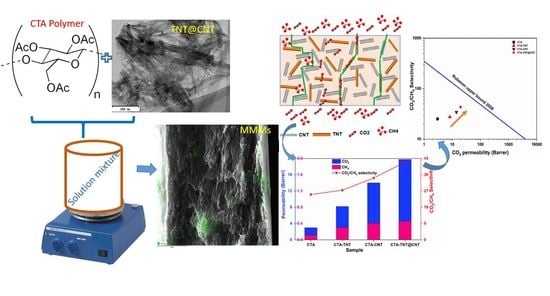
CO2/CH4 and H2/CH4 Gas Separation Performance of CTA-TNT@CNT Hybrid Mixed Matrix Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of TiO2 Nanotubes (TNT)
2.3. Modification of CNT
2.4. Synthesis of the TNT@CNT Matrix
2.5. Synthesis of Single and Hybrid Filler-Based MMMs
2.6. Characterizations
2.7. Gas Sorption and Permeation Measurements
3. Results and Discussion
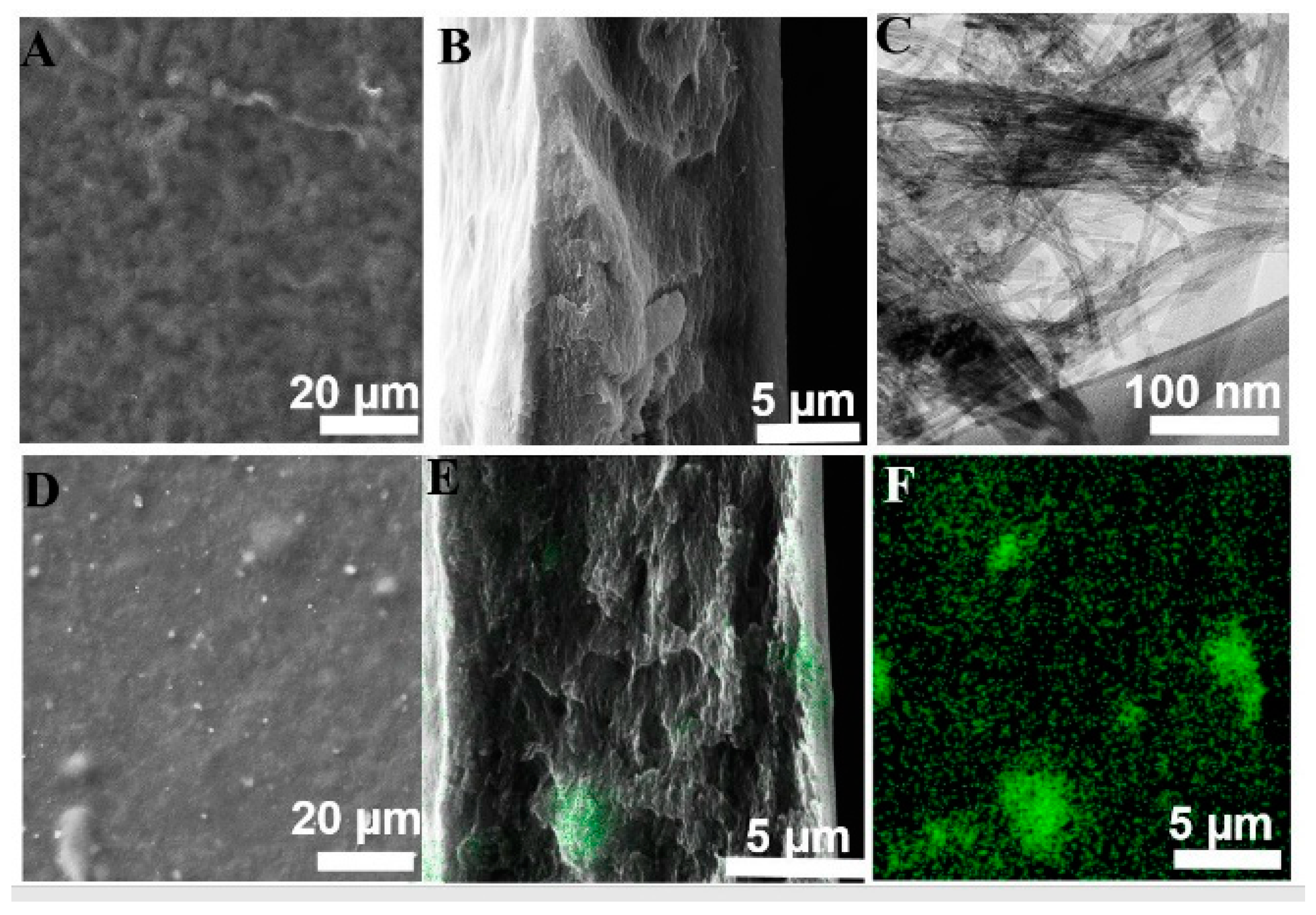
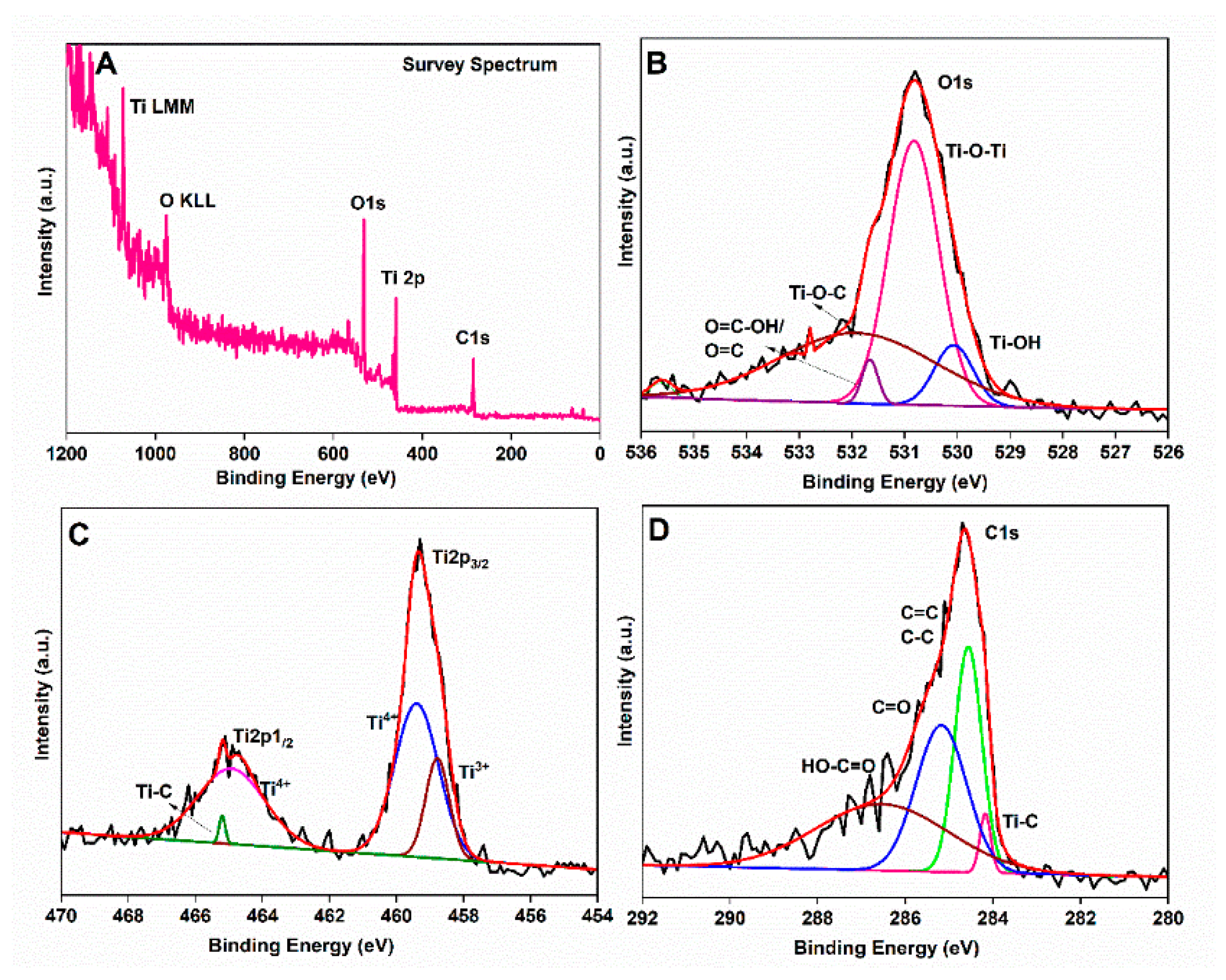
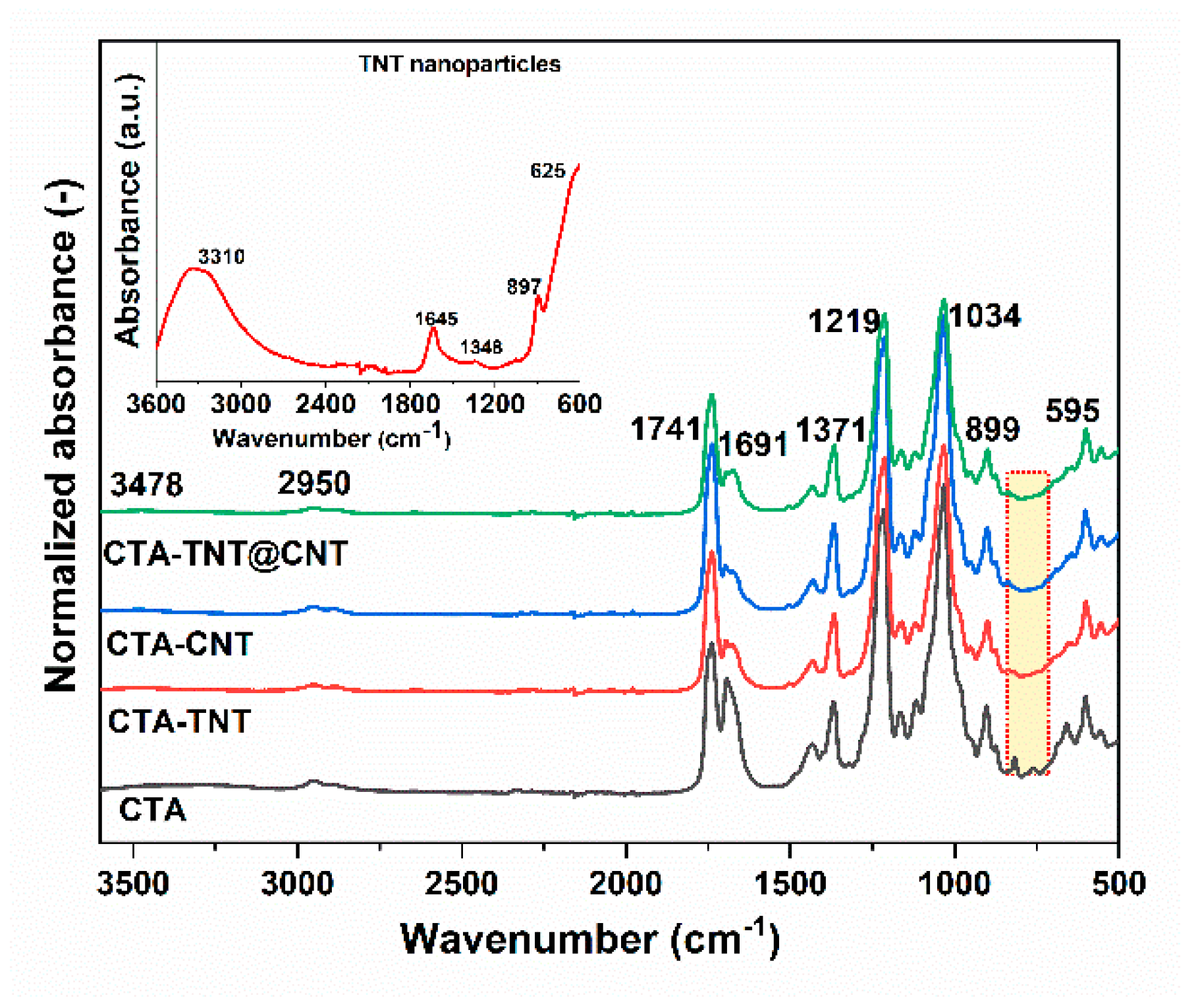
3.1. Physico-Chemical Property Evaluation
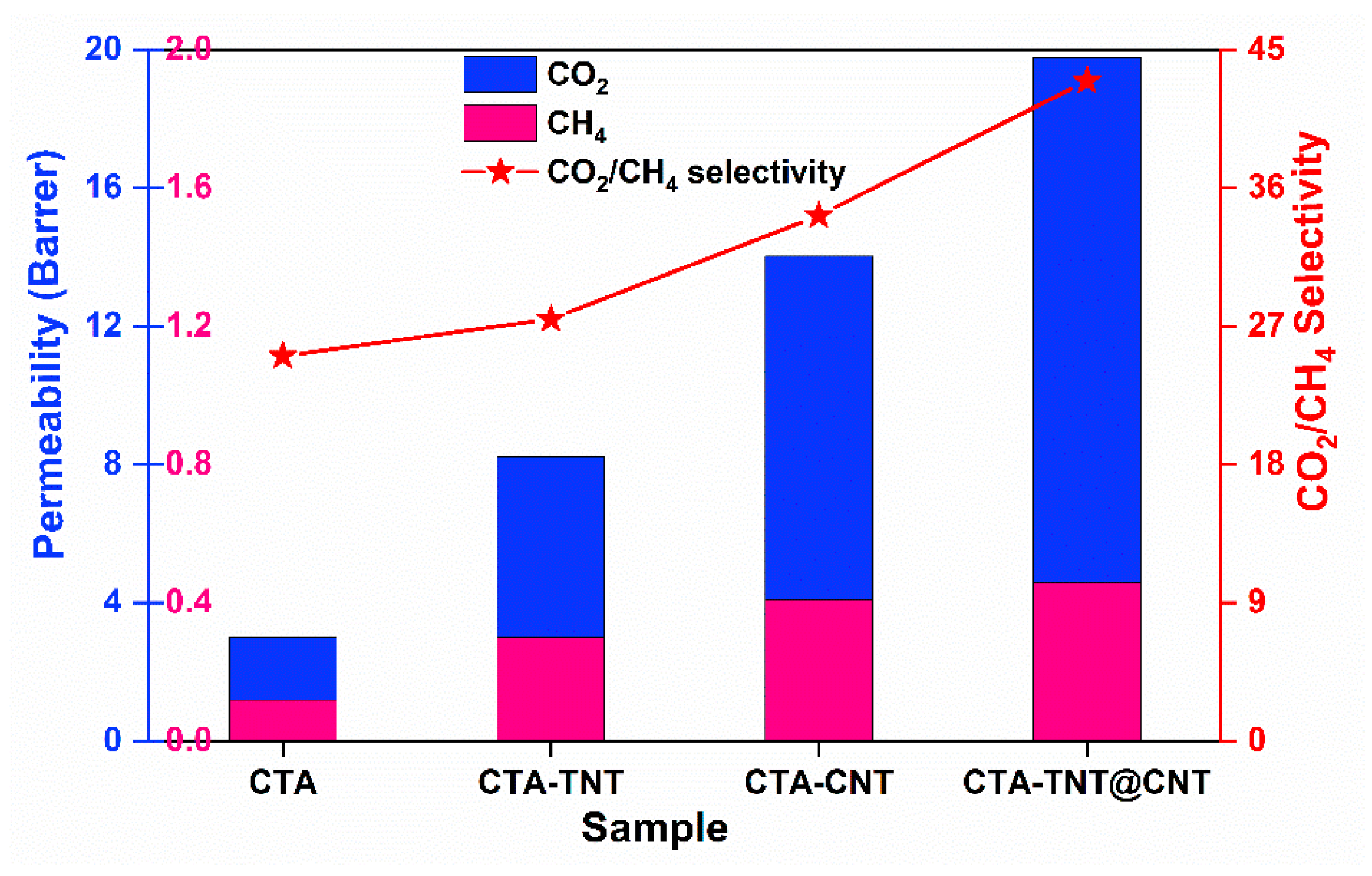
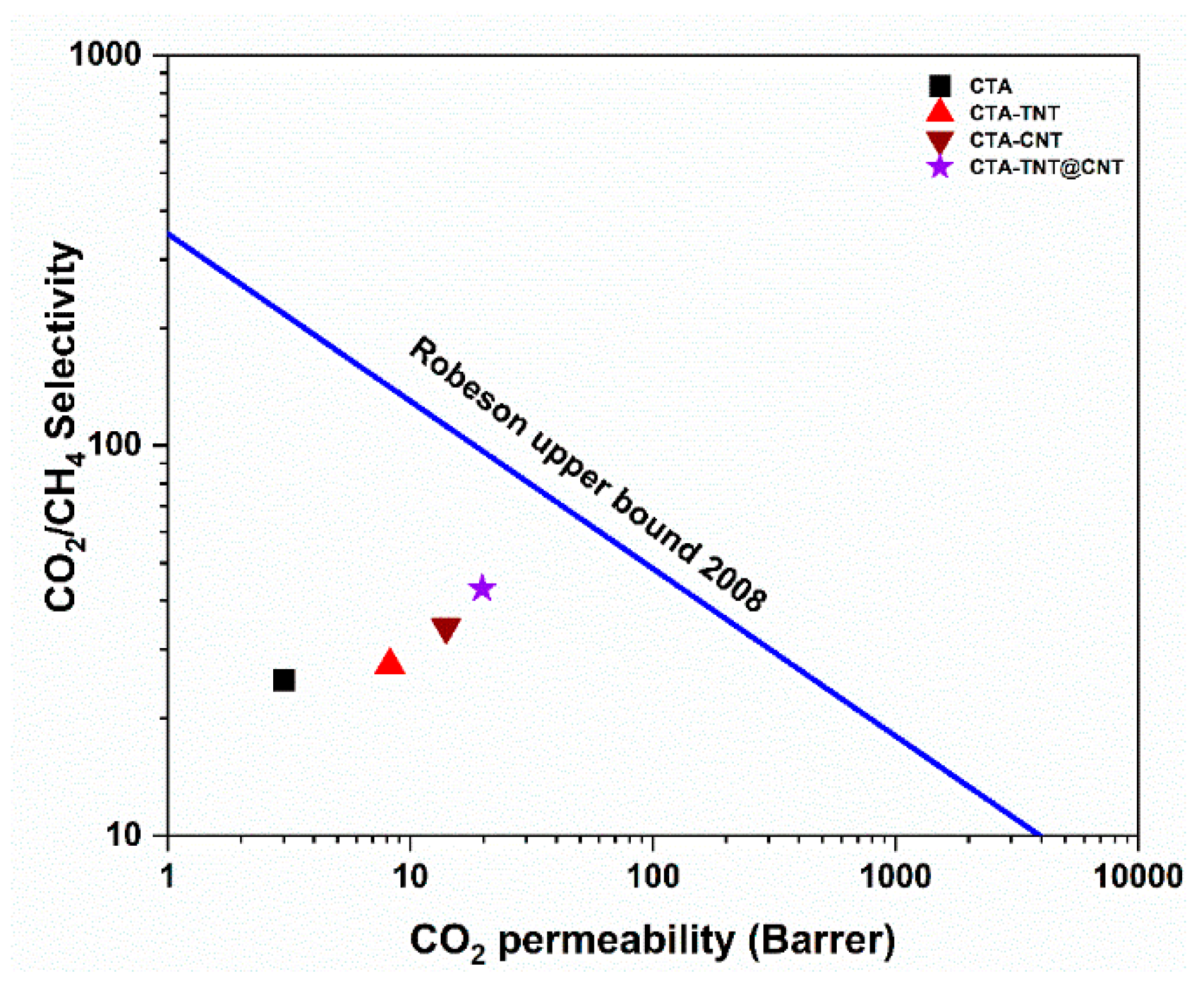
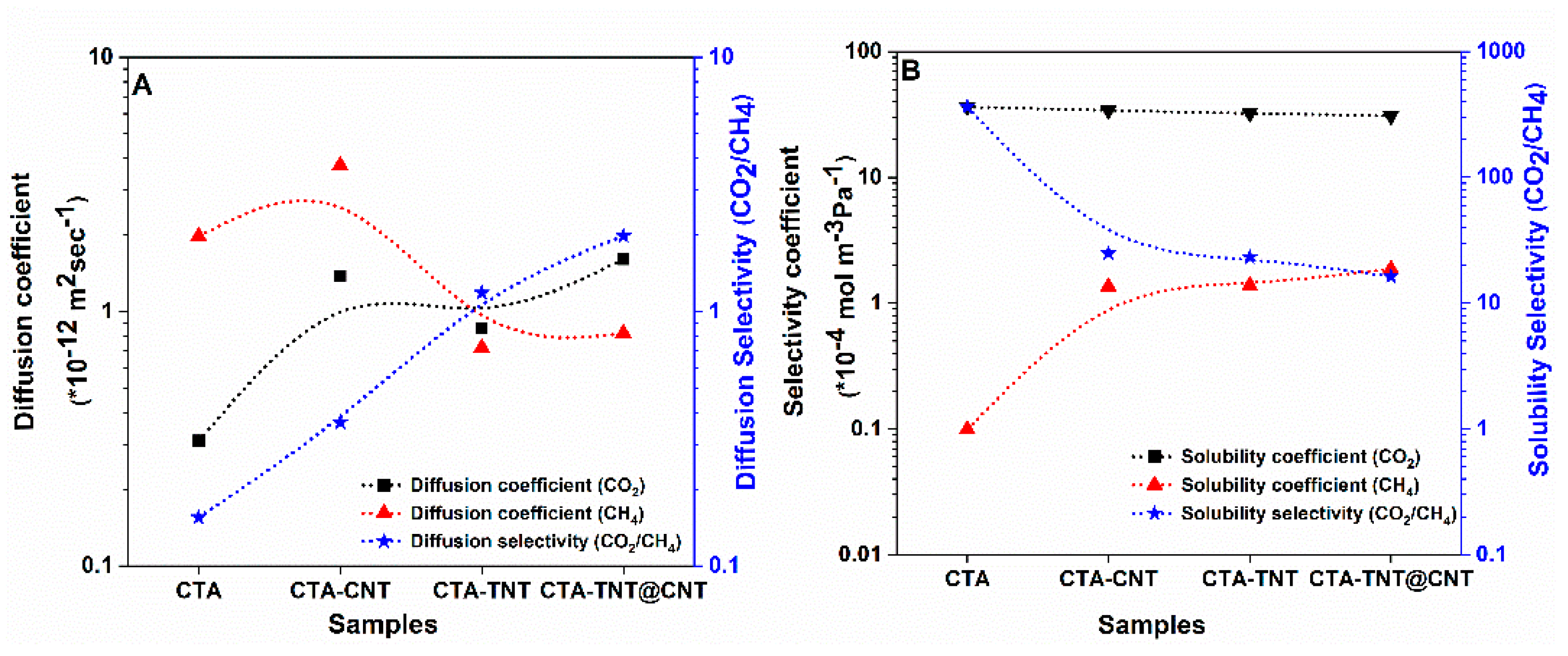
3.2. Gas Separation Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, H.; Yavari, M. Upper bound of polymeric membranes for mixed-gas CO2/CH4 separations. J. Membr. Sci. 2015, 475, 101–109. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Peters, T.A.; Konnertz, N.M.; Visser, T.; Téllez, C.; Coronas, J.; Fila, V.; de Vos, W.M.; Benes, N.E. High-pressure CO2/CH4 separation of Zr-MOFs based mixed matrix membranes. Sep. Purif. Technol. 2020, 230, 115858. [Google Scholar] [CrossRef]
- Ko, D.; Siriwardane, A.R.; Biegler, L.T. Optimization of Pressure Swing Adsorption and Fractionated Vacuum Pressure Swing Adsorption Processes for CO2 Capture. Ind. Eng. Chem. Res. 2005, 44, 8084–8094. [Google Scholar] [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An Improved CO2 Separation and Purification System Based on Cryogenic Separation and Distillation Theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef] [Green Version]
- Gleason, K.L.; Smith, Z.P.; Liu, Q.; Paul, D.R.; Freeman, B.D. Pure- and mixed-gas permeation of CO2 and CH4 in thermally rearranged polymers based on 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) and 2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA). J. Membr. Sci. 2015, 475, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Khalilpour, R.; Abbas, A.; Lai, Z.; Pinnau, I. Analysis of hollow fibre membrane systems for multicomponent gas separation. Chem. Eng. Res. Des. 2013, 91, 332–347. [Google Scholar] [CrossRef]
- Wang, D.; Teo, W.; Li, K. Preparation and characterization of high-flux polysulfone hollow fibre gas separation membranes. J. Membr. Sci. 2002, 204, 247–256. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, Reverse-Selective Nanocomposite Membranes. Science 2002, 296, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- Chuah, C.Y.; Goh, K.; Yang, Y.; Gong, H.; Li, W.; Karahan, H.E.; Guiver, M.D.; Wang, R.; Bae, T.-H. Harnessing Filler Materials for Enhancing Biogas Separation Membranes. Chem. Rev. 2018, 118, 8655–8769. [Google Scholar] [CrossRef]
- Goh, P.; Ismail, A.; Sanip, S.; Ng, B.; Aziz, M. Recent advances of inorganic fillers in mixed matrix membrane for gas separation. Sep. Purif. Technol. 2011, 81, 243–264. [Google Scholar] [CrossRef]
- Ozturk, B.; Ozen, H.A. The importance of nanomaterials in gas separation. Mater. Today Proc. 2021, 42, 1547–1549. [Google Scholar] [CrossRef]
