Performance of Polymer Electrolyte Membrane for Direct Methanol Fuel Cell Application: Perspective on Morphological Structure
Abstract
:1. Introduction
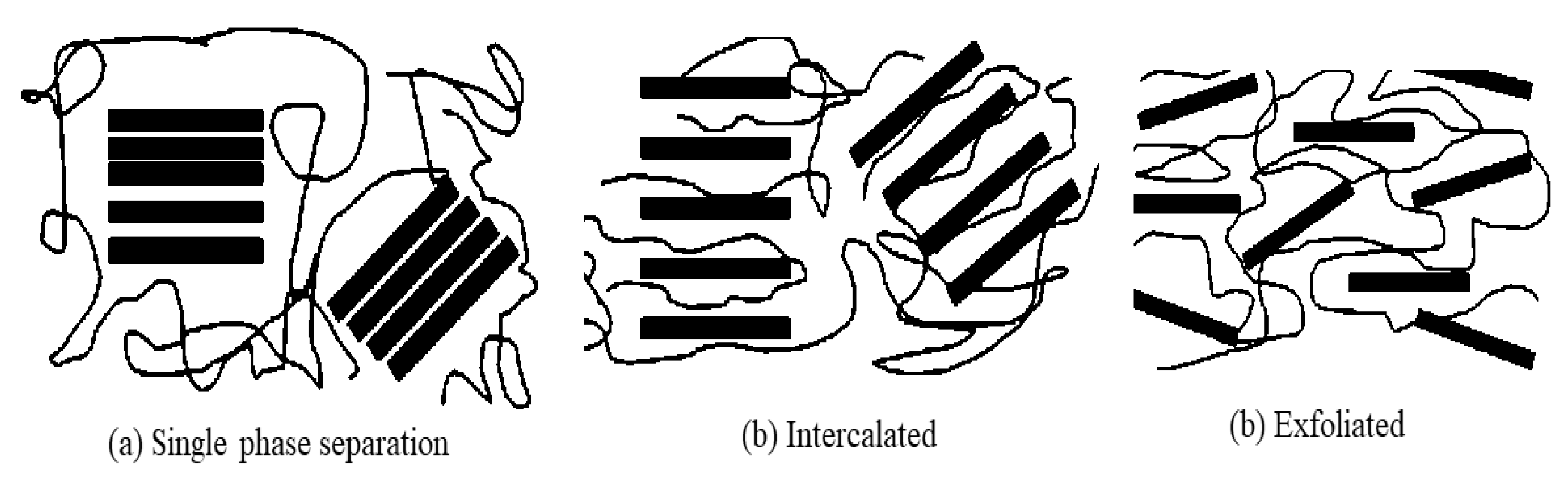
2. Morphology of PEMs
2.1. Dense Electrolyte Membranes
2.2. Thin and Thick Electrolyte Membranes
2.3. Layered Electrolyte Membranes
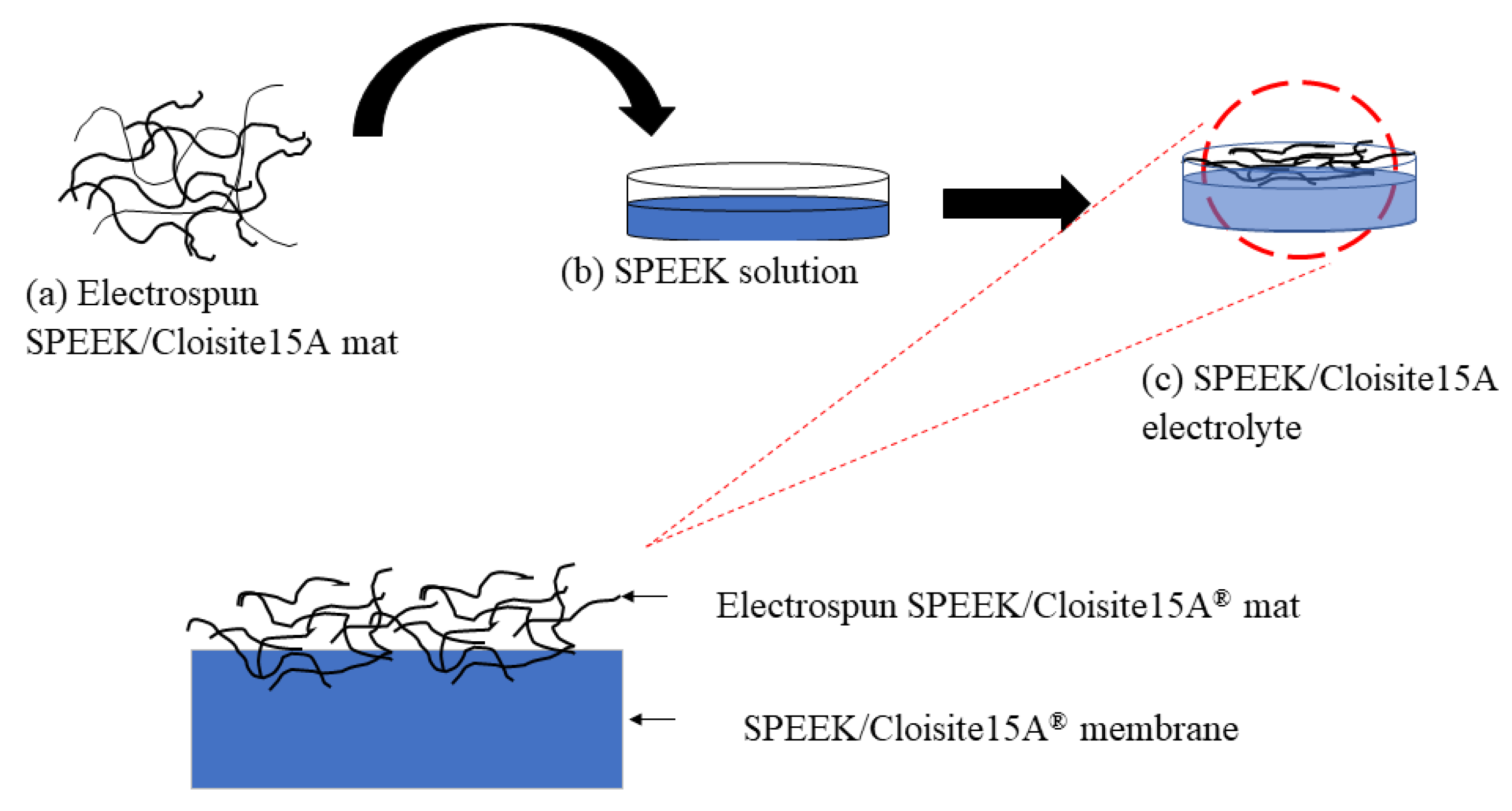
2.4. Sandwiched Electrolyte Membranes
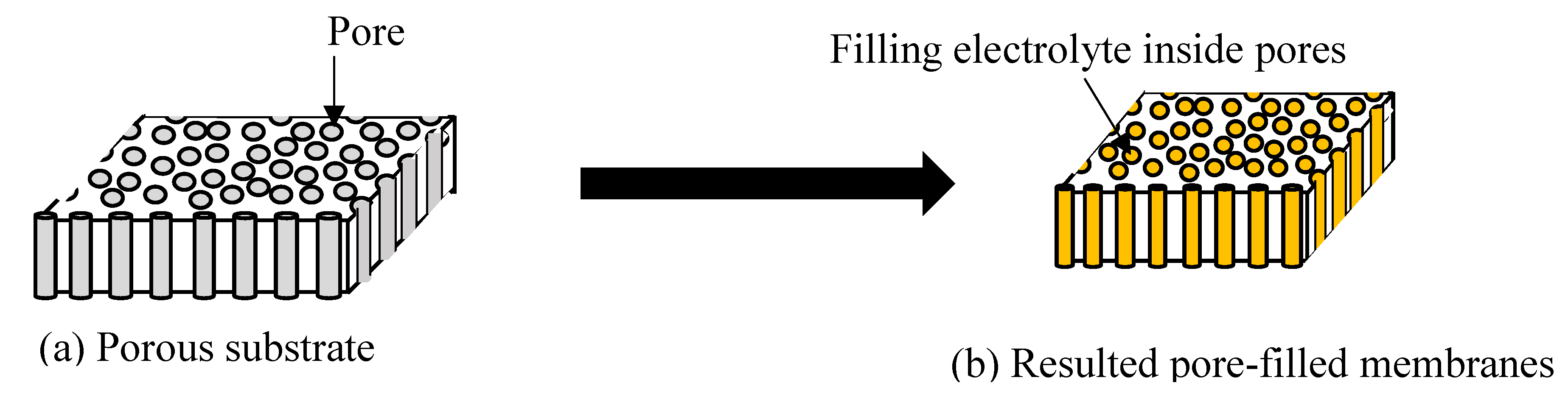
2.5. Pore-Filling Electrolyte Membranes
3. Characterization of PEMs
3.1. Conductivity Properties
3.2. Permeations of DMFC Species
3.3. Mechanical and Thermal Stability
3.4. Morphology and Elemental Analysis
3.5. DMFC Single Cell
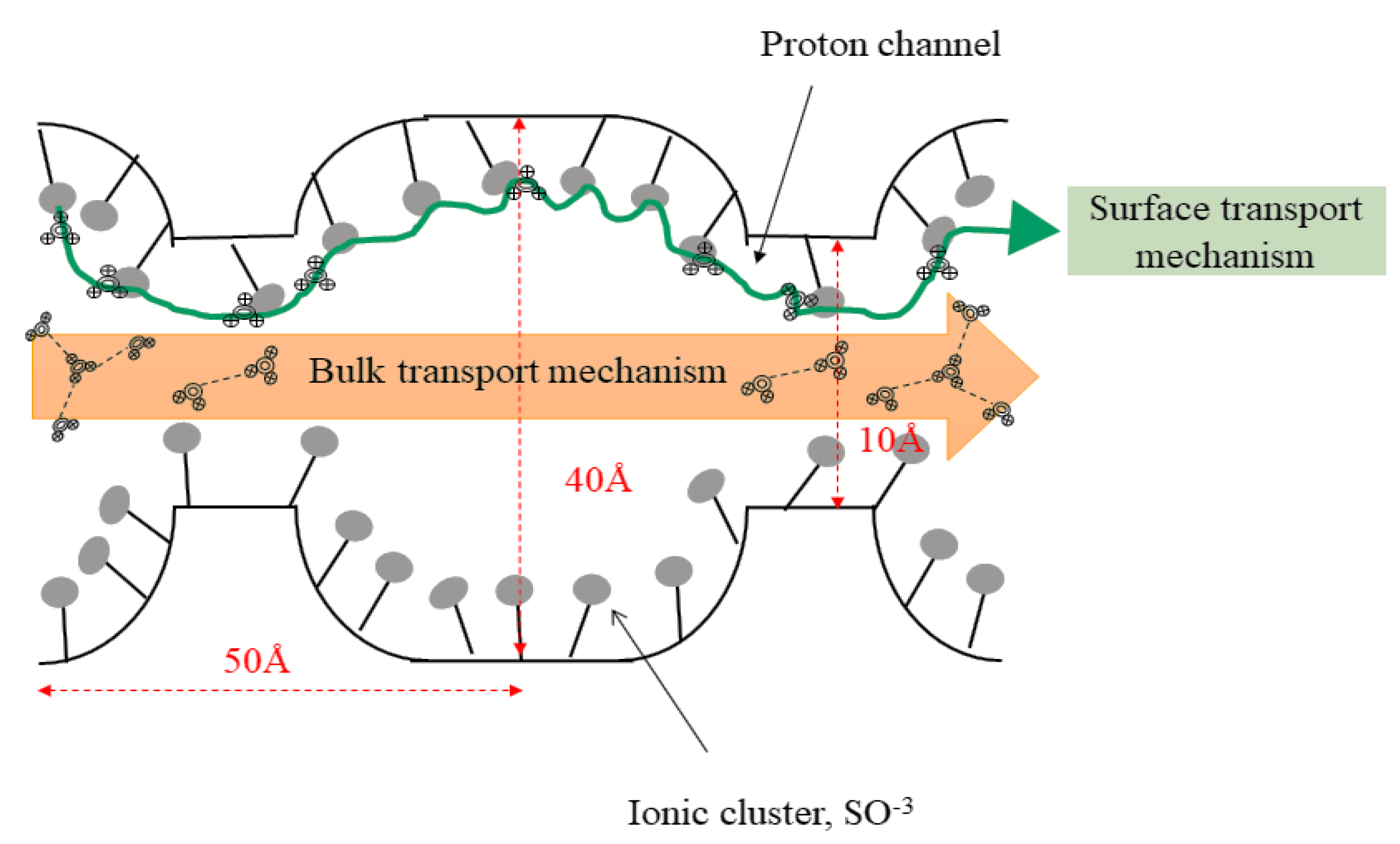
4. Transportation of Proton and Methanol within the Pores
5. Future Prospects in Electrolyte Membrane Fabrication
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Mekhilef, S.; Saidur, R.; Safari, A. Comparative study of different fuel cell technologies. Renew. Sustain. Energy Rev. 2012, 16, 981–989. [Google Scholar] [CrossRef]
- Lucia, U. Overview on fuel cells. Renew. Sustain. Energy Rev. 2014, 30, 164–169. [Google Scholar] [CrossRef]
- Daud, W.R.W.; Rosli, R.E.; Majlan, E.H.; Hamid, S.A.A.; Mohamed, R.; Husaini, T. PEM fuel cell system control: A review. Renew. Energy 2017, 113, 620–638. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Cai, W.; Xiong, J.; Ma, L.; Yang, Z.; Huang, Y.; Cheng, H. Non-destructive modification on Nafion membrane via in-situ inserting of sheared graphene oxide for direct methanol fuel cell applications. Electrochim. Acta 2018, 282, 362–368. [Google Scholar] [CrossRef]
- Hacquard, A. Improving and Understanding Direct Methanol Fuel Cell (DMFC) Performance. Master’s Thesis, Worchester Polytechnic Institute, Worchester, MA, USA, May 2005. [Google Scholar]
- Lee, L.Y.S.; Wong, K.Y. Electrocatalytic reduction of carbon dioxide. Chem 2017, 3, 717–723. [Google Scholar]
- Simakov, D.S.A. Electrocatalytic reduction of CO2. In Renewable Synthetic Fuels and Chemicals from Carbon Dioxide: Fundamentals, Catalysis, Design Consideration and Technological Challenges; Simakov, D.S.A., Ed.; Springer International Publishing: Cham, Switzerland, 2007; pp. 27–42. [Google Scholar]
- Zhang, L.; Chae, S.-R.; Hendren, Z.; Park, J.-S.; Wiesner, M.R. Recent advances in proton exchange membranes for fuel cell applications. Chem. Eng. J. 2012, 204–206, 87–97. [Google Scholar] [CrossRef]
- Kumar, G.G.; Nahm, K.S. Polymer nanocomposites—Fuel cell applications. In Advances in Nanocomposites-Synthesis, Characterization and Industrial Applications; Reddy, B., Ed.; InTech: London, UK, 2011; pp. 639–660. [Google Scholar]
- Bunlengsuwan, P.; Paradeem, N.; Sirivat, A. Influence of sulfonated graphene oxide on sulfonated polysulfone membrane for direct methanol fuel cell. Polym. Plast. Technol. Eng. 2017, 56, 1695–1703. [Google Scholar] [CrossRef]
