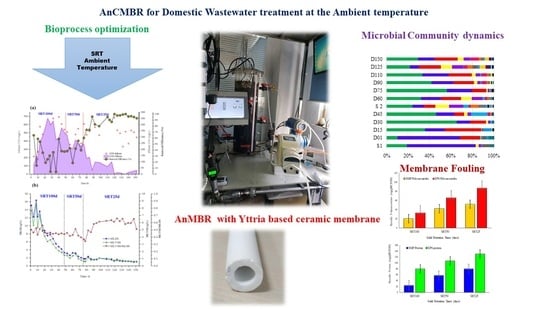
Effects of Solids Retention Time on the Anaerobic Membrane Bioreactor with Yttria-Based Ceramic Membrane Treating Domestic Wastewater at Ambient Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioreactor and Start-Up
2.2. Domestic Wastewater Characteristics
2.3. Analysis of Physico-Chemical Parameters
2.4. Microbial Community Analysis
2.5. Membrane Fouling Analysis
2.6. Statistical Analysis
3. Results and Discussions
3.1. Impact of SRT on AnMBR Performance
3.1.1. COD Removal
3.1.2. MLSS and MLVSS
3.1.3. VFA and Alkalinity
3.2. Effect of SRT on Membrane Fouling
3.2.1. TMP and Flux Evolution
3.2.2. Sludge Particle Size Distribution
3.2.3. SMPs and EPSs
3.2.4. Excitation Emission Matrix of Extracted EPS
3.2.5. FTIR Analysis
3.2.6. Factors of Membrane Fouling
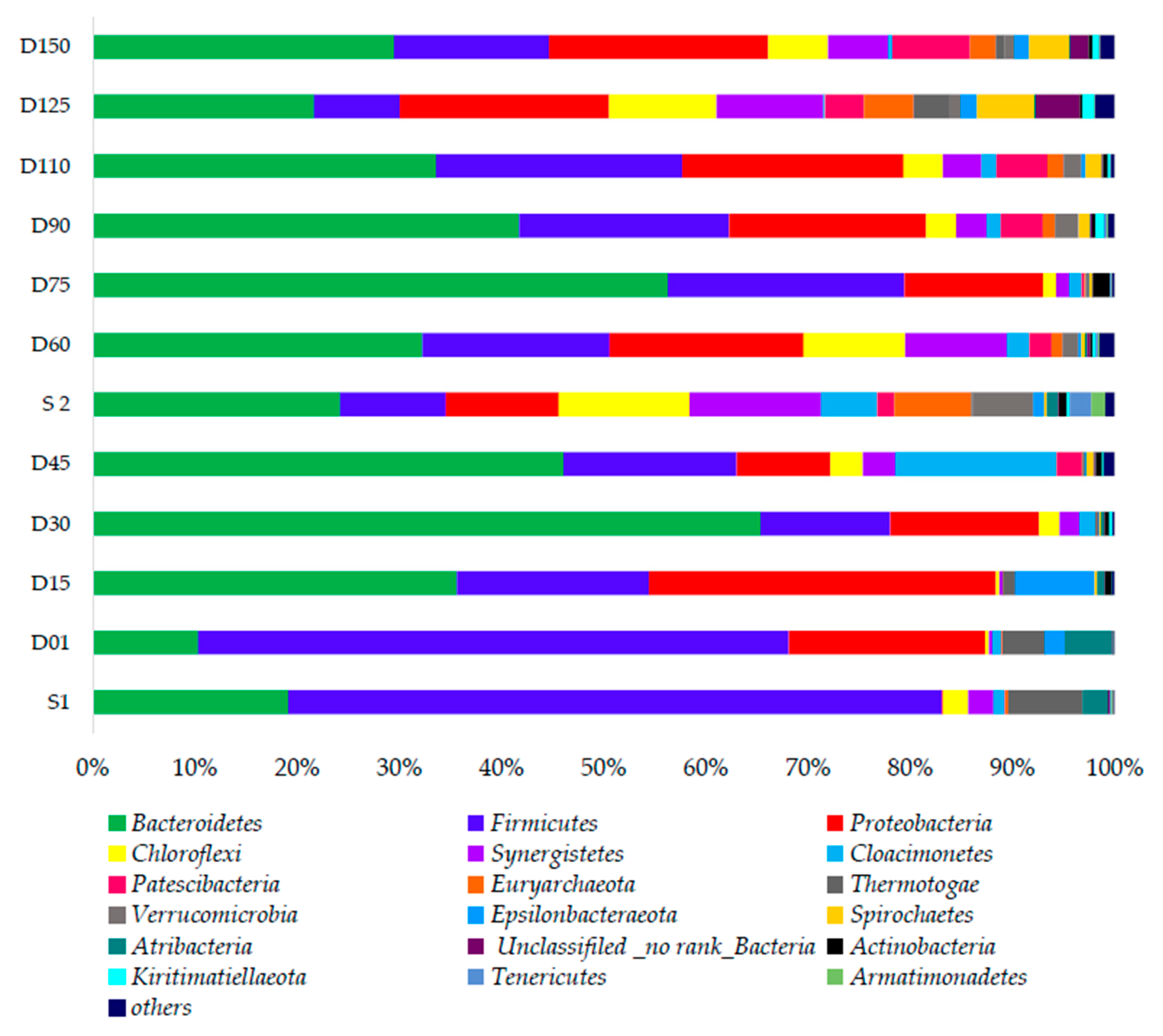
3.3. Evolution of Dominant Microbial Community
3.3.1. Bacterial Community Responses
3.3.2. Archaeal Community Responses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, A.L.; Stadler, L.B.; Love, N.G.; Skerlos, S.J.; Raskin, L. Perspectives on anaerobic membrane bioreactor treatment of domestic wastewater: A critical review. Bioresour. Technol. 2012, 122, 149–159. [Google Scholar] [CrossRef]
- Ozgun, H.; Dereli, R.K.; Ersahin, M.E.; Kinaci, C.; Spanjers, H.; van Lier, J.B. A review of anaerobic membrane bioreactors for municipal wastewater treatment: Integration options, limitations and expectations. Sep. Purif. Technol. 2013, 118, 89–104. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J. Model to couple anaerobic process kinetics with biological growth equilibrium thermodynamics. Environ. Sci. Technol. 2011, 45, 6838–6844. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Benítez, A.; Ferrer, F.J.; Greses, S.; Ruiz-Martínez, A.; Fatone, F.; Eusebi, A.L.; Mondéjar, N.; Ferrer, J.; Seco, A. AnMBR, reclaimed water and fertigation: Two case studies in Italy and Spain to assess economic and technological feasibility and CO2 emissions within the EU Innovation Deal initiative. J. Clean. Prod. 2020, 270, 122398. [Google Scholar] [CrossRef]
- Gouveia, J.; Plaza, F.; Garralon, G.; Fdz-Polanco, F.; Pena, M. Long-term operation of a pilot scale anaerobic membrane bioreactor (AnMBR) for the treatment of municipal wastewater under psychrophilic conditions. Bioresour. Technol. 2015, 185, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane Bioreactor (MBR) technology for wastewater treatment and reclamation: Membrane fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretel, R.; Robles, A.; Ruano, M.V.; Seco, A.; Ferrer, J. Economic and environmental sustainability of submerged anaerobic MBR-based (AnMBR-based) technology as compared to aerobic-based technologies for moderate-/high-loaded urban wastewater treatment. J. Environ. Manag. 2016, 166, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Skouteris, G.; Hermosilla, D.; López, P.; Negro, C.; Blanco, Á. Anaerobic membrane bioreactors for wastewater treatment: A review. Chem. Eng. J. 2012, 198, 138–148. [Google Scholar] [CrossRef]
