Immobilization of poly(vinyl pyrrolidone) in Polysulfone Membranes by Radically-Initiated Crosslinking Using Potassium Persulfate
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
2.2. Methods
2.2.1. Preparation of Polymer Dope Solution
2.2.2. Membrane Preparation
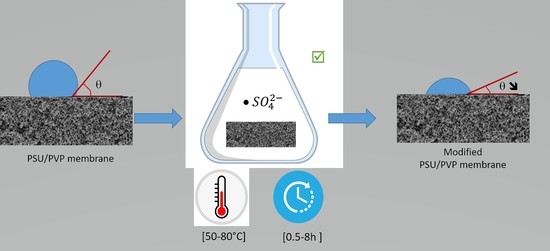
2.2.3. Crosslinking Process
2.2.4. Characterization Techniques
Structural and Chemical Characterizations
3. Results and Discussion
3.1. Investigation of PVP Cross-Linking
Attenuated Total Reflectance Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.2. Mechanical Properties
3.3. Physico-Chemical Characterizations
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Water Contact Angles Measurements
3.3.3. Pure Water Permeability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Nady, N.; Franssen, M.C.R.; Zuilhof, H.; Eldin, M.S.M.; Boom, R.; Schroën, K. Modification methods for poly(arylsulfone) membranes: A mini-review focusing on surface modification. Desalination 2011, 275, 1–9. [Google Scholar] [CrossRef]
- Hwang, J.R.; Sefton, M.V. The effects of polymer concentration and a pore-forming agent (PVP) on HEMA-MMA microcapsule structure and permeability. J. Membr. Sci. 1995, 108, 257–268. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Rosselli, A.; Drioli, E. Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD. J. Membr. Sci. 2010, 364, 219–232, Erratum in 2011, 369, 569. [Google Scholar] [CrossRef]
- Qin, J.-J.; Wong, F.-S.; Li, Y.; Liu, Y.-T. A high flux ultrafiltration membrane spun from PSU/PVP (K90)/DMF/1,2-propanediol. J. Membr. Sci. 2003, 211, 139–147. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Vankelecom, I.F. Gradual PVP leaching from PVDF/PVP blend membranes and its effects on membrane fouling in membrane bioreactors. Sep. Purif. Technol. 2019, 213, 276–282. [Google Scholar] [CrossRef]
- Singh, B.; Pal, L. Radiation crosslinking polymerization of sterculia polysaccharide–PVA–PVP for making hydrogel wound dressings. Int. J. Biol. Macromol. 2011, 48, 501–510. [Google Scholar] [CrossRef]
- Demeter, M.; Virgolici, M.; Vancea, C.; Scarisoreanu, A.; Kaya, M.G.A.; Meltzer, V. Network structure studies on γ–irradiated collagen–PVP superabsorbent hydrogels. Radiat. Phys. Chem. 2017, 131, 51–59. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, Y.-A.; Yang, S.-J.; Jeong, S.-I.; Lee, J.-Y.; Lim, Y.-M. Gamma Ray-Induced Polymerization and Cross-Linking for Optimization of PPy/PVP Hydrogel as Biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef] [Green Version]
- D’Errico, G.; De Lellis, M.; Mangiapia, G.; Tedeschi, A.; Ortona, O.; Fusco, S.; Borzacchiello, A.; Ambrosio, L. Structural and Mechanical Properties of UV-Photo-Cross-Linked Poly(N-vinyl-2-pyrrolidone) Hydrogels. Biomacromolecules 2008, 9, 231–240. [Google Scholar] [CrossRef]
- Maciejewska, B.M.; Wychowaniec, J.K.; Woźniak-Budych, M.; Popenda, Ł.; Warowicka, A.; Golba, K.; Litowczenko, J.; Fojud, Z.; Wereszczyńska, B.; Jurga, S. UV cross-linked polyvinylpyrrolidone electrospun fibres as antibacterial surfaces. Sci. Technol. Adv. Mater. 2019, 20, 979–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsi, M.; Abdelghany, A. UV-irradiation assisted control of the structural, optical and thermal properties of PEO/PVP blended gold nanoparticles. Mater. Chem. Phys. 2017, 201, 100–112. [Google Scholar] [CrossRef]
- Rosa, R.M.; Silva, J.C.; Sanches, I.S.; Henriques, C. Simultaneous photo-induced cross-linking and silver nanoparticle formation in a PVP electrospun wound dressing. Mater. Lett. 2017, 207, 145–148. [Google Scholar] [CrossRef]
- Lubasova, D.; Niu, H.; Zhao, X.; Lin, T. Hydrogel properties of electrospun polyvinylpyrrolidone and polyvinylpyrrolidone/poly(acrylic acid) blend nanofibers. RSC Adv. 2015, 5, 54481–54487. [Google Scholar] [CrossRef]
- Hatch, K.M.; Hlavatá, J.; Paulett, K.; Liavitskaya, T.; Vyazovkin, S.; Stanishevsky, A.V. Nanocrystalline Cellulose/Polyvinylpyrrolidone Fibrous Composites Prepared by Electrospinning and Thermal Crosslinking. Int. J. Polym. Sci. 2019, 2019, 7103936. [Google Scholar] [CrossRef]