- Mayahi, A.; Ismail, A.F.; Ilbeygi, H.; Othman, M.H.D.; Ghasemi, M.; Norddin, M.N.A.M.; Matsuura, T. Effect of operating temperature on the behavior of promising SPEEK/cSMM electrolyte membrane for DMFCs. Sep. Purif. Technol. 2013, 106, 72–81. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Falcão, D.S.; Oliveira, V.B.; Pinto, A.M.F.R. A one-dimensional and two-phase flow model of a proton exchange membrane fuel cell. J. Chem. Technol. Biotechnol. 2015, 90, 1547–1551. [Google Scholar] [CrossRef]
- Sajgure, M.; Kachare, B.; Gawhale, P.; Waghmare, S.; Jagadale, G. Direct Methanol Fuel Cell: A Review. Int. J. Curr. Eng. Technol. 2016, 6, 1–4. [Google Scholar]
- Azimi, M.; Peighambardoust, S.J.; Hosseini, M.G. Modification of Nafion membranes with different clays for direct methanol fuel cell applications. In Proceedings of the 6th Iranian Fuel Cell Seminar, Tehran, Iran, 12–13 March 2012. [Google Scholar]
- Heinzel, A.; Barragán, V.M. A review of the state-of-the-art of the methanol crossover in direct methanol fuel cells. J. Power Sources 1999, 84, 70–74. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Z.; Wen, F.; Cheng, Y.; Zhang, Y.; Wang, G. A bipolar passive DMFC stack for portable applications. Energy 2018, 144, 587–593. [Google Scholar] [CrossRef]
- Kim, D.J.; Choi, D.H.; Park, C.H.; Nam, S.Y. Characterization of the sulfonated PEEK/sulfonated nanoparticles composite membrane for the fuel cell application. Int. J. Hydrog. Energy 2016, 41, 5793–5802. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, H.B.; Rhim, J.W.; Lee, Y.M. Preparation and characterization of crosslinked PVA/SiO2 hybrid membranes containing sulfonic acid groups for direct methanol fuel cell applications. J. Membr. Sci. 2004, 240, 37–48. [Google Scholar] [CrossRef]
- Prashantha, K.; Park, S.G. Nanosized TiO2-filled sulfonated polyether sulfone proton conducting membranes for direct methanol fuel cells. J. Appl. Polym. Sci. 2005, 98, 1875–1878. [Google Scholar] [CrossRef]
- Kalappa, P.; Lee, J.H. Proton conducting membranes based on sulfonated poly (ether ether ketone)/TiO2 nanocomposites for direct methanol fuel cell. Polym. Int. 2007, 56, 371–375. [Google Scholar] [CrossRef]
- Helen, M.; Viswanathan, B.; Murthy, S.S. Synthesis and characterization of composite membranes based on α-zirconium phosphate and silicotungstic acid. J. Membr. Sci. 2007, 292, 98–105. [Google Scholar] [CrossRef]
- Hasanabadi, N.; Ghaffarian, S.R.; H-Sadrabadi, M.M. Magnetic field aligned nanocomposite proton exchange membranes based sulfonated poly (ether sulfone) and Fe2O3 nanoparticles for direct methanol fuel cell application. Int. J. Hydrog. Energy 2011, 36, 15323–15332. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Manthiram, A. Sulfonated poly (ether ether ketone) membranes with sulfonated graphene oxide fillers for direct methanol fuel cells. Int. J. Hydrog. Energy 2013, 38, 5875–5884. [Google Scholar] [CrossRef]
- He, Y.; Tong, C.; Geng, L.; Liu, L.; Lü, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Membr. Sci. 2014, 458, 36–46. [Google Scholar] [CrossRef]
- Amoozadeh, A.; Mazdarani, H.; Beydaghi, H.; Tabrizian, E.; Javanbakht, M. Novel nanocomposite membrane based on Fe3O4@TDI@TiO2-SO3H: Hydration, mechanical and DMFC study. New J. Chem. 2018, 42, 16855–16862. [Google Scholar] [CrossRef]