- Wang, D.; Zheng, Y.; Yao, D.; Yang, Z.; Xin, Y.; Wang, F.; Wang, Y.; Ning, H.; Wu, H.; Wang, H. Liquid-like CNT/SiO2 nanoparticle organic hybrid materials as fillers in mixed matrix composite membranes for enhanced CO2-selective separation. New J. Chem. 2019, 43, 11949–11958. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Zhai, L.; Liu, G.; Dong, J.; Wang, Y.; Christopher, M.P.; Long, S.; Wang, Y.; Zhao, D. Mixed matrix membranes containing MOF@COF hybrid fillers for efficient CO2/CH4 separation. J. Membr. Sci. 2019, 573, 97–106. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.; Matsuura, T.; Montazer-Rahmati, M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Bae, T.-H.; Liu, J.; Lee, J.S.; Koros, W.J.; Jones, C.W.; Nair, S. Facile High-Yield Solvothermal Deposition of Inorganic Nanostructures on Zeolite Crystals for Mixed Matrix Membrane Fabrication. J. Am. Chem. Soc. 2009, 131, 14662–14663. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Wong, D.; Yang, J. Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation. Polymer 2021, 13, 2539. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 570, 343–354. [Google Scholar] [CrossRef]
- Zornoza, B.; Seoane, B.; Zamaro, J.M.; Téllez, C.; Coronas, J. Combination of MOFs and Zeolites for Mixed-Matrix Membranes. ChemPhysChem 2011, 12, 2781–2785. [Google Scholar] [CrossRef]
- Süer, M.G.; Baç, N.; Yilmaz, L. Gas permeation characteristics of polymer-zeolite mixed matrix membranes. J. Membr. Sci. 1994, 91, 77–86. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.S.; Huang, Z.; Kulprathipanja, S. Dual-layer polyethersulfone (PES)/BTDA-TDI/MDI co-polyimide (P84) hollow fiber membranes with a submicron PES–zeolite beta mixed matrix dense-selective layer for gas separation. J. Membr. Sci. 2006, 277, 28–37. [Google Scholar] [CrossRef]
- Pechar, T.W.; Kim, S.; Vaughan, B.; Marand, E.; Tsapatsis, M.; Jeong, H.K.; Cornelius, C.J. Fabrication and characterization of polyimide–zeolite L mixed matrix membranes for gas separations. J. Membr. Sci. 2006, 277, 195–202. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Kim, S.; Chen, L.; Johnson, J.K.; Marand, E. Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: Theory and experiment. J. Membr. Sci. 2007, 294, 147–158. [Google Scholar] [CrossRef]
- Gomes, D.; Nunes, S.; Peinemann, K.-V. Membranes for gas separation based on poly(1-trimethylsilyl-1-propyne)–silica nanocomposites. J. Membr. Sci. 2005, 246, 13–25. [Google Scholar] [CrossRef]
- Setiawan, W.K.; Chiang, K.-Y. Silica applied as mixed matrix membrane inorganic filler for gas separation: A review. Sustain. Environ. Res. 2019, 29, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.-S.; Chan, S.S.; Wang, R.; Lu, Z.; He, C. Characterization of permeability and sorption in Matrimid/C60 mixed matrix membranes. J. Membr. Sci. 2003, 211, 91–99. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Regmi, C.; Friess, K. Interfacial Design of Mixed Matrix Membranes via Grafting PVA on UiO-66-NH2 to Enhance the Gas Separation Performance. Membranes 2021, 11, 419. [Google Scholar] [CrossRef]
