High-Degree Concentration Organic Solvent Forward Osmosis for Pharmaceutical Pre-Concentration
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Characterization of HF Support
2.2.1. Pore Size and Porosity
2.2.2. Water Permeation of Support
2.3. Fabrication of TFC-HF Membrane
2.4. Characterization of TFC-HF Membrane
2.4.1. SEM Observation
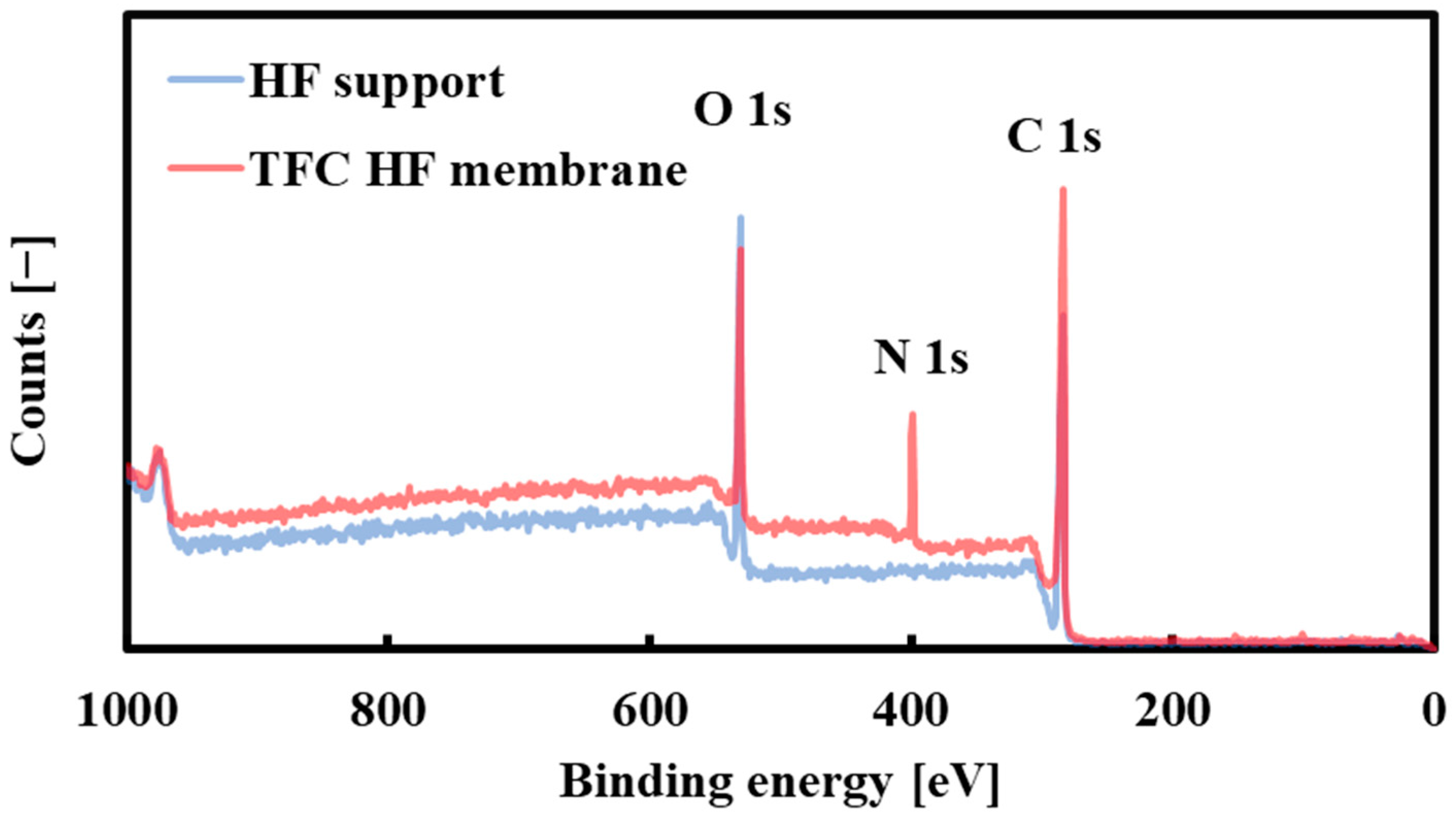