- Aricò, A.S.; Sebastian, D.; Schuster, M.; Bauer, B.; D’Urso, C.; Lufrano, F.; Baglio, V. Selectivity of direct methanol fuel cell membranes. Membranes 2015, 5, 793–809. [Google Scholar] [CrossRef] [PubMed]
- Parnian, M.J.; Gashoul, F.; Rowshanzamir, S. Studies on the SPEEK membrane with low degree of sulfonation as a stable proton exchange membrane for fuel cell applications. Iran. J. Hydrog. Fuel Cell 2016, 3, 221–232. [Google Scholar]
- Jiang, Y.; Hao, J.; Hou, M.; Hong, S.; Song, W.; Yi, B.; Shao, Z. A novel porous sulfonated poly (ether ether ketone)-based multi-layer composite membrane for proton exchange membrane fuel cell application. Sustain. Energy Fuels 2017, 1, 1405–1413. [Google Scholar] [CrossRef]
- Shin, D.W.; Guiver, M.D.; Lee, Y.M. Hydrocarbon-based polymer electrolyte membranes: Importance of morphology on ion transport and membrane stability. Am. Chem. Soc. 2016, 117, 4759–5809. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Matsuura, T.; Narbaitz, R.M.; Feng, C. Development and characterization of novel hydrophilic surface modifying macromolecule for polymeric membranes. J. Membr. Sci. 2005, 249, 103–112. [Google Scholar] [CrossRef]
- Norddin, M.N.A.M.; Ismail, A.F.; Rana, D.; Matsuura, T.; Mustafa, A.; Tabe-Mohammadi, A. Characterization and performance of proton exchange membranes for direct methanol fuel cell: Blending of sulfonated poly (ether ether ketone) with charged surface modifying macromolecule. J. Membr. Sci. 2008, 323, 404–413. [Google Scholar] [CrossRef]
- Gates, B.; Yin, Y.; Xia, Y. Fabrication and characterization of porous membranes with highly ordered three-dimensional periodic structure. Chem. Mater. 1999, 11, 2827–2836. [Google Scholar] [CrossRef]
- Bazzarelli, F.; Giorno, L.; Piacentini, E. Porous membranes. In Encyclopaedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–3. [Google Scholar]
- Ahmad, N.A.; Leo, C.P.; Ahmad, A.L.; Ramli, W.L.W. Membranes with great hydrophobicity: A review on preparation and characterization. Sep. Purif. Rev. 2015, 44, 109–134. [Google Scholar] [CrossRef]
- Moskvin, L.N. A classification of separation methods. Sep. Purif. Rev. 2016, 45, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Xing, P.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Wang, K.; Kaliaguine, S. Synthesis and characterization of sulfonated poly (ether ether ketone) for proton exchange membranes. J. Membr. Sci. 2004, 229, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons Ltd.: Newark, CA, USA, 2012. [Google Scholar]
- Othman, M.H.D.; Ismail, A.F.; Mustafa, A. Physico-chemical study of sulfonated poly (ether ether ketone) membranes for direct methanol fuel cell application. Malays. Polym. J. 2007, 2, 10–28. [Google Scholar]
- Ilbeygi, H.; Ismail, A.F.; Mayahi, A.; Nasef, M.M.; Jaafar, J.; Jalalvandi, E. Transport properties and direct methanol fuel cell performance of sulfonated poly (ether ether ketone)/Cloisite/triaminopyrimidine nanocomposite polymer electrolyte membrane at moderate temperature. Sep. Purif. Technol. 2013, 118, 567–575. [Google Scholar] [CrossRef]
