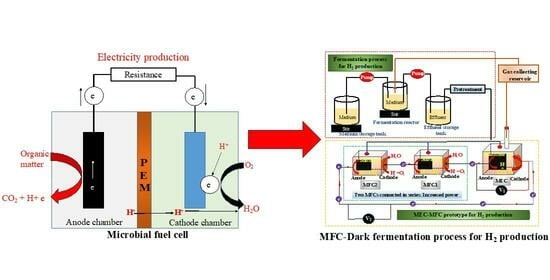
An Overview of Microbial Fuel Cell Technology for Sustainable Electricity Production
Abstract
:Highlights
- Configurations and operations of microbial fuel cells are discussed.
- Bioelectrochemical system performance depends on the type of design and electrode materials.
- Microbial fuel cells are a feasible alternative for fuel production and wastewater treatment.
- Advances in electrode materials are summarized.
- Techno-economic and life-cycle assessments of microbial fuel cells are highlighted.
Abstract
1. Introduction

2. Influence Factors of MFCs
2.1. Designs, Configurations, and Operation

| Configuration of BES | Working Volume (mL) | Operation (Days) | Type of Electrolyte | Removal Efficiency (%) | Maximum Power Generation | Refs. |
|---|---|---|---|---|---|---|
| SC-MFC | 850 | ~30 | Activated sludge | N/A | 105 mW/m2 | [61] |
| SC-MFC | 150 | 30 | Synthetic wastewaters | 89 (COD) | 450.36 mW/m2 | [68] |
| SC-MFC | 80 | N/A | Wastewater | 83 (COD) | 548 mW/m2 | [69] |
| SC-MFC | N/A | 18 | Wastewater | 81; 94 (COD) | 989 mV | [70] |
| SC-MFC | 100 | N/A | Wastewater | 73.7 HCQ | 241–280 mW/m2 | [71] |
| DC-MFC | 120 | N/A | Uranium-containing wastewater | 99.0 U(VI) | 269.5 mW/m2 | [72] |
| DC-MFC | 1000 | N/A | Wastewater | 92 (Ni); 87 (Cd) | 722 mW/m3 | [73] |
| DC-MFC | 100–200 | N/A | Wastewater | 90 (COD); 40; 60 (orgN) | 1.69 A/m2 | [74] |
| DC-MFC | 118 | 30 | Sewage sludge | 99.08 (P) | ~40 mV | [75] |
| DC-MFC | 250 | 28 | Wastewater | 95.7; 94.7; 92.37 (COD) | 1696.56 mW/m2 | [76] |
| DC-MFC | 300 | 30 | Wastewater | 70–88; 18–44 (COD) | 2.2; 44.6; 86.9 mW/m2 | [77] |
| DC-MFC | 125 (125 cm3) | 6 (144 h) | Wastewater | 99.16 (Cu+2) | 24.75 mW/m2 | [78] |
| TC-MFC | 28 | ~2 (50 h) | Synthetic municipal wastewater | 80.0 (Iron); 22.1 (Sulfur) | 576.6; 184.8 mW/m2 | [79] |
| TC-MFC | 28 | 8 | Wastewater | 86.2 (Cu+2) | 420 mW/m2 | [80] |
| Stacked MFC | 37.5 | ~9 | Barley–shochu waste | 36.7 (COD) | 15.7 mW/m2 | [81] |
| Stacked MFC | 28 | N/A | Effluent | 16.9 (COD) | 1023; 1076 mW/m2 | [82] |
| Stacked MFC | N/A | N/A | Substrate | N/A | 21,111 W/m3 | [83] |
| Stacked MFC | N/A | ~60 | Wastewater | 70.0 (Sulfide), 54.6 (COD) | 3.29 mA | [84] |
2.2. Electrode Materials
| Type of Materials | Advantage | Disadvantage | Refs. |
|---|---|---|---|
| Carbon paper | High conductivity and stability | High cost and poor environmental friendliness | [90] |
| Carbon cloth | Great porosity and high conductivity and stability | Small surface area and corrosion issues | [91] |
| Graphite felt | Great porosity and high conductivity and stability | Low surface area and corrosion issues | [92] |
| Graphite brush | |||
| Graphite rod | High conductivity and stability, very easy to use, and cheaper | Surface area not easy to increase | [93] |
| Stainless steel | High conductivity, very easy to use, and cheaper | Easy corrosion, problems with biocompatibility, and very low surface area | [94] |
| Graphene | High mechanical strength, biocompatibility, and good conductor of electricity | Susceptibility to oxidative environments and expensive and complex to process | [95] |
| Metal oxide | Low cost, high operating voltage, and ecofriendly | Expensive | [96] |
| Bamboo charcoal | Low resistance, high mechanical strength, high chemical stability and corrosion resistance, strong biocompatibility, and cheaper | High cost for large-scale implementation | [43] |
2.3. Membrane
2.4. Influence of Microorganisms
3. Bioenergy Production from MFCs
3.1. Bioelectricity Production
| Configuration Type | Electrode Materials | Membrane Type | Substrate | Working Volume (mL) | Operation (Days) | Max. Power Generation | Refs. | |
|---|---|---|---|---|---|---|---|---|
| Anode | Cathode | |||||||
| SC-MFC | Carbon felt (16 cm2) | Carbon felt (31 cm2) | Clayware | Synthetic wastewater | 150 | 30 | 995.73 mW/m3 | [131] |
| DC-MFC | Carbon fiber | Carbon fiber | SPEEK-goethite | N/A | N/A | N/A | 73.7 mW/m2 | [132] |
| T-MFC | Graphite rod | Carbon cloth coated Pt (200 cm2) | Nanocomposite | Sewage wastewater | 300 | 3 weeks | 138 mW/m2 | [117] |
| DC-MFC | Graphite | Graphite | Nation 117 | Activated strains | 500 | N/A | 12.82 mW/m2 | [133] |
| SMFC | Carbon-polymer composite | Carbon cloth (3 × 3 cm2) | N/A | Sediment from wastewater | 100 | 30 | 1056.6 W/m3 | [134] |
| MFC | Carbon fiber brushes | Carbon fiber brushes | Nafion 117 | Glucose, yeast, and MB | 800 | N/A | 5.55 W/m3 | [135] |
| SC-MFC | Carbon brush | Lignin-derived activated carbon | N/A | Sludge | 125 | N/A | 6.7–6.5 mW | [136] |
| C-MFC | Activated carbon coated carbon veil (30 mg/m2)/pressed over stainless steel mesh | Activated carbon coated carbon veil (30 mg/m2)/pressed over stainless steel mesh | Flat terracotta membrane (12.25 cm2) | Human urine and sludge | 12.5 | N/A | 492.85 μW | [32] |
| S-MFC | Graphite felt (7 × 7 × 0.4 cm) | Carbon cloth coated-Pt, plain carbon cloth, and graphite felt | N/A | Soil | N/A | ~50 | 87.3 mW/m2 | [137] |
| DC-MFC | Graphite filter | Stainless steel mesh | Carbon-ceramic composite | Wastewater | 5.3 (cm3) | N/A | 0.699 W/m3 | [138] |
| SC-MFC | Wired stainless steel 60 mesh | Wired stainless steel 60 mesh | Cylindrical terra- cotta pots | Textile effluent | N/A | N/A | 21–42 mW/m2 | [139] |
| SC-MFC | Graphite brush | graphite-based nanomaterials | N/A | Anaerobic mud | N/A | 30 | 2203 mW/m2 | [115] |
| SC-SMFC | Unidirectional Carbon Fiber (total area 81 cm2) | Unidirectional Carbon Fiber (total area 40.5 cm2) | N/A | Marine and fluvial sediments | 2000 | 30 | 70 mW/cm2 | [140] |
| SC-MFC | Graphite felt (thickness 10 mm, diameter 80 mm) | Graphite felt (thickness 10 mm, diameter 80 mm) | N/A | Oily sludge | 2000 | ~31 | 1277.90 mW/m3 | [141] |
| SC-MFC | Carbon cloth | Carbon cloth | Nafion 117 | Agro-waste | 200 | N/A | 590 mW/m2 | [116] |
3.2. Other Types of Energy Production
4. Applications and Challenges of MFCs
5. LCA of MFCs
6. Conclusions and Future Research Directions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Kundu, P.P. Biocatalysts in microbial fuel cells. Enzyme Microb. Technol. 2010, 47, 179–188. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Meyer, E.L.; Nwokolo, N. Microbial fuel cells, a renewable energy technology for bio-electricity generation: A mini-review. Electrochem. Commun. 2021, 125, 107003. [Google Scholar] [CrossRef]