- Ho, J.; Sung, S. Methanogenic activities in anaerobic membrane bioreactors (AnMBR) treating synthetic municipal wastewater. Bioresour. Technol. 2010, 101, 2191–2196. [Google Scholar] [CrossRef]
- Nges, I.A.; Liu, J. Effects of solid retention time on anaerobic digestion of dewatered-sewage sludge in mesophilic and thermophilic conditions. Renew. Energy 2010, 35, 2200–2206. [Google Scholar] [CrossRef]
- Magdalena, J.A.; Gonzalez-Fernandez, C. Archaea inhibition: Strategies for the enhancement of volatile fatty acids production from microalgae. Waste Manag. 2020, 102, 222–230. [Google Scholar] [CrossRef]
- Cho, K.; Seo, K.W.; Shin, S.G.; Lee, S.; Park, C. Process stability and comparative rDNA/rRNA community analyses in an anaerobic membrane bioreactor with silicon carbide ceramic membrane applications. Sci. Total Environ. 2019, 666, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Seib, M.D.; Berg, K.J.; Zitomer, D.H. Influent wastewater microbiota and temperature influence anaerobic membrane bioreactor microbial community. Bioresour. Technol. 2016, 216, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.L.; Skerlos, S.J.; Raskin, L. Anaerobic membrane bioreactor treatment of domestic wastewater at psychrophilic temperatures ranging from 15 °C to 3 °C. Environ. Sci. Water Res. Technol. 2015, 1, 56–64. [Google Scholar] [CrossRef]
- Bandara, W.; Kindaichi, T.; Satoh, H.; Sasakawa, M.; Nakahara, Y.; Takahashi, M.; Okabe, S. Anaerobic treatment of municipal wastewater at ambient temperature: Analysis of archaeal community structure and recovery of dissolved methane. Water Res. 2012, 46, 5756–5764. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-H.; Tsai, C.-Y.; Chen, S.-Y.; Sung, S.; Lin, J.-G. Treatment of campus domestic wastewater using ambient-temperature anaerobic fluidized membrane bioreactors with zeolites as carriers. Int. Biodeterior. Biodegrad. 2019, 136, 49–54. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Jiang, M.; Gapani, D.E.; Bi, S.; Dong, R. AnMBR as alternative to conventional CSTR to achieve efficient methane production from thermal hydrolyzed sludge at short HRTs. Energy 2018, 159, 588–598. [Google Scholar] [CrossRef]
- Yang, T.; Xiong, H.; Liu, F.; Yang, Q.; Xu, B.; Zhan, C. Effect of UV/TiO2 pretreatment on fouling alleviation and mechanisms of fouling development in a cross-flow filtration process using a ceramic UF membrane. Chem. Eng. J. 2019, 358, 1583–1593. [Google Scholar] [CrossRef]
- El-Fadel, M.; Sleem, F.; Hashisho, J.; Saikaly, P.E.; Alameddine, I.; Ghanimeh, S. Impact of SRT on the performance of MBRs for the treatment of high strength landfill leachate. Waste Manag. 2018, 73, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Y.; Bacaksiz, A.M.; Bayrak, H.; Vergili, I.; Gönder, Z.B.; Hasar, H.; Yilmaz, G. Investigation of membrane fouling in an anaerobic membrane bioreactor (AnMBR) treating pharmaceutical wastewater. J. Water Process. Eng. 2019, 31, 100822. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Performance of submerged anaerobic membrane bioreactor at different SRTs for domestic wastewater treatment. J. Biotechnol. 2013, 164, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Yurtsever, A.; Calimlioglu, B.; Sahinkaya, E. Impact of SRT on the efficiency and microbial community of sequential anaerobic and aerobic membrane bioreactors for the treatment of textile industry wastewater. Chem. Eng. J. 2017, 314, 378–387. [Google Scholar] [CrossRef]
