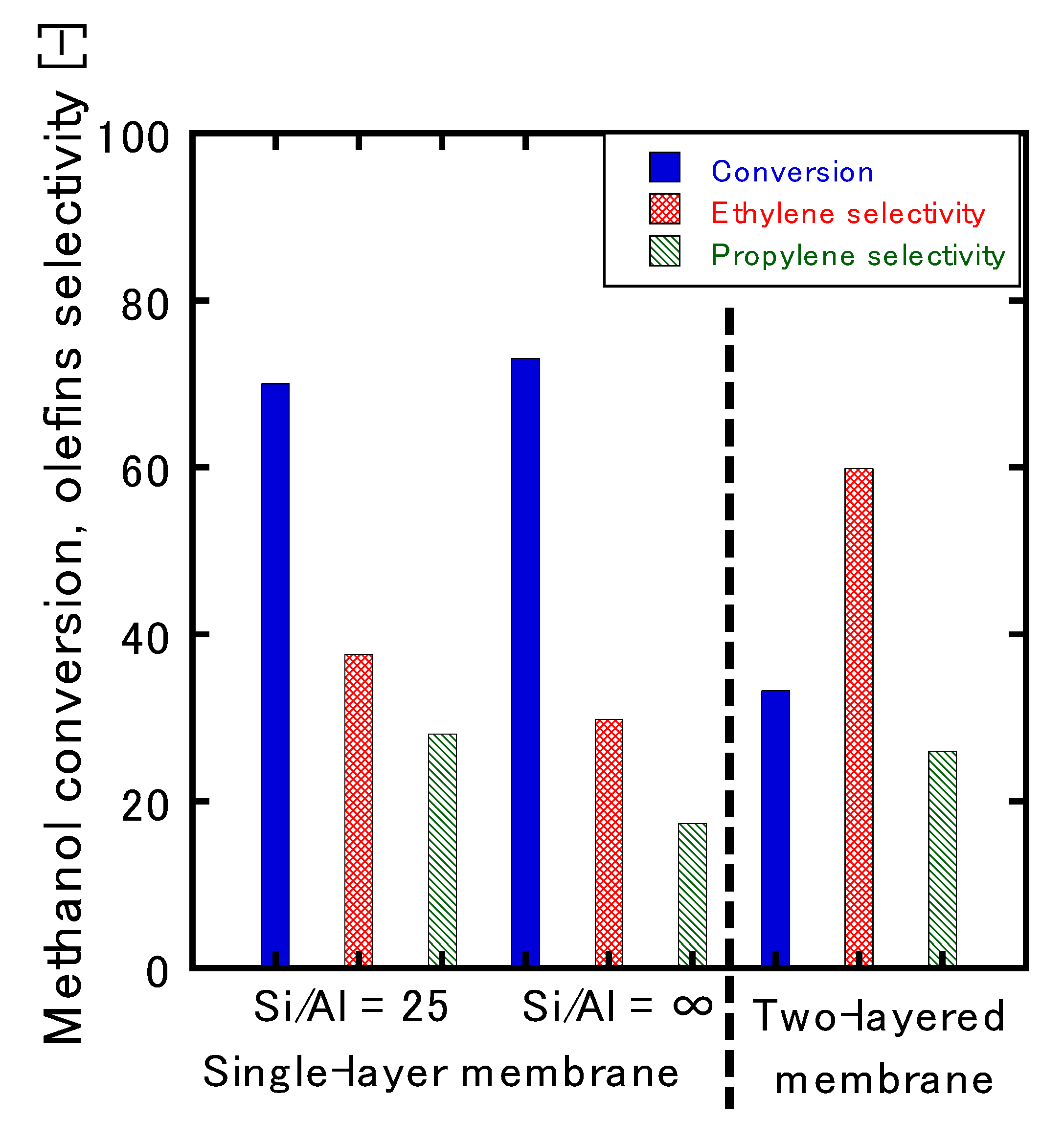
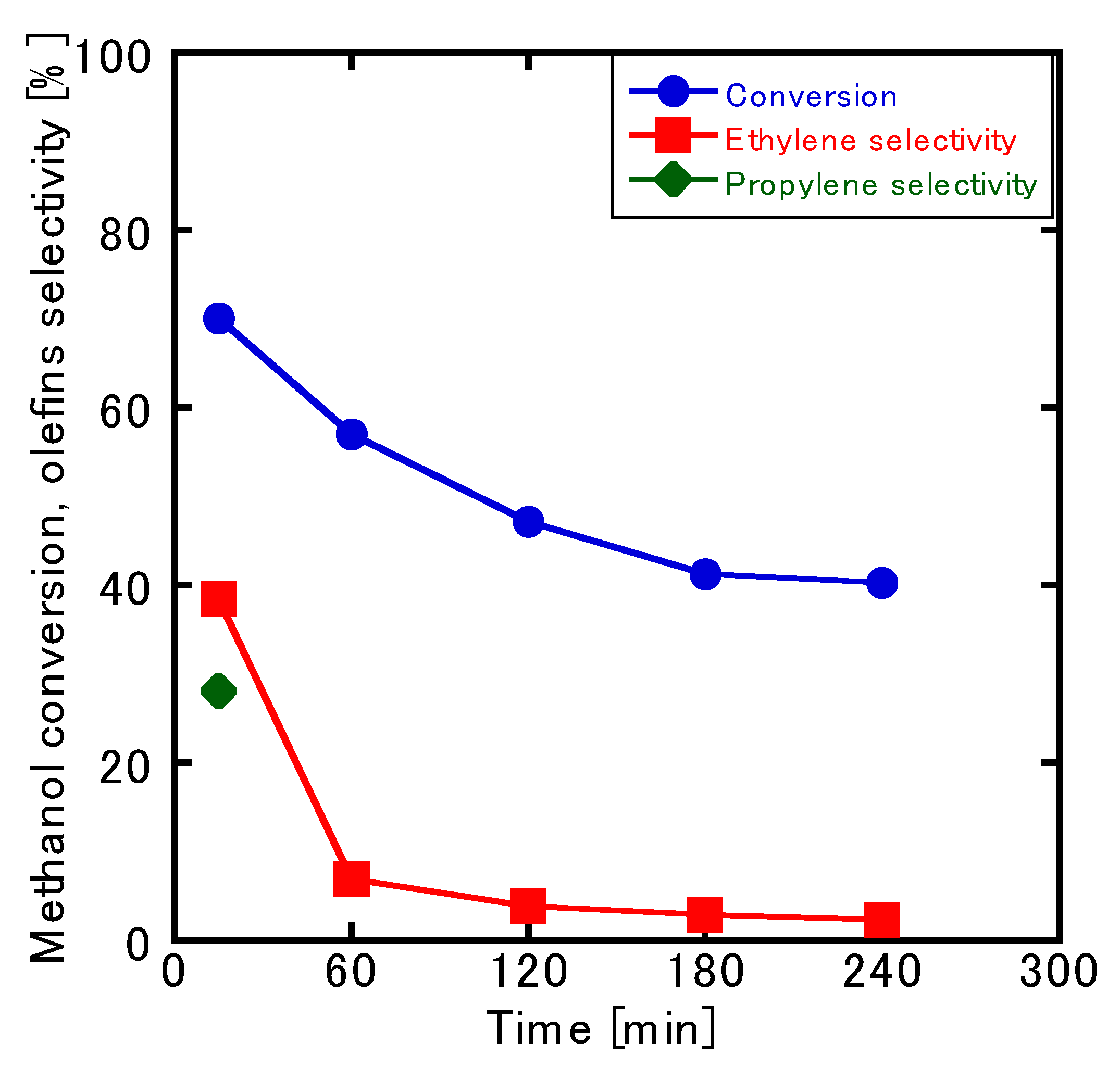
3.3. MTO Reaction
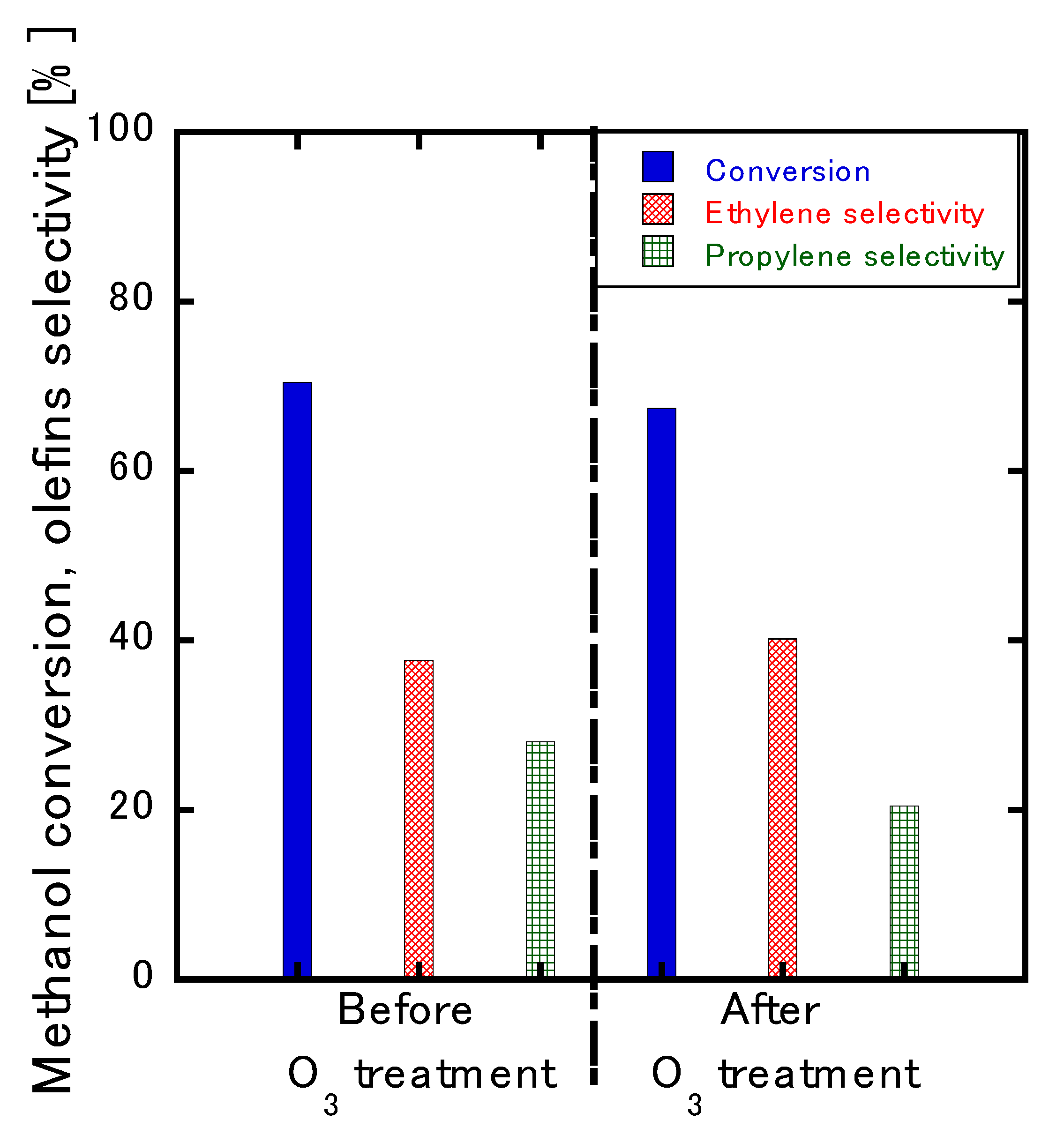
3.4. Catalyst Regeneration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhong, J.; Han, J.; Wei, Y.; Xu, S.; Sun, T.; Guo, X.; Song, C.; Liu, Z. The template-assisted zinc ion incorporation in SAPO-34 and the enhanced ethylene selectivity in MTO reaction. J. Energy Chem. 2019, 32, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Olsbye, U.; Svelle, S.; Bjorgen, M.; Beato, P.; Janssens, T.; Joensen, F.; Bordiga, S.; Lillerud, K. Conversion of Methanol to Hydrocarbons: How Zeolite Cavity and Pore Size Controls Product Selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, E.; Haghighi, M. Hydrothermal synthesis of nanostructured Ce-SAPO-34: High-performance and long-lifetime catalyst with various ceria contents for methanol to light olefins conversion. Microporous Mesoporous Mater. 2018, 270, 227–240. [Google Scholar] [CrossRef]