- Sadeq, A.M.; Ismail, Z.Z. Sustainable application of tubular photosynthesis microbial desalination cell for simultaneous desalination of seawater for potable water supply associated with sewage treatment and energy recovery. Sci. Total Environ. 2023, 875, 162630. [Google Scholar] [CrossRef]
- Imoro, A.Z.; Mensah, M.; Buamah, R. Developments in the microbial desalination cell technology: A review. Water Energy Nexus 2021, 4, 76–87. [Google Scholar] [CrossRef]
- Jatoi, A.S.; Hashmi, Z.; Mazari, S.A.; Mubarak, N.M.; Karri, R.R.; Ramesh, S.; Rezakazemi, M. A comprehensive review of microbial desalination cells for present and future challenges. Desalination 2022, 535, 115808. [Google Scholar] [CrossRef]
- Tanguay-Rioux, F.; Nwanebu, E.; Thadani, M.; Tartakovsky, B. On-line current control for continuous conversion of CO2 to CH4 in a microbial electrosynthesis cell. Biochem. Eng. J. 2023, 197, 108965. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Chen, K.; Gu, W.; Zhang, Y.H.P.; Zhu, Z. Enzymatic biofuel cell-powered iontophoretic facial mask for enhanced transdermal drug delivery. Biosens. Bioelectron. 2023, 223, 115019. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, C.; Jiang, Y.; Li, P.; Xia, C. A robust fluorine-containing ceramic cathode for direct CO2 electrolysis in solid oxide electrolysis cells. J. Energy Chem. 2023, 77, 300–309. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Van Bogaert, G.; Olsen, S.I.; Nigam, P.S.; Diels, L.; Vanbroekhoven, K. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012, 2, 1248–1263. [Google Scholar] [CrossRef]
- Apollon, W.; Kamaraj, S.K.; Silos-Espino, H.; Perales-Segovia, C.; Valera-Montero, L.L.; Maldonado-Ruelas, V.A.; Vázquez-Gutiérrez, M.A.; Ortiz-Medina, R.A.; Flores-Benítez, S.; Gómez-Leyva, J.F. Impact of Opuntia species plant bio-battery in a semi-arid environment: Demonstration of their applications. Appl. Energy 2020, 279, 115788. [Google Scholar] [CrossRef]
- Apollon, W.; Valera-Montero, L.L.; Perales-Segovia, C.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A.; Gómez-Leyva, J.F.; Vázquez-Gutiérrez, M.A.; Flores-Benítez, S.; Kamaraj, S.K. Effect of ammonium nitrate on novel cactus pear genotypes aided by biobattery in a semi-arid ecosystem. Sustain. Energy Technol. Assess. 2022, 49, 101730. [Google Scholar] [CrossRef]
- Ji, B.; Zhao, Y.; Yang, Y.; Li, Q.; Man, Y.; Dai, Y.; Fu, J.; Wei, T.; Tai, Y.; Zhang, X. Curbing per-and polyfluoroalkyl substances (PFASs): First investigation in a constructed wetland-microbial fuel cell system. Water Res. 2023, 230, 119530. [Google Scholar] [CrossRef] [PubMed]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Maldonado-Ruelas, V.A.; Ortiz-Medina, R.A. Self-sustainable nutrient recovery associated to power generation from livestock’s urine using plant-based bio-batteries. Fuel 2023, 332, 126252. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.; Wu, M.; Ding, J.; Zhang, W. Biodegradation of organic matter and anodic microbial communities analysis in sediment microbial fuel cells with/without Fe(III) oxide addition. Bioresour. Technol. 2017, 225, 402–408. [Google Scholar] [CrossRef]
- Li, M.; Zhou, M.; Tian, X.; Tan, C.; McDaniel, C.T.; Hassett, D.J.; Gu, T. Microbial fuel cell (MFC) power performance improvement through enhanced microbial electrogenicity. Biotechnol. Adv. 2018, 36, 1316–1327. [Google Scholar] [CrossRef]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial fuel cells and their electrified biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef]
- Gude, V.G. Wastewater treatment in microbial fuel cells—An overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Dai, M.; Wu, Y.; Wang, J.; Lv, Z.; Li, F.; Zhang, Y.; Kong, Q. Constructed wetland-microbial fuel cells enhanced with iron carbon fillers for ciprofloxacin wastewater treatment and power generation. Chemosphere 2022, 305, 135377. [Google Scholar] [CrossRef] [PubMed]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, S.; Das, S.; Hossain, M.T.; Islam, A.; Miah, M.R.; Awual, M.R. Sustainable approach for wastewater treatment using microbial fuel cells and green energy generation—A comprehensive review. J. Mol. Liq. 2021, 344, 117795. [Google Scholar] [CrossRef]
- Terbish, N.; Popuri, S.R.; Lee, C.H. Improved performance of organic–inorganic nanocomposite membrane for bioelectricity generation and wastewater treatment in microbial fuel cells. Fuel 2023, 332, 126167. [Google Scholar] [CrossRef]
- Abubackar, H.N.; Biryol, İ.; Ayol, A. Yeast industry wastewater treatment with microbial fuel cells: Effect of electrode materials and reactor configurations. Int. J. Hydrogen Energy 2023, 48, 12424–12432. [Google Scholar] [CrossRef]
- Wang, Y.; He, C.; Li, W.; Zong, W.; Li, Z.; Yuan, L.; Wang, G.; Mu, Y. High power generation in mixed-culture microbial fuel cells with corncob-derived three-dimensional N-doped bioanodes and the impact of N dopant states. J. Chem. Eng. 2020, 399, 125848. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Ye, J.; Li, B. Atomically dispersed Fe–N4 moieties in porous carbon as efficient cathode catalyst for enhancing the performance in microbial fuel cells. J. Power Sources 2023, 556, 232434. [Google Scholar] [CrossRef]
- Rusyn, I. Role of microbial community and plant species in performance of plant microbial fuel cells. Renew. Sustain. Energy Rev. 2021, 152, 111697. [Google Scholar] [CrossRef]
- Apollon, W.; Rusyn, I.; González-Gamboa, N.; Kuleshova, T.; Luna-Maldonado, A.I.; Vidales-Contreras, J.A.; Kamaraj, S.K. Improvement of zero waste sustainable recovery using microbial energy generation systems: A comprehensive review. Sci. Total Environ. 2022, 817, 153055. [Google Scholar] [CrossRef]
- Sharma, M.; Salama, E.S.; Thakur, N.; Alghamdi, H.; Jeon, B.H.; Li, X. Advances in the biomass valorization in bioelectrochemical systems: A sustainable approach for microbial-aided electricity and hydrogen production. Chem. Eng. J. 2023, 465, 142546. [Google Scholar] [CrossRef]
- Gadkari, S.; Fontmorin, J.M.; Yu, E.; Sadhukhan, J. Influence of temperature and other system parameters on microbial fuel cell performance: Numerical and experimental investigation. Chem. Eng. J. 2020, 388, 124176. [Google Scholar] [CrossRef]
- Rezk, H.; Olabi, A.G.; Abdelkareem, M.A.; Sayed, E.T. Artificial intelligence as a novel tool for enhancing the performance of urine fed microbial fuel cell as an emerging approach for simultaneous power generation and wastewater treatment. J. Taiwan Inst. Chem. Eng. 2023, 148, 104726. [Google Scholar] [CrossRef]