- Aslam, M.; McCarty, P.L.; Shin, C.; Bae, J.; Kim, J. Low energy single-staged anaerobic fluidized bed ceramic membrane bioreactor (AFCMBR) for wastewater treatment. Bioresour. Technol. 2017, 240, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Ma, X.; Zhong, Z.; Zhang, F.; Xing, W.; Fan, Y. Performance of ceramic nanofiltration membrane for desalination of dye solutions containing NaCl and Na2SO4. Desalination 2017, 404, 102–111. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef] [PubMed]
- Zeuner, B.; Ovtar, S.; Persson, Å.H.; Foghmoes, S.; Berendt, K.; Ma, N.; Kaiser, A.; Negra, M.D.; Pinelo, M. Surface treatments and functionalization of metal-ceramic membranes for improved enzyme immobilization performance. J. Chem. Technol. Biotechnol. 2020, 95, 993–1007. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.-J.; Chen, C.-S. Hollow fiber membrane of yttrium-stabilized zirconia and strontium-doped lanthanum manganite dual-phase composite for oxygen separation. J. Membr. Sci. 2009, 340, 266–271. [Google Scholar] [CrossRef]
- Nilusha, R.T.; Wang, T.; Wang, H.; Yu, D.; Zhang, J.; Wei, Y. Optimization of In Situ Backwashing Frequency for Stable Operation of Anaerobic Ceramic Membrane Bioreactor. Processes 2020, 8, 545. [Google Scholar] [CrossRef]
- Martin Vincent, N.; Wei, Y.; Zhang, J.; Yu, D.; Tong, J. Characterization and dynamic shift of microbial communities during start-up, overloading and steady-state in an anaerobic membrane bioreactor. Int. J. Environ. Res. Public Health 2018, 15, 1399. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wang, X.; Sun, S.; Zhao, Y.; Hu, C. Effects of influent C/N ratios and treatment technologies on integral biogas upgrading and pollutants removal from synthetic domestic sewage. Sci. Rep. 2017, 7, 10897. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Kumar, P.; Mehrotra, I. Nitrogen and Phosphorus Requirement in Anaerobic Process: A Review. Environ. Eng. Manag. J. 2015, 14, 769–780. [Google Scholar] [CrossRef]
- Zhang, C.; Qian, Y.; Yuan, L.; He, S.; Wang, Y.; Wang, L. Nutrients removal performance of a denitrifying phosphorus removal process in alternate anaerobic/anoxic-aerobic double membrane bioreactors (A2N-DMBR). Water Sci. Technol. 2018, 78, 1741–1752. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, J.; Wei, Y.; Shen, P. Effects of ferric oxide on the microbial community and functioning during anaerobic digestion of swine manure. Bioresour. Technol. 2019, 287, 121393. [Google Scholar] [CrossRef]
- Lowry, O.H. Protein measurement with the Folin Phenol reagent. J. Biol. Chem. 1951, 265–275. [Google Scholar]