- Ghalei, B.; Sakurai, K.; Kinoshita, Y.; Wakimoto, K.; Isfahani, A.P.; Song, Q.; Doitomi, K.; Furukawa, S.; Hirao, H.; Kusuda, H.; et al. Enhanced selectivity in mixed matrix membranes for CO2 capture through efficient dispersion of amine-functionalized MOF nanoparticles. Nat. Energy 2017, 2, 17086. [Google Scholar] [CrossRef]
- Boháčová, M.; Zetková, K.; Knotek, P.; Bouša, D.; Friess, K.; Číhal, P.; Lanč, M.; Hrdlička, Z.; Sofer, Z. Mildly oxidized SWCNT as new potential support membrane material for effective H2/CO2 separation. Appl. Mater. Today 2019, 15, 335–342. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, P.; Du, J.; Zhao, L.; Ajayan, P.M.; Cheng, H.-M. Increasing the electrical conductivity of carbon nanotube/polymer composites by using weak nanotube–polymer interactions. Carbon 2010, 48, 3551–3558. [Google Scholar] [CrossRef]
- Skoulidas, A.I.; Ackerman, D.M.; Johnson, J.K.; Sholl, D.S. Rapid Transport of Gases in Carbon Nanotubes. Phys. Rev. Lett. 2002, 89, 185901. [Google Scholar] [CrossRef]
- Holt, J.K. Carbon Nanotubes and Nanofluidic Transport. Adv. Mater. 2009, 21, 3542–3550. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Muhammad Asyraf, M.R.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymer 2021, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Goh, P.S.; Tee, J.C.; Sanip, S.M.; Aziz, M. A review of purification techniques for carbon nanotubes. Nano 2008, 3, 127–143. [Google Scholar] [CrossRef]
- Nejad, M.N.; Asghari, M.; Afsari, M. Investigation of Carbon Nanotubes in Mixed Matrix Membranes for Gas Separation: A Review. ChemBioEng Rev. 2016, 3, 276–298. [Google Scholar] [CrossRef]
- Choi, J.-H.; Jegal, J.; Kim, W.-N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Murali, R.S.; Sridhar, S.; Sankarshana, T.; Ravikumar, Y.V.L. Gas Permeation Behavior of Pebax-1657 Nanocomposite Membrane Incorporated with Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2010, 49, 6530–6538. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Kumar, I.A.; Weng, T.-H.; Lu, C.-Y.; Wey, M.-Y. Preparation and characterization of carbon molecular sieve membranes for gas separation—the effect of incorporated multi-wall carbon nanotubes. Desalination 2009, 240, 40–45. [Google Scholar] [CrossRef]
- Hu, Q.; Marand, E.; Dhingra, S.; Fritsch, D.; Wen, J.; Wilkes, G. Poly(amide-imide)/TiO2 nano-composite gas separation membranes: Fabrication and characterization. J. Membr. Sci. 1997, 135, 65–79. [Google Scholar] [CrossRef]
- Chowdhury, S.; Parshetti, G.K.; Balasubramanian, R. Post-combustion CO2 capture using mesoporous TiO2/graphene oxide nanocomposites. Chem. Eng. J. 2015, 263, 374–384. [Google Scholar] [CrossRef]
- Matteucci, S.; Kusuma, V.; Sanders, D.; Swinnea, S.; Freeman, B.D. Gas transport in TiO2 nanoparticle-filled poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 2008, 307, 196–217. [Google Scholar] [CrossRef]
- Moghadam, F.; Omidkhah, M.; Vasheghani-Farahani, E.; Pedram, M.Z.; Dorosti, F. The effect of TiO2 nanoparticles on gas transport properties of Matrimid5218-based mixed matrix membranes. Sep. Purif. Technol. 2011, 77, 128–136. [Google Scholar] [CrossRef]