- Wang, J.; Ren, K.; Zhu, Y.; Huang, J.; Liu, S. A review of recent advances in microbial fuel cells: Preparation, operation, and application. Biotech 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ibrahim, M.N.M.; Umar, K. Electrode Material as Anode for Improving the Electrochemical Performance of Microbial Fuel Cells. In Energy Storage Battery Systems—Fundamentals and Applications; Haider, S., Haider, A., Khodaei, M., Chen, L., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patel, V.; Shah, M.T.; Jadhav, D.A.; Munshi, N.S.; Chendake, A.D.; Pant, D. Effective power management system in stacked microbial fuel cells for onsite applications. J. Power Sources 2022, 517, 230684. [Google Scholar] [CrossRef]
- Zhang, Y.; Mo, G.; Li, X.; Zhang, W.; Zhang, J.; Ye, J.; Huang, X.; Yu, C. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources 2011, 196, 5402–5407. [Google Scholar] [CrossRef]
- Li, B.; Zhou, J.; Zhou, X.; Wang, X.; Li, B.; Santoro, C.; Grattieri, M.; Babanova, S.; Artyushkova, K.; Atanassov, P.; et al. Surface modification of microbial fuel cells anodes: Approaches to practical design. Electrochim. Acta 2014, 134, 116–126. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Wu, J.; Liu, R.; Dong, P.; Li, N.; He, W.; Feng, Y.; Liu, J. Enhanced electricity generation and storage by nitrogen-doped hierarchically porous carbon modification of the capacitive bioanode in microbial fuel cells. Sci. Total Environ. 2023, 858, 159688. [Google Scholar] [CrossRef]
- Kim, M.; Li, S.; Song, Y.E.; Park, S.Y.; Kim, H.I.; Jae, J.; Chung, I.; Kim, J.R. Polydopamine/polypyrrole-modified graphite felt enhances biocompatibility for electroactive bacteria and power density of microbial fuel cell. Chemosphere 2023, 313, 137388. [Google Scholar] [CrossRef]
- Nawaz, A.; ul Haq, I.; Qaisar, K.; Gunes, B.; Raja, S.I.; Mohyuddin, K.; Amin, H. Microbial fuel cells: Insight into simultaneous wastewater treatment and bioelectricity generation. Process Saf. Environ. Prot. 2022, 161, 357–373. [Google Scholar] [CrossRef]
- Sato, C.; Paucar, N.E.; Chiu, S.; Mahmud, M.Z.I.M.; Dudgeon, J. Single-Chamber Microbial Fuel Cell with Multiple Plates of Bamboo Charcoal Anode: Performance Evaluation. Processes 2021, 9, 2194. [Google Scholar] [CrossRef]
- Priya, R.L.; Ramachandran, T.; Suneesh, P.V. Fabrication and characterization of high power dual chamber E. coli microbial fuel cell. IOP Conf. Ser. Mater. Sci. Eng. 2016, 149, 012215. [Google Scholar] [CrossRef]
- Choudhury, P.; Bhunia, B.; Mahata, N.; Bandyopadhyay, T.K. Optimization for the improvement of power in equal volume of single chamber microbial fuel cell using dairy wastewater. J. Indian Chem. Soc. 2022, 99, 100489. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Dhillon, S.K.; Kundu, P.P. 1-D semiconducting TiO2 nanotubes supported efficient bimetallic Co-Ni cathode catalysts for power generation in single-chambered air-breathing microbial fuel cells. Sustain. Energy Technol. Assess. 2022, 53, 102479. [Google Scholar] [CrossRef]
- Zhai, D.D.; Fang, Z.; Jin, H.; Hui, M.; Kirubaharan, C.J.; Yu, Y.Y.; Yong, Y.C. Vertical alignment of polyaniline nanofibers on electrode surface for high-performance microbial fuel cells. Bioresour. Technol. 2019, 288, 121499. [Google Scholar] [CrossRef]
- Sumisha, A.; Haribabu, K. Modification of graphite felt using nano polypyrrole and polythiophene for microbial fuel cell applications—A comparative study. Int. J. Hydrogen Energy 2018, 43, 3308–3316. [Google Scholar] [CrossRef]
- Liang, H.; Han, J.; Yang, X.; Qiao, Z.; Yin, T. Performance improvement of microbial fuel cells through assembling anodes modified with nanoscale materials. Nanomater. Nanotechnol. 2022, 12, 18479804221132965. [Google Scholar] [CrossRef]
- Ouyang, T.; Liu, W.; Shi, X.; Li, Y.; Hu, X. Multi-criteria assessment and triple-objective optimization of a bio-anode microfluidic microbial fuel cell. Bioresour. Technol. 2023, 382, 129193. [Google Scholar] [CrossRef] [PubMed]
- Chiranjeevi, P.; Patil, S.A. Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies. Biotechnol. Adv. 2020, 39, 107468. [Google Scholar] [CrossRef]
- Rossi, R.; Hur, A.Y.; Page, M.A.; Thomas, B.; Butkiewicz, J.J.; Jones, D.W.; Baek, G.; Saikaly, P.E.; Cropek, D.M.; Logan, B.E. Pilot scale microbial fuel cells using air cathodes for producing electricity while treating wastewater. Water Res. 2022, 215, 118208. [Google Scholar] [CrossRef]
- Sato, C.; Apollon, W.; Luna-Maldonado, A.I.; Paucar, N.E.; Hibbert, M.; Dudgeon, J. Integrating Microbial Fuel Cell and Hydroponic Technologies Using a Ceramic Membrane Separator to Develop an Energy-Water-Food Supply System. Membranes 2023, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Marassi, R.J.; Queiroz, L.G.; Silva, D.C.V.; da Silva, F.T.; Silva, G.C.; de Paiva, T.C.B. Performance and toxicity assessment of an up-flow tubular microbial fuel cell during long-term operation with high-strength dairy wastewater. J. Clean. Prod. 2020, 259, 120882. [Google Scholar] [CrossRef]
- Apollon, W.; Kamaraj, S.-K.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Vidales-Contreras, J.A.; Mardueño-Aguilar, M.V.; Luna-Maldonado, A.I. Bio-electricity production in a single-chamber microbial fuel cell using urine as a substrate. Biofuels 2023, 1–11. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Deng, L.; Chen, Z.; Nguyen, T.V. Performance of mediator-less double chamber microbial fuel cell-based biosensor for measuring biological chemical oxygen. J. Environ. Manag. 2020, 276, 111279. [Google Scholar] [CrossRef]
- Yap, K.L.; Ho, L.N.; Guo, K.; Liew, Y.M.; Lutpi, N.A.; Azhari, A.W.; Thor, S.H.; Teoh, T.P.; Oon, Y.S.; Ong, S.A. Exploring the potential of metal oxides as cathodic catalysts in a double chambered microbial fuel cell for phenol removal and power generation. J. Water Process Eng. 2023, 53, 103639. [Google Scholar] [CrossRef]
- Singh, L.; Mahapatra, D.M. (Eds.) Waste to Sustainable Energy: MFCs—Prospects through Prognosis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar] [CrossRef]
- Arends, J.B.; Speeckaert, J.; Blondeel, E.; De Vrieze, J.; Boeckx, P.; Verstraete, W.; Rabaey, K.; Boon, N. Greenhouse gas emissions from rice microcosms amended with a plant microbial fuel cell. Appl. Microbiol. Biotechnol. 2014, 98, 3205–3217. [Google Scholar] [CrossRef]