- Donoso-Bravo, A.; Bandara, W.M.; Satoh, H.; Ruiz-Filippi, G. Explicit temperature-based model for anaerobic digestion: Application in domestic wastewater treatment in a UASB reactor. Bioresour. Technol. 2013, 133, 437–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, H.; Lee, H.S. The effect of solids retention time on dissolved methane concentration in anaerobic membrane bioreactors. Environ. Technol. 2013, 34, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Pagilla, K.R.; Kim, H.-J. Lab-scale study of an anaerobic membrane bioreactor (AnMBR) for dilute municipal wastewater treatment. Biotechnol. Bioprocess. Eng. 2010, 15, 704–708. [Google Scholar] [CrossRef]
- Kocadagistan, E.; Topcu, N. Treatment investigation of the Erzurum City municipal wastewaters with anaerobic membrane bioreactors. Desalination 2007, 216, 367–376. [Google Scholar] [CrossRef]
- Huang, Z.; Ong, S.L.; Ng, H.Y. Submerged anaerobic membrane bioreactor for low-strength wastewater treatment: Effect of HRT and SRT on treatment performance and membrane fouling. Water Res. 2011, 45, 705–713. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef] [PubMed]
- Dereli, R.K.; van der Zee, F.P.; Heffernan, B.; Grelot, A.; van Lier, J.B. Effect of sludge retention time on the biological performance of anaerobic membrane bioreactors treating corn-to-ethanol thin stillage with high lipid content. Water Res. 2014, 49, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Dzakpasu, M.; Li, Q.; Chen, R. Anaerobic membrane bioreactors for domestic wastewater treatment. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–165. [Google Scholar] [CrossRef]
- Meng, F.; Chae, S.R.; Drews, A.; Kraume, M.; Shin, H.S.; Yang, F. Recent advances in membrane bioreactors (MBRs): Membrane fouling and membrane material. Water Res. 2009, 43, 1489–1512. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, S.; Fukushima, T.; Ishii, S.; Okabe, S. Membrane fouling potentials and cellular properties of bacteria isolated from fouled membranes in a MBR treating municipal wastewater. Water Res. 2016, 100, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Song, Y.; Yu, H.; Xia, S.; Hermanowicz, S.W. The effect of solids retention times on the characterization of extracellular polymeric substances and soluble microbial products in a submerged membrane bioreactor. Bioresour. Technol. 2014, 163, 395–398. [Google Scholar] [CrossRef]
- Zhen, G.; Pan, Y.; Lu, X.; Li, Y.-Y.; Zhang, Z.; Niu, C.; Kumar, G.; Kobayashi, T.; Zhao, Y.; Xu, K. Anaerobic membrane bioreactor towards biowaste biorefinery and chemical energy harvest: Recent progress, membrane fouling and future perspectives. Renew. Sustain. Energy Rev. 2019, 115, 109392. [Google Scholar] [CrossRef]
- Watanabe, R.; Nie, Y.; Wakahara, S.; Komori, D.; Li, Y.Y. Investigation on the response of anaerobic membrane bioreactor to temperature decrease from 25 degrees C to 10 degrees C in sewage treatment. Bioresour. Technol. 2017, 243, 747–754. [Google Scholar] [CrossRef]
- Bae, T.-H.; Tak, T.-M. Interpretation of fouling characteristics of ultrafiltration membranes during the filtration of membrane bioreactor mixed liquor. J. Membr. Sci. 2005, 264, 151–160. [Google Scholar] [CrossRef]
- Itonaga, T.; Kimura, K.; Watanabe, Y. Influence of suspension viscosity and colloidal particles on permeability of membrane used in membrane bioreactor (MBR). Water Sci. Technol. 2004, 50, 301–309. [Google Scholar] [CrossRef]