- Liang, C.Y.; Uchytil, P.; Petrychkovych, R.; Lai, Y.C.; Friess, K.; Sipek, M.; Suen, S.Y. A comparison on gas separation between PES (polyethersulfone)/MMT (Na-montmorillonite) and PES/TiO2 mixed matrix membranes. Sep. Purif. Technol. 2012, 92, 57–63. [Google Scholar] [CrossRef]
- Imai, H.; Takei, Y.; Shimizu, K.; Matsuda, M.; Hirashima, H. Direct preparation of anatase TiO2 nanotubes in porous alumina membranes. J. Mater. Chem. 1999, 9, 2971–2972. [Google Scholar] [CrossRef]
- Jun, Y.; Zarrin, H.; Fowler, M.; Chen, Z. Functionalized titania nanotube composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2011, 36, 6073–6081. [Google Scholar] [CrossRef]
- Wu, C.-H.; Kuo, C.-Y.; Chen, S.-T. Synergistic effects between TiO2 and carbon nanotubes (CNTs) in a TiO2/CNTs system under visible light irradiation. Environ. Technol. 2013, 34, 2513–2519. [Google Scholar] [CrossRef]
- Scholes, C.A.; Chen, G.Q.; Tao, W.X.; Bacus, J.; Anderson, C.; Stevens, G.W.; Kentish, S.E. The effects of minor components on the gas separation performance of membranes for carbon capture. Energy Procedia 2011, 4, 681–687. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.U.; Noor, T.; Othman MH, D.; Yousaf, M.F. Development of high performance amine functionalized zeolitic imidazolate framework (ZIF-8)/cellulose triacetate mixed matrix membranes for CO2/CH4 separation. Int. J. Energy Res. 2020, 44, 7989–7999. [Google Scholar] [CrossRef]
- Puleo, A.; Paul, D.; Kelley, S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Membr. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Lam, B.; Wei, M.; Zhu, L.; Luo, S.; Guo, R.; Morisato, A.; Alexandridis, P.; Lin, H. Cellulose triacetate doped with ionic liquids for membrane gas separation. Polymer 2016, 89, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; White, L.S.; Lokhandwala, K.; Baker, R.W. Natural Gas Purification. In Encyclopedia of Membrane Science and Technology; Wiley: Hoboken, NJ, USA, 2013; pp. 1–25. [Google Scholar]
- Liu, Y.; Liu, Z.; Morisato, A.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using a cellulose triacetate hollow fiber membrane illustrating controlled plasticization benefits. J. Membr. Sci. 2020, 601, 117910. [Google Scholar] [CrossRef]
- Lu, H.T.; Kanehashi, S.; Scholes, C.; Kentish, S. The potential for use of cellulose triacetate membranes in post combustion capture. Int. J. Greenh. Gas Control. 2016, 55, 97–104. [Google Scholar] [CrossRef]
- Galve, A.; Sieffert, D.; Staudt, C.; Ferrando, M.; Güell, C.; Téllez, C.; Coronas, J. Combination of ordered mesoporous silica MCM-41 and layered titanosilicate JDF-L1 fillers for 6FDA-based copolyimide mixed matrix membranes. J. Membr. Sci. 2013, 431, 163–170. [Google Scholar] [CrossRef]
- Valero, M.; Zornoza, B.; Téllez, C.; Coronas, J. Mixed matrix membranes for gas separation by combination of silica MCM-41 and MOF NH2-MIL-53(Al) in glassy polymers. Microporous Mesoporous Mater. 2014, 192, 23–28. [Google Scholar] [CrossRef]
- Shaban, M.; Ashraf, A.M.; AbdAllah, H.; El-Salam, H.A. Titanium dioxide nanoribbons/multi-walled carbon nanotube nanocomposite blended polyethersulfone membrane for brackish water desalination. Desalination 2018, 444, 129–141. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, S.; Wang, Y. Atomic layer deposition of TiO2 on carbon-nanotube membranes for enhanced capacitive deionization. Sep. Purif. Technol. 2019, 213, 70–77. [Google Scholar] [CrossRef]