- Anderson, C.C.; Rodriguez, F.; Thurston, D.A. Crosslinking aqueous poly(vinyl pyrrolidone) solutions by persulfate. J. Appl. Polym. Sci. 1979, 23, 2453–2462. [Google Scholar] [CrossRef]
- Xu, W.; Chen, H.; Li, H.; Wang, M. Fabrication of carbon black/crosslinked poly(vinyl pyrrolidone) core-shell nanoparticles stable in water. Colloids Surf. A Physicochem. Eng. Asp. 2005, 266, 68–72. [Google Scholar] [CrossRef]
- Bi, Q.; Li, Q.; Tian, Y.; Lin, Y.; Wang, X. Hydrophilic modification of poly(vinylidene fluoride) membrane with poly(vinyl pyrrolidone) via a cross-linking reaction. J. Appl. Polym. Sci. 2012, 127, 394–401. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, X.; Li, F.; Zhao, X. Poly(vinyl pyrrolidone) modified poly(vinylidene fluoride) ultrafiltration membrane via a two-step surface grafting for radioactive wastewater treatment. Sep. Purif. Technol. 2018, 194, 404–409. [Google Scholar] [CrossRef]
- Muller, H.-J.; Wang, D.; Elbaz, N. Monopersulfate Treatment of Membranes. WO Patent 2007/006104 A1, 18 January 2007. [Google Scholar]
- Muller, H.-J.; Wang, D.; Kumar, A. Cross-Linking Treatment of Polymer Membranes. WO Patent 2006/135966 A1, 28 December 2006. [Google Scholar]
- Zhu, X.; Lu, P.; Chen, W.; Dong, J. Studies of UV crosslinked poly(N-vinylpyrrolidone) hydrogels by FTIR, Raman and solid-state NMR spectroscopies. Polymer 2010, 51, 3054–3063. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Cotman, J.D. The Kinetics of the Decomposition of Potassium Persulfate in Aqueous Solutions of Methanol. J. Am. Chem. Soc. 1949, 71, 1419–1422. [Google Scholar] [CrossRef]
- Fogaça, R.; Catalani, L.H. PVP Hydrogel Membranes Produced by Electrospinning for Protein Release Devices. Soft Mater. 2013, 11, 61–68. [Google Scholar] [CrossRef]
- Salih, S.I.; Jabur, A.R.; Mohammed, T. The Effect of PVP Addition on the Mechanical Properties of Ternary Polymer Blends. IOP Conf. Series: Mater. Sci. Eng. 2018, 433, 012071. [Google Scholar] [CrossRef]













| Polymer | Time (h) | Temperature (°C) | [KPS] (g/L) |
|---|---|---|---|
| PSU | 0.51 2 4 8 10 * | 50 | 0 ** |
| 3 | |||
| 50 | |||
| 80 | 0 ** | ||
| 3 | |||
| 50 |
| Membrane | Top Surface Pore Size (nm) | Selective Layer Thickness (µm) |
|---|---|---|
| Non-treated | n.d | 3.07 ± 0.85 |
| 80 °C, 0 g/L, 30 min | 26 ± 4 | 0.36 ± 0.04 |
| 80 °C, 50 g/L, 1 h | 25 ± 1 | 0.32 ± 0.06 |
| 80 °C, 50 g/L, 2 h | 24 ± 0.1 | 0.26 ± 0.04 |
| 80 °C, 50 g/L, 4 h | 24 ± 7 | 0.22 ± 0.02 |
| Membrane | %PVPms at the Surface | Total %PVPms |
|---|---|---|
| 80 °C, 50 g/L, 30 min | 32.2 ± 1.6 | 7.6 ± 0.6 |
| 80 °C, 50 g/L, 1 h | 25.9 ± 2.6 | 7.5 ± 0.4 |
| 80 °C, 50 g/L, 2 h | 47.8 ± 0.6 | 9.6 ± 0.8 |
| 80 °C, 50 g/L, 4 h | 44.3 ± 0.6 | 7.3 ± 0.4 |
| Membrane | P0 (0 g/L) (L·bar−1 h−1 m−2) | P (50 g/L) (L·bar−1 h−1 m−2) | P/P0 |
|---|---|---|---|
| 80 °C, 30 min | 86 ± 24 | 539 ± 129 | 6.3 |
| 80 °C, 1 h | 58 ± 4 | 330 ± 40 | 5.7 |
| 80 °C, 2 h | 126 ± 32 | 268 ± 60 | 2.1 |
| 80 °C, 4 h | 71 ± 8 | 195 ± 41 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez Ortiz, D.; Nouxet, M.; Maréchal, W.; Lorain, O.; Deratani, A.; Pochat-Bohatier, C. Immobilization of poly(vinyl pyrrolidone) in Polysulfone Membranes by Radically-Initiated Crosslinking Using Potassium Persulfate. Membranes 2022, 12, 664. https://doi.org/10.3390/membranes12070664
Gonzalez Ortiz D, Nouxet M, Maréchal W, Lorain O, Deratani A, Pochat-Bohatier C. Immobilization of poly(vinyl pyrrolidone) in Polysulfone Membranes by Radically-Initiated Crosslinking Using Potassium Persulfate. Membranes. 2022; 12(7):664. https://doi.org/10.3390/membranes12070664
Chicago/Turabian StyleGonzalez Ortiz, Danae, Morgan Nouxet, William Maréchal, Olivier Lorain, André Deratani, and Céline Pochat-Bohatier. 2022. "Immobilization of poly(vinyl pyrrolidone) in Polysulfone Membranes by Radically-Initiated Crosslinking Using Potassium Persulfate" Membranes 12, no. 7: 664. https://doi.org/10.3390/membranes12070664