- Xia, T.; Gao, X.; Wang, C.; Xu, X.; Zhu, L. An enhanced anaerobic membrane bioreactor treating bamboo industry wastewater by bamboo charcoal addition: Performance and microbial community analysis. Bioresour. Technol. 2016, 220, 26–33. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation-Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef] [PubMed]
- Hudson, N.; Baker, A.; Reynolds, D. Fluorescence analysis of dissolved organic matter in natural, waste and polluted waters—A review. River Res. Appl. 2007, 23, 631–649. [Google Scholar] [CrossRef]
- Zheng, L.; Jiao, Y.; Zhong, H.; Zhang, C.; Wang, J.; Wei, Y. Insight into the magnetic lime coagulation-membrane distillation process for desulfurization wastewater treatment: From pollutant removal feature to membrane fouling. J. Hazard. Mater. 2020, 391, 122202. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, T.; Ly, Q.V.; Asif, M.B.; Ng, H.Y.; Zhang, Z. Fate and role of fluorescence moieties in extracellular polymeric substances during biological wastewater treatment: A review. Sci. Total Environ. 2020, 718, 137291. [Google Scholar] [CrossRef]
- Yu, J.; Xiao, K.; Xue, W.; Shen, Y.-X.; Tan, J.; Liang, S.; Wang, Y.; Huang, X. Excitation-emission matrix (EEM) fluorescence spectroscopy for characterization of organic matter in membrane bioreactors: Principles, methods and applications. Front. Environ. Sci. Eng. 2020, 14, 1–19. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Kim, J. Recent developments in biofouling control in membrane bioreactors for domestic wastewater treatment. Sep. Purif. Technol. 2018, 206, 297–315. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q. Advances in Energy-Producing Anaerobic Biotechnologies for Municipal Wastewater Treatment. Engineering 2016, 2, 438–446. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, R.; Tian, X.; Li, Y.-Y. Characterization of the effect of surfactant on biomass adaptation and microbial community in sewage treatment by anaerobic membrane bioreactor. J. Ind. Eng. Chem. 2019, 76, 268–276. [Google Scholar] [CrossRef]
- Takada, K.; Shiba, T.; Yamaguchi, T.; Akane, Y.; Nakayama, Y.; Soda, S.; Inoue, D.; Ike, M. Cake layer bacterial communities during different biofouling stages in full-scale membrane bioreactors. Bioresour. Technol. 2018, 259, 259–267. [Google Scholar] [CrossRef]
- Riviere, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.; Jeong, Y.; Seo, K.W.; Lee, S.; Smith, A.L.; Shin, S.G.; Cho, S.K.; Park, C. Effects of changes in temperature on treatment performance and energy recovery at mainstream anaerobic ceramic membrane bioreactor for food waste recycling wastewater treatment. Bioresour. Technol. 2018, 256, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ahlert, S.; Zimmermann, R.; Ebling, J.; Konig, H. Analysis of propionate-degrading consortia from agricultural biogas plants. Microbiologyopen 2016, 5, 1027–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bialek, K.; Kumar, A.; Mahony, T.; Lens, P.N.; O’Flaherty, V. Microbial community structure and dynamics in anaerobic fluidized-bed and granular sludge-bed reactors: Influence of operational temperature and reactor configuration. Microb. Biotechnol. 2012, 5, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Greses, S.; Gaby, J.C.; Aguado, D.; Ferrer, J.; Seco, A.; Horn, S.J. Microbial community characterization during anaerobic digestion of Scenedesmus spp. under mesophilic and thermophilic conditions. Algal Res. 2017, 27, 121–130. [Google Scholar] [CrossRef]