- Veréb, G.; Kassai, P.; Santos, E.N.; Arthanareeswaran, G.; Hodúr, C.; László, Z. Intensification of the ultrafiltration of real oil-contaminated (produced) water with pre-ozonation and/or with TiO2, TiO2/CNT nanomaterial-coated membrane surfaces. Environ. Sci. Pollut. Res. 2020, 27, 22195–22205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gyawali, G.; Son, J.; Hao, N.H.; Cho, S.H.; Lee, S.W.; Kim, T.-H. Synthesis of TiO2 nanotubes using different alkaline media and their applications in photocatalysis and DSSCs. Res. Chem. Intermed. 2017, 43, 5055–5065. [Google Scholar] [CrossRef]
- Ashtiani, S.; Sofer, Z.; Průša, F.; Friess, K. Molecular-level fabrication of highly selective composite ZIF-8-CNT-PDMS membranes for effective CO2/N2, CO2/H2 and olefin/paraffin separations. Sep. Purif. Technol. 2021, 274, 119003. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Shaliutina-Kolešová, A.; Bouša, D.; Sofer, Z.; Friess, K. Co0.5Ni0.5FeCrO4 spinel nanoparticles decorated with UiO-66-based metal-organic frameworks grafted onto GO and O-SWCNT for gas adsorption and water purification. Chemosphere 2020, 255, 126966. [Google Scholar] [CrossRef]
- Regmi, C.; Ashtiani, S.; Sofer, Z.; Friess, K. Improved CO2/CH4 separation properties of cellulose triacetate mixed–matrix membranes with CeO2@GO hybrid fillers. Membranes 2021, 11, 777. [Google Scholar] [CrossRef] [PubMed]
- Friess, K.; Hynek, V.; Šípek, M.; Kujawski, W.M.; Vopička, O.; Zgažar, M.; Kujawski, M.W. Permeation and sorption properties of poly(ether-block-amide) membranes filled by two types of zeolites. Sep. Purif. Technol. 2011, 80, 418–427. [Google Scholar] [CrossRef]
- Vopička, O.; Friess, K.; Hynek, V.; Sysel, P.; Zgažar, M.; Šípek, M.; Budd, P.M. Equilibrium and transient sorption of vapours and gases in the polymer of intrinsic microporosity PIM-1. J. Membr. Sci. 2013, 434, 148–160. [Google Scholar] [CrossRef]
- Jansen, J.C.; Friess, K.; Drioli, E. Organic vapour transport in glassy perfluoropolymer membranes: A simple semi-quantitative approach to analyze clustering phenomena by time lag measurements. J. Membr. Sci. 2011, 367, 141–151. [Google Scholar] [CrossRef]
- Friess, K.; Jansen, J.C.; Bazzarelli, F.; Izák, P.; Jarmarová, V.; Kačírková, M.; Bernardo, P. High ionic liquid content polymeric gel membranes: Correlation of membrane structure with gas and vapour transport properties. J. Membr. Sci. 2012, 415, 801–809. [Google Scholar] [CrossRef]
- Xia, Y.; Xiong, W.-S.; Jiang, Y.; Sun, W.; Sang, H.-Q.; He, R.-X.; Tai, Q.; Chen, B.; Liu, Y.; Zhao, X.-Z. Multi-walled carbon nanotubes induced a controllable TiO2 morphology transformation for high-rate and long-life lithium-ion batteries. RSC Adv. 2017, 7, 21988–21996. [Google Scholar] [CrossRef] [Green Version]
- Abbas, N.; Shao, G.N.; Haider, M.S.; Imran, S.M.; Park, S.S.; Jeon, S.J.; Kim, H.T. Inexpensive sol-gel synthesis of multiwalled carbon nanotube-TiO2 hybrids for high performance antibacterial materials. Mater. Sci. Eng. C 2016, 68, 780–788. [Google Scholar] [CrossRef]