- Eslami, S.; Bahrami, M.; Zandi, M.; Fakhar, J.; Gavagsaz-Ghoachani, R.; Noorollahi, Y.; Phattanasak, M.; Nahid-Mobarakeh, B. Performance investigation and comparison of polypropylene to Nafion117 as the membrane of a dual-chamber microbial fuel cell. Clean. Mater. 2023, 8, 100184. [Google Scholar] [CrossRef]
- Papillon, J.; Olivier, O.; Éric, M. Scale up of single-chamber microbial fuel cells with stainless steel 3D anode: Effect of electrode surface areas and electrode spacing. Bioresour. Technol. Rep. 2021, 13, 100632. [Google Scholar] [CrossRef]
- Li, D.; Sun, Y.; Shi, Y.; Wang, Z.; Okeke, S.; Yang, L.; Xiao, L. Structure evolution of air cathodes and their application in electrochemical sensor development and wastewater treatment. Sci. Total Environ. 2023, 869, 161689. [Google Scholar] [CrossRef]
- Al Lawati, M.J.; Jafary, T.; Baawain, M.S.; Al-Mamun, A. A mini review on biofouling on air cathode of single chamber microbial fuel cell; prevention and mitigation strategies. Biocatal. Agric. Biotechnol. 2019, 22, 101370. [Google Scholar] [CrossRef]
- Hidayat, A.R.P.; Widyanto, A.R.; Asranudin, A.; Ediati, R.; Sulistiono, D.O.; Putro, H.S.; Sugiarso, D.; Prasetyoko, D.; Purnomo, A.S.; Bahruji, H.; et al. Recent development of double chamber microbial fuel cell for hexavalent chromium waste removal. J. Environ. Chem. Eng. 2022, 10, 107505. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Removal of heavy metals from industrial wastewater using microbial fuel cell. Eng. Life Sci. 2022, 22, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, S.; Wang, K.; Wang, M.; Chen, B.; Wang, Z.; Tan, T. Improved energy efficiency in microbial fuel cells by bioethanol and electricity co-generation. Biotechnol. Biofuels Bioprod. 2022, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Rudra, R.; Hait, S. Sulfonated polyvinylidene fluoride-crosslinked-aniline-2-sulfonic acid as ion exchange membrane in single-chambered microbial fuel cell. J. Environ. Chem. Eng. 2021, 9, 106467. [Google Scholar] [CrossRef]
- Kolubah, P.D.; Mohamed, H.O.; Ayach, M.; Hari, A.R.; Alshareef, H.N.; Saikaly, P.; Chae, K.-J.; Castaño, P. W2N-MXene composite anode catalyst for efficient microbial fuel cells using domestic wastewater. Chem. Eng. J. 2023, 461, 141821. [Google Scholar] [CrossRef]
- Adnan, M.Y.; Hassoon Ali, A. Investigating the Performance of Single Chamber Microbial Fuel Cell (SCMFC) as Sustainable-Innovative Technique for Electricity Generation and Wastewater Treatment. Defect Diffus. Forum 2023, 425, 107–117. [Google Scholar] [CrossRef]
- Wang, C.; Xing, Y.; Zhang, K.; Zheng, H.; Zhang, Y.N.; Zhu, X.; Yuan, X.; Qu, J. Evaluation of photocathode coupling-mediated hydroxychloroquine degradation in a single-chamber microbial fuel cell based on electron transfer mechanism and power generation. J. Power Sources 2023, 559, 232625. [Google Scholar] [CrossRef]
- Wu, X.; Lv, C.; Ye, J.; Li, M.; Zhang, X.; Lv, J.; Fang, Q.; Yu, S.; Xie, W. Glycine-hydrochloric acid buffer promotes simultaneous U (VI) reduction and bioelectricity generation in dual chamber microbial fuel cell. J. Taiwan Inst. Chem. Eng. 2021, 127, 236–247. [Google Scholar] [CrossRef]
- Singh, A.; Kaushik, A. Removal of Cd and Ni with enhanced energy generation using biocathode microbial fuel cell: Insights from molecular characterization of biofilm communities. J. Clean. Prod. 2021, 315, 127940. [Google Scholar] [CrossRef]
- Burns, M.; Qin, M. Ammonia recovery from organic nitrogen in synthetic dairy manure with a microbial fuel cell. Chemosphere 2023, 325, 138388. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tai, Y.; Zhao, X.; He, Q.; Hu, Z.; Li, M. Phosphorus recovery directly from sewage sludge as vivianite and simultaneous electricity production in a dual chamber microbial fuel cell. J. Environ. Chem. Eng. 2023, 11, 110152. [Google Scholar] [CrossRef]
- Huang, S.J.; Dwivedi, K.A.; Kumar, S.; Wang, C.T.; Yadav, A.K. Binder-free NiO/MnO2 coated carbon based anodes for simultaneous norfloxacin removal, wastewater treatment and power generation in dual-chamber microbial fuel cell. Environ. Pollut. 2023, 317, 120578. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Mishra, V. Bioelectricity generation by microbial degradation of banana peel waste biomass in a dual-chamber S. cerevisiae-based microbial fuel cell. Biomass Bioenergy 2023, 168, 106677. [Google Scholar] [CrossRef]
- Wang, H.; Chai, G.; Zhang, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Li, J.; Lin, Y.; et al. Copper removal from wastewater and electricity generation using dual-chamber microbial fuel cells with shrimp shell as the substrate. Electrochim. Acta 2023, 441, 141849. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, L.; Zhu, Y.; Li, X.; Ren, Y. A triple-chamber microbial fuel cell enabled to synchronously recover iron and sulfur elements from sulfide tailings. J. Hazard. Mater. 2021, 401, 123307. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Chen, F.; Xu, L.; Jin, Y.; Xu, S.; Wang, J.; Shen, X.; Zhang, L.; Song, Y. Bioelectrochemical process for simultaneous removal of copper, ammonium and organic matter using an algae-assisted triple-chamber microbial fuel cell. Sci. Total Environ. 2021, 798, 149327. [Google Scholar] [CrossRef]
- Fujimura, S.; Kamitori, K.; Kamei, I.; Nagamine, M.; Miyoshi, K.; Inoue, K. Performance of stacked microbial fuel cells with barley–shochu waste. J. Biosci. Bioeng. 2022, 133, 467–473. [Google Scholar] [CrossRef]
- Lin, N.; Yang, Q.; Feng, Y. Optimization of catalyst dosage and total volume for extendible stacked microbial fuel cell reactors using spacer. J. Power Sources 2022, 517, 230697. [Google Scholar] [CrossRef]
- Shirkosh, M.; Hojjat, Y.; Mardanpour, M.M. Evaluation and optimization of the Zn-based microfluidic microbial fuel cells to power various electronic devices. Biosens. Bioelectron. X 2022, 12, 100254. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, B.; Liu, Y.; Wang, Z.; Hao, L. Continuous bioelectricity generation with simultaneous sulfide and organics removals in an anaerobic baffled stacking microbial fuel cell. Int. J. Hydrogen Energy 2015, 40, 8128–8136. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Naveenkumar, M.; Ratnam, M.V.; Samraj, S. A review on scaling-up of microbial fuel cell: Challenges and opportunities. In Scaling Up of Microbial Electrochemical Systems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 13–28. [Google Scholar] [CrossRef]