- Dyballa, M.; Obenaus, U.; Blum, M.; Dai, W. Alkali metal ion exchanged ZSM-5 catalyst: On acidity and methanol-to-olefin performance. Catal. Sci. Technol. 2018, 8, 4440–4449. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Yaripour, F.; Hazrati, H. High efficient mesoporous HZSM-5 nanocatalyst development through desilication with mixed alkaline solution for methanol to olefin reaction. J. Porous Mater. 2018, 25, 1287–1299. [Google Scholar] [CrossRef]
- Wang, F.; Chu, X.; Zhu, F.; Li, Q.; Liu, B.; Xiao, G. Producing BTX aromatics-enriched oil from biomass derived glycerol using dealuminated HZSM-5 by successive steaming and acid leaching as catalyst: Reactivity, acidity and product distribution. Microporous Mesoporous Mater. 2019, 277, 289–294. [Google Scholar] [CrossRef]
- Masuda, T.; Asanuma, T.; Shouji, M.; Kawase, M.; Hashimoto, K. Methanol to olefins using ZSM-5 zeolite catalyst membrane reactor. Chem. Eng. Sci. 2003, 58, 649–656. [Google Scholar] [CrossRef]
- Tago, T.; Iwakai, K.; Morita, K.; Tanaka, K.; Masuda, T. Control of acid-site location of ZSM-5 zeolite membrane and its application to the MTO reaction. Catal. Today 2005, 105, 662–666. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Ikarugi, S.; Oura, K.; Ikeda, A.; Matsuyama, E.; Ono, R.; Nomura, M.; Tawarayama, H.; Saito, T.; Kuwahara, K. MFI zeolite membranes prepared on novel silica substrates. J. Chem. Eng. Jpn. 2015, 48, 891–896. [Google Scholar] [CrossRef]
- Ueno, K.; Negishi, H.; Miyamoto, M.; Uemiya, S.; Oumi, Y. Effect of deposition seed crystal amount on the α-Al2O3 support and separation performance of silicalite-1 membranes for acetic acid/water mixture. Sep. Purif. Technol. 2010, 174, 57–65. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, X.; Kita, H. Inexpensive synthesis of silicalite-1 membranes with high pervaporation performance. Chem. Lett. 2010, 39, 388–389. [Google Scholar] [CrossRef]
- Khanghham, S.; Julcour, C.; Damronglerd, S.; Ngamcharussrivichai, C.; Manero, M.; Delmas, H. Regeneration of coked zeolite from PMMA cracking process by ozonation. Appl. Catal. B 2013, 140–141, 396–405. [Google Scholar] [CrossRef] [Green Version]




| Name | Experimental Method | O/P Ratio (-) |
|---|---|---|
| Single-layer membrane (Si/Al = 25) | Cross-flow | 13.1 |
| Previous study [7] | Dead-end | 3.5 |
| Two-layered membrane | Cross-flow | 26.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanizume, S.; Yoshimura, T.; Ishii, K.; Nomura, M. Control of Sequential MTO Reactions through an MFI-Type Zeolite Membrane Contactor. Membranes 2020, 10, 26. https://doi.org/10.3390/membranes10020026
Tanizume S, Yoshimura T, Ishii K, Nomura M. Control of Sequential MTO Reactions through an MFI-Type Zeolite Membrane Contactor. Membranes. 2020; 10(2):26. https://doi.org/10.3390/membranes10020026
Chicago/Turabian StyleTanizume, Shusei, Toshihiro Yoshimura, Katsunori Ishii, and Mikihiro Nomura. 2020. "Control of Sequential MTO Reactions through an MFI-Type Zeolite Membrane Contactor" Membranes 10, no. 2: 26. https://doi.org/10.3390/membranes10020026