- Al-Batty, S.; Dawson, C.; Shanmukham, S.P.; Roberts, E.P.L.; Holmes, S.M. Improvement of direct methanol fuel cell performance using a novel mordenite barrier layer. R. Soc. Chem. 2016, 4, 10850–10857. [Google Scholar] [CrossRef] [Green Version]
- Yee, R.S.L.; Zhang, K.; Ladewig, B.P. The effect of sulfonated poly (ether ether ketone) ion exchange preparation conditions on membrane properties. Membranes 2013, 3, 182–195. [Google Scholar] [CrossRef]
- Shao, Z.-G.; Hsing, I.-M. Nafion membrane coated with sulfonated poly (vinyl alcohol)-Nafion film for direct methanol fuel cells. Electrochem. Solid-State Lett. 2002, 5, A185–A187. [Google Scholar] [CrossRef]
- Mondal, S.; Soam, S.; Kundu, P.P. Reduction of methanol crossover and improved electrical efficiency in direct methanol fuel cell by the formation of a thin layer on Nafion 117 membrane: Effect of dip-coating of a blend of sulfonated PvdF-co-HFP and PBI. J. Membr. Sci. 2015, 474, 140–147. [Google Scholar] [CrossRef]
- Zhang, S.; Tanioka, A.; Matsumoto, H. Nanofibers as novel platform for high-functional ion exchanger. J. Chem. Technol. Biotechnol. 2018, 93, 2791–2803. [Google Scholar] [CrossRef]
- Awang, N.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Aziz, F.; Salleh, W.N.W.; Suradi, S.S.; Ilbeygi, H.; et al. Development of dense void-free electrospun SPEEK-Cloisite15A membrane for direct methanol fuel cell application: Optimization using response surface methodology. Int. J. Hydrog. Energy 2017, 42, 26496–26510. [Google Scholar] [CrossRef]
- Padmavathi, R.; Karthikumar, R.; Sangeetha, D. Multilayered sulfonated polysulfone/silica composite membranes for fuel cell applications. Electrochim. Acta 2012, 71, 283–293. [Google Scholar] [CrossRef]
- Neelakandan, S.; Kanagaraj, P.; Nagendran, A.; Rana, D.; Matsuura, T.; Muthumeenal, A. Enhancing proton conduction of sulfonated poly (phenylene ether ether sulfone) membrane by charged surface modifying macromolecules for H2/O2 fuel cells. Renew. Energy 2015, 78, 306–313. [Google Scholar] [CrossRef]
- Wang, L.; He, M.; Hu, Y.; Zhang, Y.; Liu, X.; Wang, G. A “4-cell” modular passive DMFC (direct methanol fuel cell) stack for portable applications. Energy 2015, 82, 229–235. [Google Scholar] [CrossRef]
- Divya, K.; Rana, D.; Alwarappan, S.; Saraswathi, M.S.S.A.; Nagendran, A. Investigating the usefulness of chitosan-based proton exchange membranes tailored with exfoliated molybdenum disulphide nanosheets for clean energy applications. Carbohydr. Polym. 2019, 208, 504–512. [Google Scholar] [CrossRef]
- Bakangura, E.; Ge, L.; Muhammad, M.; Pan, J.; Wu, L.; Xu, T. Sandwich structure SPPO/BPPO proton exchange membranes for fuel cells: Morphology-electrochemical properties relationship. J. Membr. Sci. 2015, 475, 30–38. [Google Scholar] [CrossRef]
- Li, C.; Huang, N.; Jiang, Z.; Tian, X.; Zhao, X.; Xu, Z.-L.; Yang, H.; Jiang, Z.-J. Sulfonated holey graphene oxide paper with SPEEK membranes on its both sides: A sandwiched membrane with high performance for semi-passive direct methanol fuel cells. Electrochim. Acta 2017, 250, 68–76. [Google Scholar] [CrossRef]