- Mustakeem. Electrode materials for microbial fuel cells: Nanomaterial approach. Mater. Renew. Sustain. Energy 2015, 4, 22. [Google Scholar] [CrossRef]
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef]
- Santoro, C.; Kodali, M.; Herrera, S.; Serov, A.; Ieropoulos, I.; Atanassov, P. Power generation in microbial fuel cells using platinum group metal-free cathode catalyst: Effect of the catalyst loading on performance and costs. J. Power Sources 2018, 378, 169–175. [Google Scholar] [CrossRef]
- Tan, K.L.; Reeves, S.; Hashemi, N.; Thomas, D.G.; Kavak, E.; Montazami, R.; Hashemi, N. Graphene as Flexible Electrode: Review of Fabrication Approaches. J. Mater. Chem. 2017, 5, 17777–17803. [Google Scholar] [CrossRef]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 61, 106716. [Google Scholar] [CrossRef]
- Li, D.; Feng, Y.; Li, F.; Tang, J.; Hua, T. Carbon Fibers for Bioelectrochemical: Precursors, Bioelectrochemical System, and Biosensors. Adv. Fiber Mater 2023, 5, 699–730. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Cretin, M. Carbon felt based-electrodes for energy and environmental applications: A review. Carbon 2017, 122, 564–591. [Google Scholar] [CrossRef]
- Roy, H.; Rahman, T.U.; Tasnim, N.; Arju, J.; Rafid, M.M.; Islam, M.R.; Pervez, M.N.; Cai, Y.; Naddeo, V.; Islam, M.S. Microbial Fuel Cell Construction Features and Application for Sustainable Wastewater Treatment. Membranes 2023, 13, 490. [Google Scholar] [CrossRef]
- Dumas, C.; Mollica, A.; Fron, D.; Bassguy, R.; Etcheverry, L.; Bergel, A. Marine microbial fuel cell: Use of stainless steel electrodes as anode and cathode materials. Electrochim. Acta 2007, 53, 468. [Google Scholar] [CrossRef]
- IAS EXPRESS. [In-Depth] Graphene: Applications, Advantages, Disadvantages. Available online: https://www.iasexpress.net/graphene-features-advantages-disadvantages-upsc-ias-gk/ (accessed on 16 October 2023).
- Ullah, K.; Shah, N.; Wadood, R.; Khan, B.M.; Oh, W.C. Recent trends in graphene based transition metal oxides as anode materials for rechargeable lithium-ion batteries. Nano Trends 2023, 1, 100004. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim, M.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent Advances in Anodes for Microbial Fuel Cells: An Overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Apollon, W.; Luna-Maldonado, A.I.; Kamaraj, S.K.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Aranda-Ruíz, J. Progress and recent trends in photosynthetic assisted microbial fuel cells: A review. Biomass Bioenergy 2021, 148, 106028. [Google Scholar] [CrossRef]
- Scott, K. An Introduction to Microbial Fuel Cells. In Microbial Electrochemical and Fuel Cells; Woodhead Publishing: Sawston, UK, 2016; pp. 3–27. [Google Scholar] [CrossRef]
- Scott, K. Membranes and Separators for Microbial Fuel Cells; Woodhead Publishing: Sawston, UK, 2016; pp. 153–178. [Google Scholar] [CrossRef]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2009, 26, 450–459. [Google Scholar] [CrossRef]
- Heijne, T.A.; Hamelers, H.V.M.; De Wilde, V.; Rozendal, R.A.; Buisman, C.J.N. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Lefebvre, O.; Shen, Y.; Tan, Z.; Uzabiaga, A.; Chang, I.S.; Ng, H.Y. A comparison of membranes and enrichment strategies for microbial fuel cells. Bioresour. Technol. 2011, 102, 6291–6294. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Ismail, A.F.; Jafari, Y.; Ismail, M.; Mayahi, A.; Othman, J. Simultaneous wastewater treatment and electricity generation by microbial fuel cell: Performance comparison and cost investigation of using Nafion 117 and SPEEK as separators. Desalination 2013, 325, 1–6. [Google Scholar] [CrossRef]
- Tang, H.; Guo, K.; Li, H.; Du, Z.; Tian, J. Microfiltration membrane performance in two-chamber microbial fuel cells. Biochem. Eng. J. 2010, 52, 194–198. [Google Scholar] [CrossRef]
- Dharmalingam, S.; Kugarajah, V.; Sugumar, M. Membranes for Microbial Fuel Cells. Microb. Electrochem. Technol. 2019, 143–194. [Google Scholar] [CrossRef]
- Sondhi, R.; Bhave, R.; Jung, G. Applications and benefits of ceramic membranes. Membr. Technol. 2003, 11, 5–8. [Google Scholar] [CrossRef]
- Kamaraj, S.-K.; Rivera, A.E.; Murugesan, S.; García-Mena, J.; Maya, O.; Frausto-Reyes, C.; Tapia-Ramírez, J.; Espino, H.S.; Caballero-Briones, F. Electricity generation from Nopal biogas effluent using a surface modified clay cup (cantarito) microbial fuel cell. Heliyon 2019, 5, e01506. [Google Scholar] [CrossRef] [PubMed]
- Lovley, D.R. Bug juice: Harvesting electricity with microorganisms. Nat. Rev. Microbiol. 2006, 4, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, A. Modelling the impact of operating mode and electron transfer mechanism in microbial fuel cells with two-species anodic biofilm. Biochem. Eng. J. 2020, 158, 107560. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, H.; Liu, W.; Zhang, R.; Guo, J.; Xian, M.; Liu, H. Electricigens in the anode of microbial fuel cells: Pure cultures versus mixed communities. Microb. Cell Factories 2019, 18, 39. [Google Scholar] [CrossRef]
- Sherafatmand, M.; Ng, H.Y. Using sediment microbial fuel cells (SMFCs) for bioremediation of polycyclic aromatic hydrocarbons (PAHs). Bioresour. Technol. 2015, 195, 122–130. [Google Scholar] [CrossRef]
- Xu, J.Z.; Zhang, W.G. Strategies used for genetically modifying bacterial genome: Site-directed mutagenesis, gene inactivation, and gene over-expression. J. Zhejiang Univ. Sci. B 2016, 17, 83–99. [Google Scholar] [CrossRef]
- Sathish, T.; Sathyamurthy, R.; Kumar, S.S.; Huynh, G.B.; Saravanan, R.; Rajasimman, M. Amplifying power generation in microbial fuel cells with cathode catalyst of graphite-based nanomaterials. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Subran, N.; Ajit, K.; Krishnan, H.; Pachiyappan, S.; Ramaswamy, P. Synthesis and performance of a cathode catalyst derived from areca nut husk in microbial fuel cell. Chemosphere 2023, 312, 137303. [Google Scholar] [CrossRef]
- Sugumar, M.; Dharmalingam, S. Statistical assessment of operational parameters using optimized sulphonated titanium nanotubes incorporated sulphonated polystyrene ethylene butylene polystyrene nanocomposite membrane for efficient electricity generation in microbial fuel cell. Energy 2022, 242, 123000. [Google Scholar] [CrossRef]