- Qin, Q.; Shi, Q.; Sun, W.; Wan, J.; Hu, Z. Fabrication and interfacial electron transfer of ultrathin g-C3N4 nanosheet/TNT@CNTs ternary nanostructure heterojunction for high-efficiency visible-light-driven photocatalysis. J. Mater. Sci. Mater. Electron. 2018, 29, 8673–8687. [Google Scholar] [CrossRef]
- Nabili, A.; Fattoum, A.; Brochier-Salon, M.-C.; Bras, J.; Elaloui, E. Synthesis of cellulose triacetate-I from microfibrillated date seeds cellulose (Phoenix dactylifera L.). Iran. Polym. J. 2017, 26, 137–147. [Google Scholar] [CrossRef]
- Zhuang, G.-L.; Tseng, H.-H.; Wey, M.-Y. Preparation of PPO-silica mixed matrix membranes by in-situ sol–gel method for H2/CO2 separation. Int. J. Hydrogen Energy 2014, 39, 17178–17190. [Google Scholar] [CrossRef]
- Alsharaeh, E.H.; Bora, T.; Soliman, A.; Ahmed, F.; Bharath, G.; Ghoniem, M.G.; Abu-Salah, K.M.; Dutta, J. Sol-Gel-Assisted Microwave-Derived Synthesis of Anatase Ag/TiO2/GO Nanohybrids toward Efficient Visible Light Phenol Degradation. Catalysts 2017, 7, 133. [Google Scholar] [CrossRef]
- Szabo, L.; Čík, G.; Lesný, J. Thermal stability increase of doped poly (3-hexadecylthiophene) by γ-radiation. Synth. Met. 1996, 78, 149–153. [Google Scholar] [CrossRef]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Regmi, C.; Ashtiani, S.; Sofer, Z.; Hrdlička, Z.; Průša, F.; Vopička, O.; Friess, K. CeO2-blended cellulose triacetate mixed-matrix membranes for selective CO2 separation. Membranes 2021, 11, 632. [Google Scholar] [CrossRef]
- Chae, D.W.; Nam, Y.W.; Wang, S.S.; Hong, S.M. Structures and physical properties of multi-walled carbon nanotube-filled PVDF ihermoplastic composites. Solid State Phenom. 2007, 464, 195–777. [Google Scholar]
- Shin, J.E.; Lee, S.K.; Cho, Y.H.; Park, H.B. Effect of PEG-MEA and graphene oxide additives on the performance of Pebax®1657 mixed matrix membranes for CO2 separation. J. Membr. Sci. 2019, 572, 300–308. [Google Scholar] [CrossRef]
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.; Peinemann, K.-V. CO2-Philic Polymer Membrane with Extremely High Separation Performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Alentiev, A.; Drioli, E.; Gokzhaev, M.; Golemme, G.; Ilinich, O.; Lapkin, A.; Volkov, V.; Yampolskii, Y. Gas permeation properties of phenylene oxide polymers. J. Membr. Sci. 1998, 138, 99–107. [Google Scholar] [CrossRef]
- Sawada, H.; Takahashi, Y.; Miyata, S.; Kanehashi, S.; Sato, S.; Nagai, K. Gas Transport Properties and Crystalline Structures of Poly(lactic acid) Membranes. Trans. Mater. Res. Soc. Jpn. 2010, 35, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Van Goethem, C.; Mulunda, M.M.; Verbeke, R.; Koschine, T.; Wübbenhorst, M.; Zhang, Z.; Nies, E.; Dickmann, M.; Egger, W.; Vankelecom, I.F.J.; et al. Increasing Membrane Permeability by Increasing the Polymer Crystallinity: The Unique Case of Polythiophenes. Macromolecules 2018, 51, 9943–9950. [Google Scholar] [CrossRef]