- Yan, X.H.; Wu, R.; Xu, J.B.; Luo, Z.; Zhao, T.S. A monolayer graphene-Nafion sandwich membrane for direct methanol fuel cells. J. Power Sources 2016, 311, 188–194. [Google Scholar] [CrossRef]
- Liu, S.; Yue, Z.; Liu, Y. Incorporation of imidazole within the metal-organic framework UiO-67 for enhanced anhydrous proton conductivity. Dalton Trans. 2015, 44, 12976–12980. [Google Scholar] [CrossRef]
- Dewi, A.U.; Divya, K.; Rana, D.; Saaraswathi, M.S.A.; Nagendran, A. Highly selective and methanol resistance polypyrrole laminated SPVdF-co-HFP/PWA proton exchange membranes for DMFC applications. Mater. Chem. Phys. 2018, 212, 533–542. [Google Scholar]
- Nguyen, T.H.; Wang, X. Fabrication of the porous polyimide film as a matrix of the composite membrane of the direct methanol fuel cell. Sep. Purif. Technol. 2009, 67, 208–212. [Google Scholar] [CrossRef]
- Shahmiradi, M.A.A.; Hosseini, S.S.; Ruan, G.; Tan, N.R. Tailoring PES nanofiltration membranes through systematic investigations on the effect of prominent design, fabrication and operational parameters. R. Soc. Chem. 2015, 5, 49080–49097. [Google Scholar]
- Salim, N.E.; Jaafar, J.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Yusof, N.; Qtaishat, M.; Matsuura, T.; Aziz, F.; Salleh, W.N.W. Preparation and characterization of hydrophilic surface modifier macromolecule modified poly (ether sulfone) photocatalytic membrane for phenol removal. Chem. Eng. J. 2018, 335, 236–247. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Zhou, H.; Nazakawa, S.; Hara, N. An extremely low methanol crossover and highly durable aromatic pore-filling electrolyte membrane for direct methanol fuel cells. Adv. Mater. 2007, 19, 592–596. [Google Scholar] [CrossRef]
- Alwin, S.; Bhat, S.D.; Sahu, A.K.; Jalajakshi, A.; Sridhar, P.; Pitchumani, S.; Shukla, A.K. Modified-pore-filled-PVDF- membrane electrolytes for direct methanol fuel cells. J. Electrochem. Soc. 2011, 158, B91–B98. [Google Scholar] [CrossRef]
- Khabibullin, A.; Minteer, S.D.; Zharov, I. The effect of sulfonic acid group content in pore-filled silica colloidal membranes on their proton conductivity and direct methanol fuel cell performance. J. Mater. Chem. A 2014, 2, 12761–12769. [Google Scholar] [CrossRef]
- Pandey, J.; Seepana, M.M.; Shukla, A. Zirconium phosphate-based proton conducting membrane for DMFC application. Int. J. Hydrog. Energy 2015, 40, 9410–9421. [Google Scholar] [CrossRef]
- Yang, T.; Li, Z.; Lyu, H.; Zheng, J.; Liu, J.; Liu, F.; Zhang, Z.; Rao, H. A graphene oxide polymer brush based cross-linked nanocomposite proton exchange membrane for direct methanol fuel cell. Rsc Adv. 2018, 8, 15740–15753. [Google Scholar] [CrossRef] [Green Version]
- Tawalbeh, M.; Al-Othman, A.; Assad, M.E.H. Grahene oxide-Nafion composite membrane for effective methanol crossover reduction in passive direct methanol fuel cells. In Proceedings of the 2018 5th International Conference on Renewable Energy: Generation and Applications (ICREGA), Al Ain, UAE, 25–28 February 2018. [Google Scholar]