- Bensaida, K.; Maamoun, I.; Eljamal, R.; Falyouna, O.; Sugihara, Y.; Eljamal, O. New insight for electricity amplification in microbial fuel cells (MFCs) applying magnesium hydroxide coated iron nanoparticles. Energy Convers. Manag. 2021, 249, 114877. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Production of electricity from acetate or butyrate using a single-chamber microbial fuel cell. Environ. Sci. Technol. 2005, 39, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Khater, D.; El-Khatib, K.M.; Hazaa, M.; Hassan, R. Electricity generation using Glucose as substrate in microbial fuel cell. J. Environ. Sci. 2015, 2, 84–98. [Google Scholar]
- Hashmi, Z.; Jatoi, A.S.; Aziz, S.; Soomro, S.A.; Abbasi, S.A.; Usto, M.A.; Alam, M.S.; Anjum, A.; Iqbal, A.; Usman, M.T. Bio-assisted treatment of hazardous spent wash via microbial fuel cell. Environmental friendly approach. Biomass Convers. Biorefinery 2021, 13, 5981–5989. [Google Scholar] [CrossRef]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N.L. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sustain. Energy Rev. 2020, 132, 110041. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.-H.; Zhang, J.-T.; Chi, Z.-Y.; Kong, F.T.; Zhang, Q. The long overlooked microalgal nitrous oxide emission: Characteristics, mechanisms, and influencing factors in microalgae-based wastewater treatment scenarios. Sci. Total Environ. 2023, 856, 159153. [Google Scholar] [CrossRef]
- Shukla, M.; Kumar, S. Algal growth in photosynthetic algal microbial fuel cell and its subsequent utilization for biofuels. Renew. Sustain. Energy Rev. 2018, 82, 402–414. [Google Scholar] [CrossRef]
- Mahmoud, R.H.; Samhan, F.A.; Ibrahim, M.K.; Ali, G.H.; Hassan, R.Y. Waste to energy conversion utilizing nanostructured Algal-based microbial fuel cells. Electrochem. Sci. Adv. 2021, 2, e2100071. [Google Scholar] [CrossRef]
- Ribeiro, V.R.; Osório, H.D.D.; Ulrich, A.C.; Rizzetti, T.M.; Barrios, A.S.; de Cassia de Souza Schneider, R.; Benitez, L.B. The use of microalgae-microbial fuel cells in wastewater bioremediation and bioelectricity generation. J. Water Process. Eng. 2022, 48, 102882. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Al-Zaqri, N.; Yaakop, A.S.; Umar, K. Potato waste as an effective source of electron generation and bioremediation of pollutant through benthic microbial fuel cell. Sustain. Energy Technol. Assess. 2022, 53, 102560. [Google Scholar] [CrossRef]
- Du, H.; Li, F. Enhancement of solid potato waste treatment by microbial fuel cell with mixed feeding of waste activated sludge. J. Clean. Prod. 2017, 143, 336–344. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Guerrero–Barajas, C.; Ibrahim, M.N.M.; Umar, K.; Yaakop, A.S. Local fruit wastes driven benthic microbial fuel cell: A sustainable 859 approach to toxic metal removal and bioelectricity generation. Environ. Sci. Pollut. Res. 2022, 29, 32913–32928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.; Ding, L.; Xu, K.; Ren, H. Influences of initial pH on performance and anodic microbes of fed-batch microbial fuel cells. J. Chem. Technol. Biotechnol. 2011, 86, 1226–1232. [Google Scholar] [CrossRef]
- Suransh, J.; Mungray, A.K. Reduction in particle size of vermiculite and production of the low-cost earthen membrane to achieve enhancement in the microbial fuel cell performance. J. Environ. Chem. Eng. 2022, 10, 108787. [Google Scholar] [CrossRef]
- Saniei, N.; Ghasemi, N.; Zinatizadeh, A.A.; Zinadini, S.; Ramezani, M.; Derakhshan, A.A. Electricity generation enhancement in microbial fuel cell via employing a new SPEEK based proton exchange membrane modified by goethite nanoparticles functionalized with tannic acid and sulfanilic acid. Environ. Technol. Innov. 2022, 25, 102168. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Xiao, X.; Zhang, D.; Zhang, Z.; Zhou, Z.; Liu, S. (R)−3-hydroxybutyrate production by Burkholderia cepacia in the cathode chamber of ethanol-producing microbial fuel cells. Biochem. Eng. J. 2022, 186, 108581. [Google Scholar] [CrossRef]
- Mejía-López, M.; Lastres, O.; Alemán-Ramirez, J.L.; Lobato-Peralta, D.R.; Verde, A.; Gámez, J.M.; de Paz, P.L.; Verea, L. Conductive carbon-polymer composite for bioelectrodes and electricity generation in a sedimentary microbial fuel cell. Biochem. Eng. J. 2023, 193, 108856. [Google Scholar] [CrossRef]
- Yuan, J.; Huang, H.; Chatterjee, S.G.; Wang, Z.; Wang, S. Effective factors for the performance of a co-generation system for bioethanol and electricity production via microbial fuel cell technology. Biochem. Eng. J. 2022, 178, 108309. [Google Scholar] [CrossRef]
- Poli, F.; Santoro, C.; Soavi, F. Improving microbial fuel cells power density using internal and external optimized, tailored and totally green supercapacitor. J. Power Sources 2023, 564, 232780. [Google Scholar] [CrossRef]
- Nandy, A.; Farkas, D.; Pepió-Tárrega, B.; Martinez-Crespiera, S.; Borràs, E.; Avignone-Rossa, C.; Di Lorenzo, M. Influence of carbon-based cathodes on biofilm composition and electrochemical performance in soil microbial fuel cells. Environ. Sci. Ecotechnol. 2023, 16, 100276. [Google Scholar] [CrossRef]
- Sabina-Delgado, A.; Kamaraj, S.K.; Hernández-Montoya, V.; Cervantes, F.J. Novel carbon-ceramic composite membranes with high cation exchange properties for use in microbial fuel cell and electricity generation. Int. J. Hydrogen Energy 2023, 48, 25512–25526. [Google Scholar] [CrossRef]
- Ravinuthala, S.; Anilbhai, B.V.; Settu, S. Fabrication of Low-cost, green Material-Based Microbial Fuel Cell for bioelectricity production through textile wastewater remediation. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Feregrino-Rivas, M.; Ramirez-Pereda, B.; Estrada-Godoy, F.; Cuesta-Zedeño, L.F.; Rochín-Medina, J.J.; Bustos-Terrones, Y.A.; Gonzalez-Huitron, V.A. Performance of a sediment microbial fuel cell for bioenergy production: Comparison of fluvial and marine sediments. Biomass Bioenergy 2023, 168, 106657. [Google Scholar] [CrossRef]
- Guo, H.; Huang, C.; Geng, X.; Jia, X.; Huo, H.; Yue, W. Influence of the original electrogenic bacteria on the performance of oily sludge Microbial Fuel Cells. Energy Rep. 2022, 8, 14374–14381. [Google Scholar] [CrossRef]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, X.; Du, Q.; Wang, H.; Ngo, H.H.; Guo, W.; Zhang, Y.; Long, T.; Qi, L. A new integrated single-chamber air-cathode microbial fuel cell-Anaerobic membrane bioreactor system for improving methane production and membrane fouling mitigation. J. Membr. Sci. 2022, 655, 120591. [Google Scholar] [CrossRef]