- Gracia-Fernández, C.A.; Gómez-Barreiro, S.; López-Beceiro, J.; Saavedra, J.T.; Naya, S.; Artiaga, R. Comparative study of the dynamic glass transition temperature by DMA and TMDSC. Polym. Test. 2010, 29, 1002–1006. [Google Scholar] [CrossRef]
- Keusch, S.; Haessler, R. Influence of surface treatment of glass fibres on the dynamic mechanical properties of epoxy resin composites. Compos. Part A Appl. Sci. Manuf. 1999, 30, 997–1002. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F.J. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Tol, R.T.; Groeninckx, G.; Vinckier, I.; Moldenaers, P.; Mewis, J. Phase morphology and stability of co-continuous (PPE/PS)/PA6 and PS/PA6 blends: Effect of rheology and reactive compatibilization. Polymer 2004, 45, 2587–2601. [Google Scholar] [CrossRef]
- Fauzan, N.A.B.; Mukhtar, H.; Nasir, R.; Mohshim, D.F.B.; Arasu, N.; Man, Z.; Mannan, H.A. Composite amine mixed matrix membranes for high-pressure CO2-CH4 separation: Synthesis, characterization and performance evaluation. R. Soc. Open Sci. 2020, 7, 200795. [Google Scholar] [CrossRef]
- Flyagina, I.S.; Mahdi, E.M.; Titov, K.; Tan, J.-C. Thermo-mechanical properties of mixed-matrix membranes encompassing zeolitic imidazolate framework-90 and polyvinylidine difluoride: ZIF-90/PVDF nanocomposites. APL Mater. 2017, 5, 086104. [Google Scholar] [CrossRef] [Green Version]
- Aframehr, W.M.; Molki, B.; Bagheri, R.; Heidarian, P.; Davodi, S.M. Characterization and enhancement of the gas separation properties of mixed matrix membranes: Polyimide with nickel oxide nanoparticles. Chem. Eng. Res. Des. 2020, 153, 789–805. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Chen, H.; Sholl, D.S. Predictions of selectivity and flux for CH4/H2 separations using single walled carbon nanotubes as membranes. J. Membr. Sci. 2006, 269, 152–160. [Google Scholar] [CrossRef]
- Magueijo, V.; Anderson, L.; Fletcher, A.; Shilton, S. Polysulfone mixed matrix gas separation hollow fibre membranes filled with polymer and carbon xerogels. Chem. Eng. Sci. 2013, 92, 13–20. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Nikolaeva, D.; Azcune, I.; Tanczyk, M.; Warmuzinski, K.; Jaschik, M.; Sandru, M.; Vankelecom, I.F. The performance of affordable and stable cellulose-based poly-ionic membranes in CO2/N2 and CO2/CH4 gas separation. J. Membr. Sci. 2018, 564, 552–561. [Google Scholar] [CrossRef]
- Číhal, P.; Vopička, O.; Lanč, M.; Kludský, M.; Velas, J.; Hrdlička, Z.; Michalcová, A.; Dendisová, M.; Friess, K. Poly(butylene succinate)-cellulose triacetate blends: Permeation, pervaporation, sorption and physical structure. Polym. Test. 2018, 65, 468–479. [Google Scholar] [CrossRef]
- Raza, A.; Japip, S.; Liang, C.Z.; Farrukh, S.; Hussain, A.; Chung, T.-S. Novel Cellulose Triacetate (CTA)/Cellulose Diacetate (CDA) Blend Membranes Enhanced by Amine Functionalized ZIF-8 for CO2 Separation. Polymer 2021, 13, 2946. [Google Scholar] [CrossRef]
- Raza, A.; Farrukh, S.; Hussain, A.; Khan, I.; Othman, M.; Ahsan, M. Performance Analysis of Blended Membranes of Cellulose Acetate with Variable Degree of Acetylation for CO2/CH4 Separation. Membrances 2021, 11, 245. [Google Scholar] [CrossRef]