- Barique, M.A.; Tsuchida, E.; Ohira, A.; Tashiro, K. Effect of elevated temperatures on the states of water and their correlation with the proton conductivity of Nafion. ACS Omega 2018, 3, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Aricò, A.S.; Baglio, V.; Antonucci, V. Direct methanol fuel cells: History, status and perspectives. In Electrocatalysis of Direct Methanol Fuel Cells; Liu, H., Zhang, J., Eds.; Verlag GmbH & Co.: Wienheim, Germany, 2009; pp. 1–78. [Google Scholar]
- Mohanty, P.M.S.; Nayak, S.K. Polymer electrolyte membrane from Cloisite 30B based solid proton conductor and sulfonated polyether ether ketone/polyvinylidene fluoride-co-hexafluoro propylene blends for direct methanol fuel cell. RSC Adv. 2014, 4, 61178–61186. [Google Scholar]
- Bagheri, A.; Javanbakht, M.; Beydaghi, H.; Salarizadeh, P.; Shabanikia, A.; Amoli, H.S. Sulfonated poly(etheretherketone) and sulfonated polyvinylidene fluoride-co-hexafluoropropylene based blend proton exchange membranes for direct methanol fuel cell applications. RSC Adv. 2016, 6, 39500. [Google Scholar] [CrossRef]
- Sonpingkam, S.; Pattavarakorn, D. Mechanical properties of sulfonated poly (ether ether ketone) nanocomposite membranes. Int. J. Chem. Eng. Appl. 2014, 5, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Pagidi, A.; Arthanareeswaran, G.; Seepana, M.M. Synthesis of highly stable PTFE-ZrP-PVA composite membrane for high-temperature direct methanol fuel cell. Int. J. Hydrogen Energy 2019, article in press. [Google Scholar] [CrossRef]
- Ercelik, M.; Ozden, A.; Devrim, Y.; Colpan, C.O. Investigation of Nafion based composite membranes on the performance of DMFCs. Int. J. Hydrogen Energy 2017, 42, 2658–2668. [Google Scholar] [CrossRef]
- Yang, C.C.; Lee, Y.-J.; Yang, J.M. Direct methanol fuel cell (DMFC) based on PVA/MMT composite polymer membranes. J. Power Sources 2009, 188, 30–37. [Google Scholar] [CrossRef]
- Hwang, H.Y.; Kim, S.J.; Oh, D.Y.; Nam, S.Y. Proton conduction and methanol transport through sulfonated poly(styrene-b-ethylene/butylenes-b-styrene) copolymer/clay nanocomposite. Macromol. Res. 2011, 19, 1–6. [Google Scholar] [CrossRef]
- Cheng, T.; Feng, M.; Huang, Y.; Liu, X. SGO-SPEN-based highly selective polyer electrolyte membranes for direct methanol fuel cells. Ionics 2017, 23, 2143–2152. [Google Scholar] [CrossRef]
- Jaafar, J.; Ismail, A.F.; Matsuura, T.; Nagai, K. Performance of SPEEK based polymer-nanoclay inorganic membrane for DMFC. J. Membr. Sci. 2011, 382, 202–211. [Google Scholar] [CrossRef]
- Muthumeenal, A.; Saraswathi, M.S.A.; Rana, D.; Negendran, A. Fabrication and electrochemical properties of highly selective SPES/GO composite membranes for direct methanol fuel cells. J. Envrion. Chem. Eng. 2017, 5, 3828–3833. [Google Scholar] [CrossRef]
- Parthiban, V.; Akula, S.; Sahu, A.K. Surfactant templated nanoporous carbon-nafion hybrid membranes for direct methanol fuel cells with reduced methanol crossover. J. Membr. Sci. 2017, 541, 127–136. [Google Scholar] [CrossRef]
- Ranjini, M.; Yoo, D.J.; Kumar, G.G. Sulfonated Fe3O4@SiO2 nanorods incorporated sPVdF nanocomposite membranes for DMFC applications. J. Membr. Sci. 2018, 555, 407–506. [Google Scholar] [CrossRef]