- Vu, H.T.; Min, B. Integration of submersible microbial fuel cell in anaerobic digestion for enhanced production of methane and current at varying glucose levels. Int. J. Hydrogen Energy 2019, 44, 7574–7582. [Google Scholar] [CrossRef]
- Nguyen, P.K.T.; Das, G.; Kim, J.; Yoon, H.H. Hydrogen production from macroalgae by simultaneous dark fermentation and microbial electrolysis cell. Bioresour. Technol. 2020, 315, 123795. [Google Scholar] [CrossRef] [PubMed]
- Gebreslassie, T.R.; Nguyen, P.K.T.; Yoon, H.H.; Kim, J. Co-production of hydrogen and electricity from macroalgae by simultaneous dark fermentation and microbial fuel cell. Bioresour. Technol. 2021, 336, 125269. [Google Scholar] [CrossRef]
- Yun, W.H.; Yoon, Y.S.; Yoon, H.H.; Nguyen, P.K.T.; Hur, J. Hydrogen production from macroalgae by simultaneous dark fermentation and microbial electrolysis cell with surface-modified stainless steel mesh cathode. Int. J. Hydrogen Energy 2021, 46, 39136–39145. [Google Scholar] [CrossRef]
- Li, W.; He, L.; Cheng, C.; Cao, G.; Ren, N. Effects of biochar on ethanol-type and butyrate-type fermentative hydrogen productions. Bioresour. Technol. 2020, 306, 123088. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, J.; Zhao, W.; Zang, L. Improved Fermentative hydrogen production with the addition of calcium-lignosulfonate-derived biochar. Energy Fuels 2019, 33, 7406–7414. [Google Scholar] [CrossRef]
- Harun, K.; Adhikari, S.; Jahromi, H. Hydrogen production via thermocatalytic decomposition of methane using carbon-based catalysts. RSC Adv. 2020, 10, 40882–40893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, P.; Liu, J.; Zhou, X.; Li, X.; Lu, J.; Hou, B. Study on performance and mechanisms of anaerobic oxidation of methane-microbial fuel cells (AOM-MFCs) with acetate-acclimatizing or formate-acclimatizing electroactive culture. Bioelectrochemistry 2023, 151, 108404. [Google Scholar] [CrossRef]
- Liu, J.; Yun, S.; Wang, K.; Liu, L.; An, J.; Ke, T.; Gao, T.; Zhang, X. Enhanced methane production in microbial electrolysis cell coupled anaerobic digestion system with MXene accelerants. Bioresour. Technol. 2023, 380, 129089. [Google Scholar] [CrossRef] [PubMed]
- Aboelela, D.; Soliman, M.A. Hydrogen production from microbial electrolysis cells powered with microbial fuel cells. J. King Saud Univ. Eng. Sci. 2022. [Google Scholar] [CrossRef]
- guyen, P.K.T.; Kim, J.; Das, G.; Yoon, H.H.; Lee, D.H. Optimization of simultaneous dark fermentation and microbial electrolysis cell for hydrogen production from macroalgae using response surface methodology. Biochem. Eng. J. 2021, 171, 108029. [Google Scholar]
- Sun, R.; Zhou, A.; Jia, J.; Liang, Q.; Liu, Q.; Xing, D.; Ren, N. Characterization of methane production and microbial community shifts during waste activated sludge degradation in microbial electrolysis cells. Bioresour. Technol. 2015, 175, 68–74. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, X.; Luo, H.; Li, X.; Chen, W.; Chen, J.; Mo, Y.; Wang, W. CH4 control and associated microbial process from constructed wetland (CW) by microbial fuel cells (MFC). J. Environ. Manag. 2020, 260, 110071. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, M.; Li, D. Removal of sulfide and production of methane from carbon dioxide in microbial fuel cells–microbial electrolysis cell (MFCs–MEC) coupled system. Appl. Biochem. Biotechnol. 2014, 172, 2720–2731. [Google Scholar] [CrossRef] [PubMed]
- Durna Pişkin, E.; Genç, N. Multi response optimization of waste activated sludge oxidation and azo dye reduction in microbial fuel cell. Environ. Technol. 2023, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Bose, D.; Yadav, J.; Sharma, B.; Sangli, E.; Patel, A.; Mukherjee, A.; Singh, A.A. Bioremediation and bioelectricity from Himalayan rock soil in sediment-microbial fuel cell using carbon rich substrates. Fuel 2023, 341, 127019. [Google Scholar] [CrossRef]
- Habibul, N.; Hu, Y.; Sheng, G.P. Microbial fuel cell driving electrokinetic remediation of toxic metal contaminated soils. J. Hazard. Mater. 2016, 318, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, N.M.; Alardhi, S.M.; Al-Jadir, T.; Dhahad, H.A. Contaminants Removal from Real Refinery Wastewater Associated with Energy Generation in Microbial Fuel Cell. J. Ecol. Eng. 2023, 24, 107–114. [Google Scholar] [CrossRef]
- Apollon, W.; Luna-Maldonado, A.I.; Vidales-Contreras, J.A.; Rodríguez-Fuentes, H.; Gómez-Leyva, J.F.; Kamaraj, S.K. Application of microbial fuel cells and other bioelectrochemical systems: A comparative study. In Microbial Fuel Cells: Emerging Trends in Electrochemical Applications; IOP Publishing: Bristol, UK, 2022. [Google Scholar] [CrossRef]
- You, S.; Zhao, Q.; Zhang, J.; Jiang, J.; Zhao, S. A microbial fuel cell using permanganate as the cathodic electron acceptor. J. Power Sources 2006, 162, 1409–1415. [Google Scholar] [CrossRef]
- Kim, B.H.; Chang, I.S.; Gadd, G.M. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 2007, 76, 485–494. [Google Scholar] [CrossRef]
- Logan, B.E.; Regan, J.M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006, 14, 512–518. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef]
- Naha, A.; Debroy, R.; Sharma, D.; Shah, M.P.; Nath, S. Microbial Fuel Cell: A State-of-the-Art and Revolutionizing Technology for efficient Energy Recovery. Clean. Circ. Bioecon. 2023, 5, 100050. [Google Scholar] [CrossRef]
- Sabin, J.M.; Leverenz, H.; Bischel, H.N. Microbial fuel cell treatment energy-offset for fertilizer production from human urine. Chemosphere 2022, 294, 133594. [Google Scholar] [CrossRef]
- Sharma, R.; Kumari, R.; Pant, D.; Malaviya, P. Bioelectricity generation from human urine and simultaneous nutrient recovery: Role of Microbial Fuel Cells. Chemosphere 2022, 292, 133437. [Google Scholar] [CrossRef] [PubMed]
- Walter, X.A.; You, J.; Gajda, I.; Greenman, J.; Ieropoulos, I. Impact of feedstock dilution on the performance of urine-fed ceramic and membrane-less microbial fuel cell cascades designs. J. Power Sources 2023, 561, 232708. [Google Scholar] [CrossRef]