- Ariesyady, H.D.; Ito, T.; Okabe, S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007, 41, 1554–1568. [Google Scholar] [CrossRef]
- Khanal, S.K.; Giri, B.; Nitayavardhana, S.; Gadhamshetty, V. Anaerobic bioreactors/digesters. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–279. [Google Scholar] [CrossRef]
- Wu, Z.; Miao, Y.; Wang, Z.; Mei, X. A pilot-scale anaerobic membrane bioreactor under short hydraulic retention time for municipal wastewater treatment: Performance and microbial community identification. J. Water Reuse Desalin. 2018, 8, 58–67. [Google Scholar] [CrossRef] [Green Version]







| Parameters | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|
| Time (days) | 1–50 | 51–75 | 76–150 |
| Effective volume (L) | 15 | 15 | 15 |
| HRT (h) | 48 | 48 | 48 |
| OLR (kg COD m−3 d−1) | 0.23 ± 0.09 | 0.21 ± 0.05 | 0.29 ± 0.10 |
| Cross flow rate (m/s) | 2.48 ± 0.04 | 2.45 ± 0.04 | 2.53 ± 0.08 |
| Backwashing (s d−1) | 60 | 60 | 60 |
| Temperature (°C) | 31.6 ± 2.46 | 32.01 ± 1.53 | 30.5 ± 3.14 |
| SRT (days) | 100 | 50 | 25 |
| MLSS (g/L) | 9.08 ± 4.18 | 2.70 ± 0.50 | 1.45 ± 0.36 |
| MLVSS (g/L) | 4.37 ± 2.24 | 1.21 ± 0.31 | 0.84 ± 0.27 |
| MLVSS/MLSS | 0.46 ± 0.04 | 0.43 ± 0.04 | 0.58 ± 0.12 |
| TMP (kPa) | 73.33 ± 2.01 | 75.22 ± 1.18 | 76.54 ± 4.94 |
| Flux (Lm−2 h−1) | 50.82 ± 4.39 | 49.68 ± 2.38 | 34.40 ± 13.46 |
| Permeability (Lm−2 h−1/kPa) | 0.69 ± 0.06 | 0.66 ± 0.03 | 0.44 ± 0.15 |
| Chemical | Concentration (g/L) |
|---|---|
| D (+) – Glucose monohydrate | 200 |
| Triptone | 15 |
| Calcium chloride dehydrate (CaCl.2H2O) | 25 |
| Potassium dihydrogen phosphate (KH2PO4) | 17.5 |
| Sodium hydrogen carbonate (NaHCO3) | 137.5 |
| Ammonium chloride (NH4Cl) | 95.5 |
| Sodium acetate (C2H3NaO2) | 100 |
| Magnesium sulfate (MgSO4.7H2O) | 50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilusha, R.T.; Yu, D.; Zhang, J.; Wei, Y. Effects of Solids Retention Time on the Anaerobic Membrane Bioreactor with Yttria-Based Ceramic Membrane Treating Domestic Wastewater at Ambient Temperature. Membranes 2020, 10, 196. https://doi.org/10.3390/membranes10090196
Nilusha RT, Yu D, Zhang J, Wei Y. Effects of Solids Retention Time on the Anaerobic Membrane Bioreactor with Yttria-Based Ceramic Membrane Treating Domestic Wastewater at Ambient Temperature. Membranes. 2020; 10(9):196. https://doi.org/10.3390/membranes10090196
Chicago/Turabian StyleNilusha, Rathmalgodage Thejani, Dawei Yu, Junya Zhang, and Yuansong Wei. 2020. "Effects of Solids Retention Time on the Anaerobic Membrane Bioreactor with Yttria-Based Ceramic Membrane Treating Domestic Wastewater at Ambient Temperature" Membranes 10, no. 9: 196. https://doi.org/10.3390/membranes10090196