- Sun, H.; Tang, B.; Wu, P. Two-dimensional zeolitic imidazolate framework/carbon nanotube hybrid networks modified proton exchange membranes for improving transport properties. Appl. Mater. Interfaces 2017, 9, 35075–35085. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, Z.; Ying, W.; Chen, L.; Liu, Y.; Wang, X.; Jiang, Z.-J.; Chen, B.; Peng, X. A DNA-threaded ZIF-8 membrane with high proton conductivity and low methanol permeability. Adv. Mater. 2017, 1705155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.-P.; Hwang, B.-J. Synthesis and characterization of hydrated phosphor-silicate glass membrane prepared by an accelerated sol-gel process with water/vapor management. J. Mater. Chem. 2005, 34. [Google Scholar] [CrossRef]
- Yoshida, T.; Tokumasu, T. Molecular dynamic study of proton transfer including Grotthuss mechanism in polymer electrolyte membrane. ECS Trans. 2010, 33, 1055–1065. [Google Scholar]
- Luduena, G.A.T.; Kuhne, D.; Sebastiani, D. Mixed Grotthuss and vehicle transport mechanism in proton conducting polymers from Ab initio molecular dynamic simulations. Chem. Mater. 2011, 23, 1424–1429. [Google Scholar] [CrossRef]
- Zhai, S.; Dai, W.; Lin, J.; He, S.; Zhang, B.; Chen, L. Enhanced proton conductivity in sulfonated poly (ether ether ketone) membranes by incorporating sodium dodecyl benzene sulfonate. Polym. J. 2019, 11, 203. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Lu, T.; Liu, C.; Xing, W. Low methanol permeable composite Nafion/silica/PWA membranes for low temperature direct methanol fuel cells. Electrochim. Acta 2005, 50, 3280–3285. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Wu, H.; Zheng, B.; Jiang, Z.; Hao, X.; Wang, B. Effect of zeolites on chitosan/zeolite hybrid membranes for direct methanol fuel cell. J. Power Sources 2008, 178, 9–19. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Rambabu, G.; Bhat, S.D.; Raj, V. Pectin based nanocomposite membranes as green electrolytes for direct methanol fuel cells. Arabian J. Chem. 2020, 13, 2024–2040. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K.; Basri, S.; Shyuan, L.K.; Masdar, M.S.; Nordin, D. Enhanced proton conductivity and methanol permeability reduction via sodium alginate electrolyte-sulfonated graphene oxide bio-membrane. Nanoscale Res. Lett. 2018, 13, 1–16. [Google Scholar] [CrossRef]
- Balsara, N.P.; Beers, K.M. Proton conduction in materials comprising conducting domains with widths less than 6 nm. Eur. Polym. J. 2011, 47, 647–650. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Luo, Q.; Chen, Z.; Yao, L.; Fu, P.; Lin, Z. The effect of electrolyte concentration on electrochemical impedance for evaluating polysulfone membranes. Envr. Sci. Water Res. Technol. 2018, 4, 1145–1151. [Google Scholar] [CrossRef]
- Barbosa, P.; Rosero-Navarro, N.C.; Shi, F.-N.; Figueiredo, M.L. Protonic conductivity of nanocrystalline zeolitic imidazolate framework 8. Electrochim. Acta 2015, 153, 19–27. [Google Scholar] [CrossRef]