- Du, H.; Shao, Z. Synergistic effects between solid potato waste and waste activated sludge for waste-to-power conversion in microbial fuel cells. Appl. Energy 2022, 314, 118994. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, D.; Zhan, Y.; Cao, L.; Liu, Y. A systematic study on self-powered microbial fuel cell based BOD biosensors running under different temperatures. Biochem. Eng. J. 2022, 180, 108372. [Google Scholar] [CrossRef]
- Nath, D.; Kallepalli, S.; Rao, L.T.; Dubey, S.K.; Javed, A.; Goel, S. Microfluidic paper microbial fuel cell powered by Shewanella putrefaciens in IoT cloud framework. Int. J. Hydrogen Energy 2021, 46, 3230–3239. [Google Scholar] [CrossRef]
- Prasad, J.; Tripathi, R.K. Scale-up and control the voltage of sediment microbial fuel cell for charging a cell phone. Biosens. Bioelectron. 2021, 172, 112767. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.; Prasad Uday, U.S.; Bandyopadhyay, T.K.; Ray, R.N.; Bhunia, B. Performance improvement of microbial fuel cell (MFC) using suitable electrode and Bioengineered organisms: A review. Bioengineered 2017, 8, 471–487. [Google Scholar] [CrossRef]
- Davidson, D.J. Exnovating for a renewable energy transition. Nat. Energy 2019, 4, 254–256. [Google Scholar] [CrossRef]
- Liu, L.J.; Jiang, H.D.; Liang, Q.M.; Creutzig, F.; Liao, H.; Yao, Y.F.; Qian, X.Y.; Ren, Z.Y.; Qing, J.; Cai, Q.R. Carbon emissions and economic impacts of an EU embargo on Russian fossil fuels. Nat. Clim. Chang. 2023, 13, 290–296. [Google Scholar] [CrossRef]
- Savla, N.; Pandit, S.; Verma, J.P.; Awasthi, A.K.; Sana, S.S.; Prasad, R. Techno-economical evaluation and life cycle assessment of microbial electrochemical systems: A review. Curr. Res. Green Sustain. Chem. 2021, 4, 100111. [Google Scholar] [CrossRef]
- Harnisch, F.; Schröder, U. Selectivity versus mobility: Separation of anode and cathode in microbial bioelectrochemical systems. ChemSusChem 2009, 2, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, M. The International Standards as the Constitution of Life Cycle Assessment: The ISO 14040 Series and its Offspring. In Background and Future Prospects in Life Cycle Assessment. LCA Compendium—The Complete World of Life Cycle Assessment; Klöpffer, W., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 85–106. [Google Scholar]
- Chin, M.Y.; Phuang, Z.X.; Hanafiah, M.; Zhang, Z.; Woon, K.S. Exploring the life cycle environmental performance of different microbial fuel cell configurations. Chem. Eng. Trans. 2021, 89, 175–180. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Nghiem, L.D.; Zhang, X.; Wang, J. Effect of organic loading rate on the recovery of nutrients and energy in a dual-chamber microbial fuel cell. Bioresour. Technol. 2019, 281, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Divya, S.; Rusyn, I.; Solorza-Feria, O.; Sathish-Kumar, K. Sustainable SMART fertilizers in agriculture systems: A review on fundamentals to in-field applications. Sci. Total Environ. 2023, 904, 166729. [Google Scholar] [CrossRef]
- Asensio, Y.; Fernandez-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Influence of the ion-exchange membrane on the performance of double-compartment microbial fuel cells. J. Electroanal. Chem. 2018, 808, 427–432. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Abu-Reesh, I.M.; He, Z.; Yuan, C. Life Cycle Environmental Impact Comparison of Bioelectrochemical Systems for Wastewater Treatment. Procedia CIRP 2019, 80, 382–388. [Google Scholar] [CrossRef]
- Alseroury, F.A. Microbial desalination cells: Progress and impacts. J. Biochem. Technol. 2018, 9, 40. [Google Scholar]
- Zhang, J.; Yuan, H.; Deng, Y.; Abu-Reesh, I.M.; He, Z.; Yuan, C. Life cycle assessment of osmotic microbial fuel cells for simultaneous wastewater treatment and resource recovery. Int. J. Life Cycle Assess 2019, 24, 1962–1975. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J.; Garfí, M. Life cycle assessment of constructed wetland systems for wastewater treatment coupled with microbial fuel cells. Sci. Total Environ. 2017, 584, 355–362. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Hiloidhari, M.; Gware, P.; Singh, A.; Pant, D. Development and life cycle assessment of an auto circulating bioelectrochemical reactor for energy positive continuous wastewater treatment. Bioresour. Technol. 2020, 304, 122959. [Google Scholar] [CrossRef]
- Pant, D.; Singh, A.; Bogaert, G.V.; Alvarez-Gallego, Y.; Diels, L.; Vanbroekhoven, K. An introduction to the life cycle assessment (LCA) of bioelectrochemical systems (BES) for sustainable energy and product generation: Relevance and key aspects. Renew. Sustain. Energy Rev. 2011, 15, 1305–1313. [Google Scholar] [CrossRef]
- Shemfe, M.; Gadkari, S.; Yu, E.; Rasul, S.; Scott, K.; Head, I.M.; Gu, S.; Sadhukhan, J. Life cycle, techno-economic and dynamic simulation assessment of bioelectrochemical systems: A case of formic acid synthesis. Bioresour. Technol. 2018, 255, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Weidema, B.P. The social footprint—A practical approach to comprehensive and consistent social LCA. Int. J. Life Cycle Assess 2018, 23, 700–709. [Google Scholar] [CrossRef]






| Type of Reactor | Applied Energy | Production Rate | Refs. | |
|---|---|---|---|---|
| H2 | CH4 | |||
| MEC-MFC (units in series) | 7.473 × 10−4 A | 7.32 mL/d | N/A | [153] |
| MEC-MFC (units in series) | 6.631 × 10−4 A | 6.50 mL/d | - | [153] |
| MEC-MFC (units in parallel) | 4.062 × 10−4 A | 3.98 mL/d | - | [153] |
| MEC-MFC (units in parallel) | 3.849 × 10−4 A | 3.77 mL/d | N/A | [153] |
| sDF-MFC | N/A | 0.21 L/L/d | N/A | [146] |
| sDF-MFC | - | 0.34 L/L/d | - | [146] |
| sDF-MEC | 0.8 V | 438.7 mL/g-TS | N/A | [145] |
| DF-MEC | 0.8 V | 403.5 mL/g-TS | - | [145] |
| sDFMEC | 0.8 V | 492.3 mL/g-TS | N/A | [154] |
| BES/MEC | N/A | N/A | 77.13 L/kg | [155] |
| CW-MFC | 0.27 W m3 | N/A | 9.5 mg/m2/h | [156] |
| MFC-MEC | 0.7 V | N/A | 0.354 mL/h/L | [157] |
| MEC | 0.8 V | 1.22 L/L/d | - | [147] |
| sDF-MEC | 0.8 V | 0.45 L/L/d | - | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apollon, W. An Overview of Microbial Fuel Cell Technology for Sustainable Electricity Production. Membranes 2023, 13, 884. https://doi.org/10.3390/membranes13110884
Apollon W. An Overview of Microbial Fuel Cell Technology for Sustainable Electricity Production. Membranes. 2023; 13(11):884. https://doi.org/10.3390/membranes13110884
Chicago/Turabian StyleApollon, Wilgince. 2023. "An Overview of Microbial Fuel Cell Technology for Sustainable Electricity Production" Membranes 13, no. 11: 884. https://doi.org/10.3390/membranes13110884