- Soleimany, A.; Karimi-Sabet, J.; Hosseini, S.S. Experimental and modeling investigations towards tailoring cellulose triacetate membranes for high performance helium separation. Chem. Eng. Res. Des. 2018, 137, 194–212. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Kargari, A.; Nasernejad, B. Aminosilane-functionalization of a nanoporous Y-type zeolite for application in a cellulose acetate based mixed matrix membrane for CO2 separation. RSC Adv. 2014, 4, 63966–63976. [Google Scholar] [CrossRef]
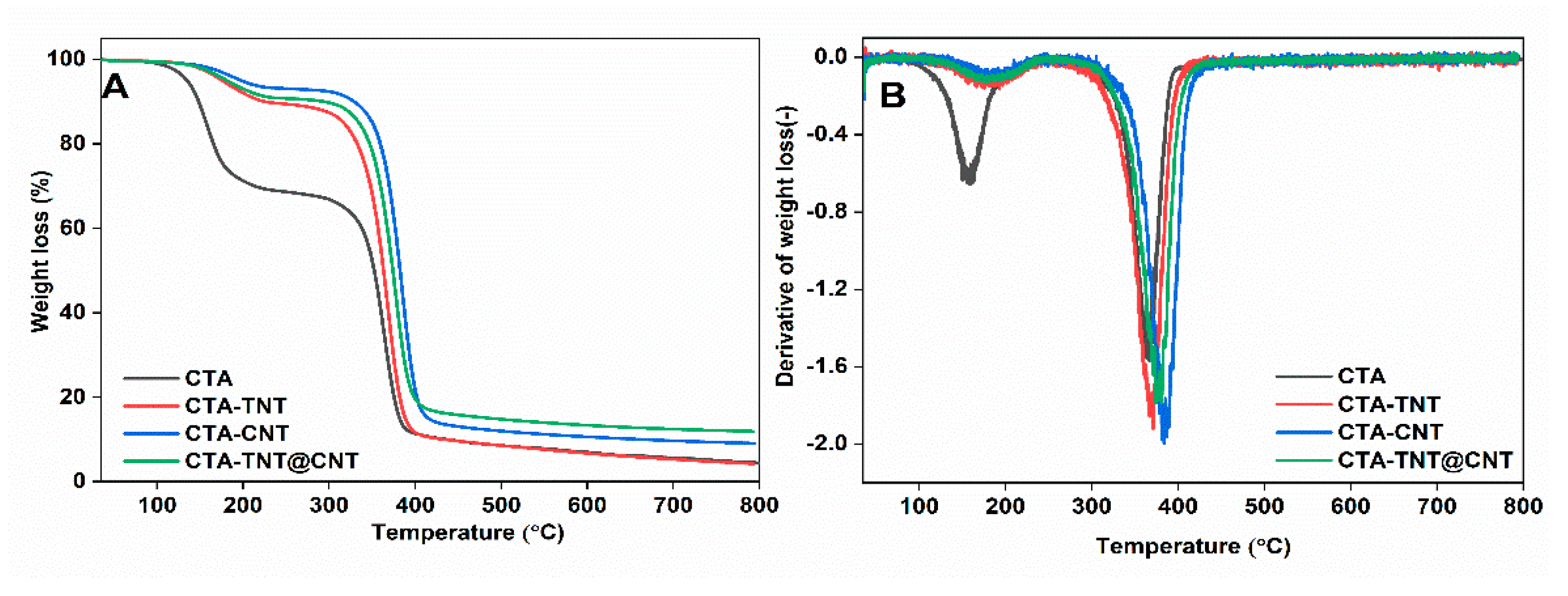


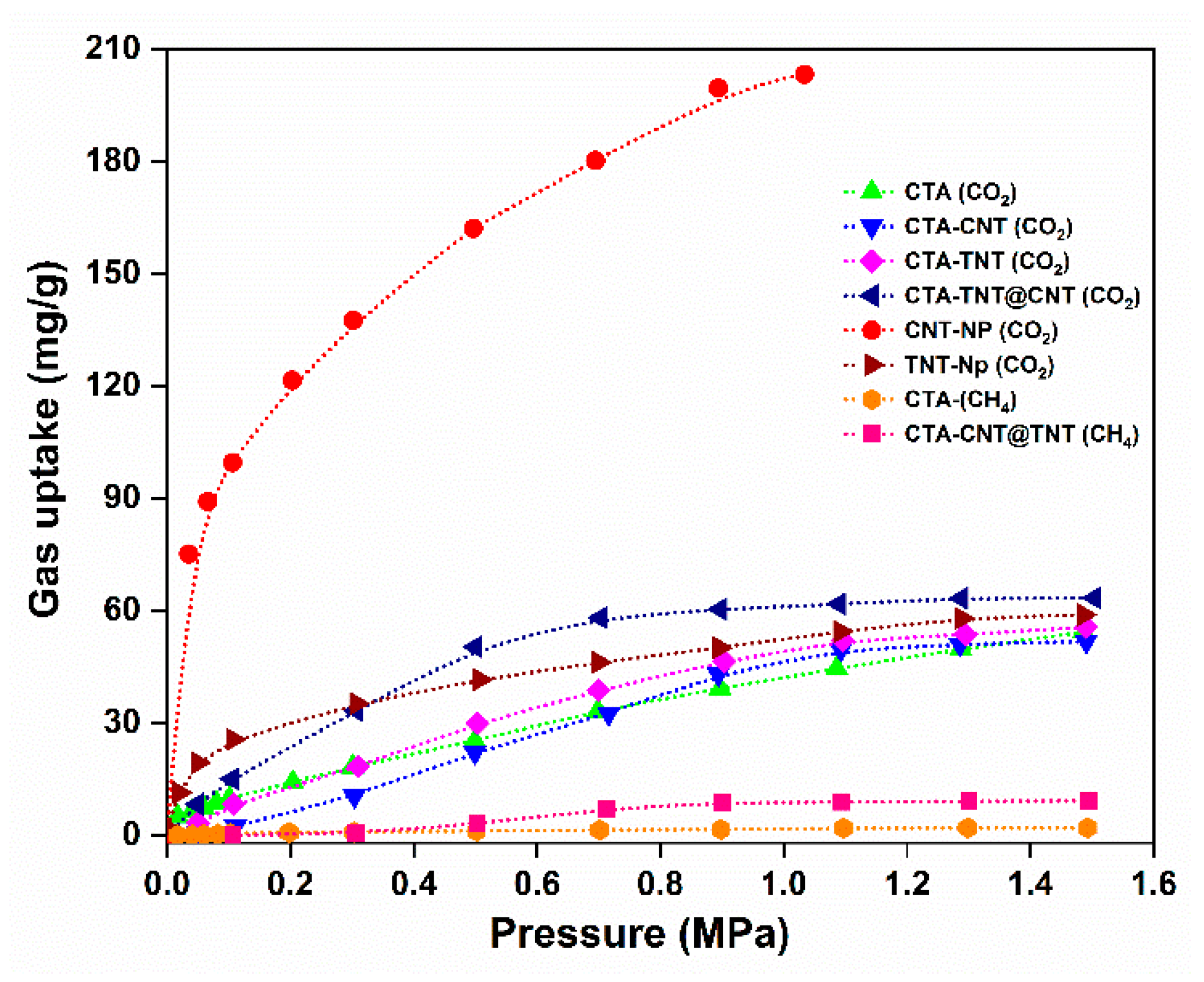

| Sample Code | Glass Transition Temperature (Tg) (°C) | Mechanical Properties (25 °C) | ||
|---|---|---|---|---|
| Young’s Modulus (GPa) | Elongation Strain at Break (%) | Tensile Strength (MPa) | ||
| CTA | 188.5 | 1.24 ± 0.46 | 51.29 ± 3.06 | 31.09 ± 2.28 |
| CTA-CNT | 194.7 | 0.70 ± 0.19 | 6.19 ± 1.32 | 37.95 ± 3.41 |
| CTA-TNT | 198.7 | 0.73 ± 0.30 | 5.28 ± 0.21 | 36.94 ± 3.24 |
| CTA-CNT@TNT | 198.1 | 1.20 ± 0.22 | 15.32 ± 1.32 | 41.21 ± 1.01 |
| Samples | Permeability (Barrer) | Selectivity (-) | |||
|---|---|---|---|---|---|
| H2 | CO2 | CH4 | H2/CH4 | CO2/CH4 | |
| CTA | 4.39 | 3.01 | 0.12 | 36.58 | 25.08 |
| CTA-TNT | 12.83 | 8.24 | 0.30 | 42.77 | 27.47 |
| CTA-CNT | 15.83 | 14.03 | 0.41 | 38.61 | 34.22 |
| CTA-TNT@CNT | 22.28 | 19.77 | 0.46 | 48.43 | 42.98 |
| Membrane Type | Permeability (Barrer) /Selectivity (--) | References |
|---|---|---|
| Cellulose-based poly-ionic liquid membranes P[CA][Tf2N] | PCO2 = 8.9 CO2/CH4 = 22.3 | Nikolaeva et al. [97] |
| Poly(butylene succinate)–cellulose triacetate blends [CTA + 10wt% PBS] | PCO2 = 3.5 CO2/CH4 = 35.0 | Cihal et al. [98] |
| CeO2@GO blended CTA membrane | PCO2 = 10.14 CO2/CH4 = 50.7 | Regmi et al. [66] |
| CTA/CDA blend membrane by amine functionalized ZIF-8 [CTA/CDA-NH2-ZIF-8 (15 wt%)] | PCO2 = 11.33 CO2/CH4 = 33 | Raza et al. [99] |
| CTA and CDA blended membrane [CTA (80 wt.%): CDA(20 wt.%) | PCO2 = 17.32 CO2/CH4 = 18.55 | Raza et al. [100] |
| CTA doped with ionic liquids of [emim][BF4] and [emim][dca] | PCO2 = 7.3 CO2/CH4 = 26 | Lam et al. [54] |
| PEG and ZIF-8 tailored CTA membranes [CTA/PEG/ZIF-8 (60/20/20 wt.%)] | PHe = 73.25 He/CH4 = 40 | Soleimany et al. [101] |
| APDEMS modified NaY zeolite blended CA membranes [CA/NaY-sm 20 wt.%] | PCO2 = 4.04 CO2/N2 = 26 | Sanaeepur et al. [102] |
| Hybrid TNT@CNT blended CTA membrane | PCO2 = 19.77 CO2/CH4 = 42.98 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regmi, C.; Ashtiani, S.; Hrdlička, Z.; Friess, K. CO2/CH4 and H2/CH4 Gas Separation Performance of CTA-TNT@CNT Hybrid Mixed Matrix Membranes. Membranes 2021, 11, 862. https://doi.org/10.3390/membranes11110862
Regmi C, Ashtiani S, Hrdlička Z, Friess K. CO2/CH4 and H2/CH4 Gas Separation Performance of CTA-TNT@CNT Hybrid Mixed Matrix Membranes. Membranes. 2021; 11(11):862. https://doi.org/10.3390/membranes11110862
Chicago/Turabian StyleRegmi, Chhabilal, Saeed Ashtiani, Zdeněk Hrdlička, and Karel Friess. 2021. "CO2/CH4 and H2/CH4 Gas Separation Performance of CTA-TNT@CNT Hybrid Mixed Matrix Membranes" Membranes 11, no. 11: 862. https://doi.org/10.3390/membranes11110862