- Yang, L.; Tang, B.; Wu, P. Metal organic framework-graphene oxide composites: A facile method to highly improve the proton conductivity of PEM operated under low humidity. J. Mater. Chem. A 2015, 3, 15838–15842. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.R.; Chang, C.-H.; Wu, K.C.-W.; Tung, K.-L.; Lue, S.J. Highly zeolite-loaded polyvinyl alcohol composite membranes for alkaline fuel-cell electrolytes. Polym. J. 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Alberti, G.; Casciola, M. Composite membranes for medium-temperature PEM fuel cells. Annu. Rev. Mater. Res. 2003, 33, 129–154. [Google Scholar] [CrossRef]
- Kumar, M.; Khan, M.A.; Al-Othman, Z.A.; Choong, T.S.Y. Recent developments in ion-exchange membranes and their applications in electrochemical processes for in-situ ion substitutions, separation and water splitting. Sep. Purif. Rev. 2013, 42, 187–261. [Google Scholar] [CrossRef]








| PEMs | Proton Conductivity (mS/cm) | Methanol Permeability (× 10−7 cm2/s) | Water Uptake | Methanol Uptake | Swelling Ratio | Ref. |
|---|---|---|---|---|---|---|
| Poly (vinyl alcohol) /montmorillonite (PVA/MMT) | 36.8 | 36.7 | NA | NA | improved | [72] |
| Sulfonated poly (styrene-b-ethylene/butylenes-b-styrene) copolymer/Cloisite®Na+ (S-SEBS/Na+) | 142 | 6.2 | Relatively high | NA | NA | [73] |
| Sulfonated poly (arylene ether nitrile)/sulfonated graphene oxide (SPEN/SGO) | 109 | 1.7 | 42.6 | NA | 13.57 | [74] |
| Sulfonated poly (ether ether ketone)/Cloisite15A®/triaminopyrimidine (SPEEK/Cloisite15A®/TAP) | 16.3 | 1.3 | 26.19 | NA | NA | [75] |
| Sulfonated poly (ether sulfone)/graphene oxide (SPES/GO) | 4.3 | 0.492 | 40.1 | NA | NA | [76] |
| Nafion/Nanoporous carbon (Nafion/NPC) | 75.1 | 9.8 | NA | NA | NA | [77] |
| Sulfonated poly (cinlidene fluoride)/sulfonated magnetite @silica (sPVdF/sFe3O4@SiO2 | 64 | 20 | 33.6 | NA | NA | [78] |
| Zeolitic imidazolate framework-carbon nanotube hybrid/sulfonated poly (ether ether ketone) (ZCN/SPEEK) | 206 | 0.0245 | 40.2 | NA | 8.6 | [79] |
| Zeolitic imidazolate framework-8/deoxyribonucleic acid (ZIF-8@DNA) | 170 | 0.125 | NA | NA | No swelling | [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junoh, H.; Jaafar, J.; Nordin, N.A.H.M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N. Performance of Polymer Electrolyte Membrane for Direct Methanol Fuel Cell Application: Perspective on Morphological Structure. Membranes 2020, 10, 34. https://doi.org/10.3390/membranes10030034
Junoh H, Jaafar J, Nordin NAHM, Ismail AF, Othman MHD, Rahman MA, Aziz F, Yusof N. Performance of Polymer Electrolyte Membrane for Direct Methanol Fuel Cell Application: Perspective on Morphological Structure. Membranes. 2020; 10(3):34. https://doi.org/10.3390/membranes10030034
Chicago/Turabian StyleJunoh, Hazlina, Juhana Jaafar, Nik Abdul Hadi Md Nordin, Ahmad Fauzi Ismail, Mohd Hafiz Dzarfan Othman, Mukhlis A. Rahman, Farhana Aziz, and Norhaniza Yusof. 2020. "Performance of Polymer Electrolyte Membrane for Direct Methanol Fuel Cell Application: Perspective on Morphological Structure" Membranes 10, no. 3: 34. https://doi.org/10.3390/membranes10030034