2.4.2. XPS Analysis
2.4.3. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.5. Performance of TFC-HF Membrane
2.5.1. Rejection Performance under Pressurized Condition
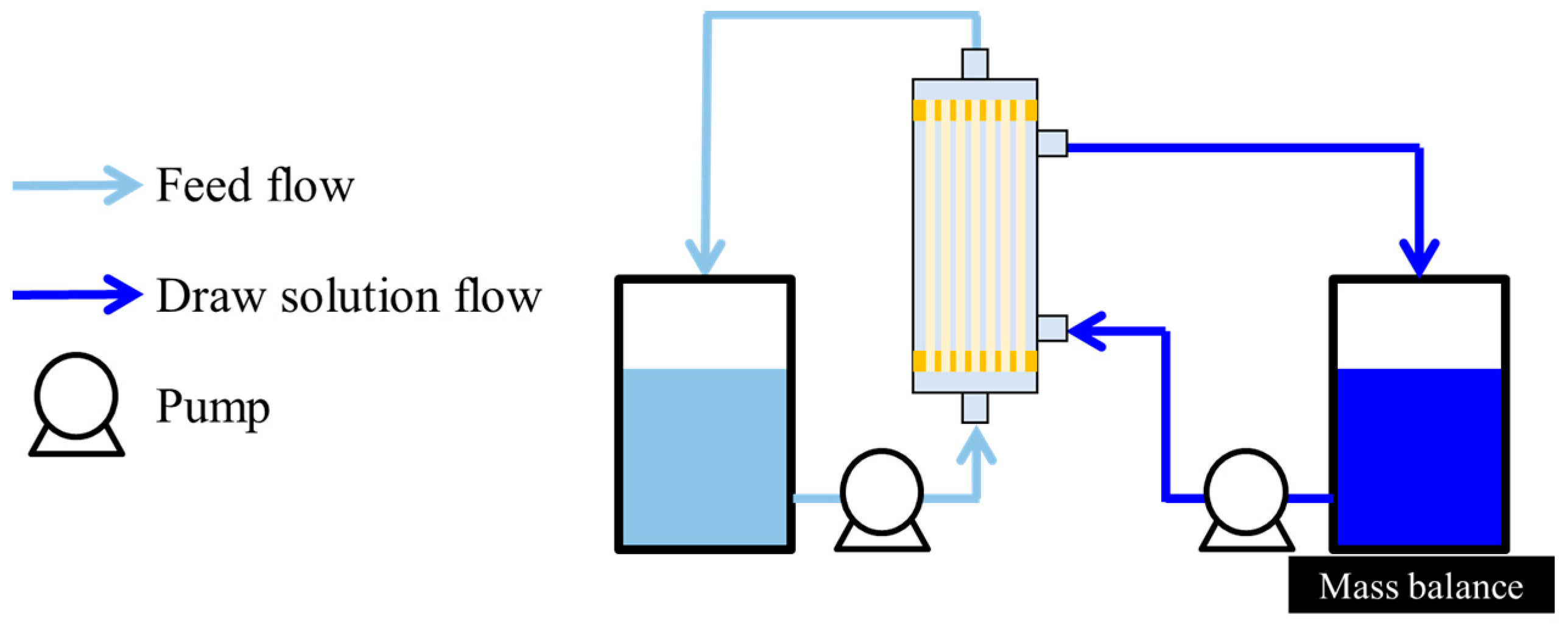
2.5.2. OSFO Performance
2.5.3. High-Degree Concentration by OSFO
3. Results and Discussion
3.1. Characterization of HF Support
3.2. Characterization of the Selective Layer
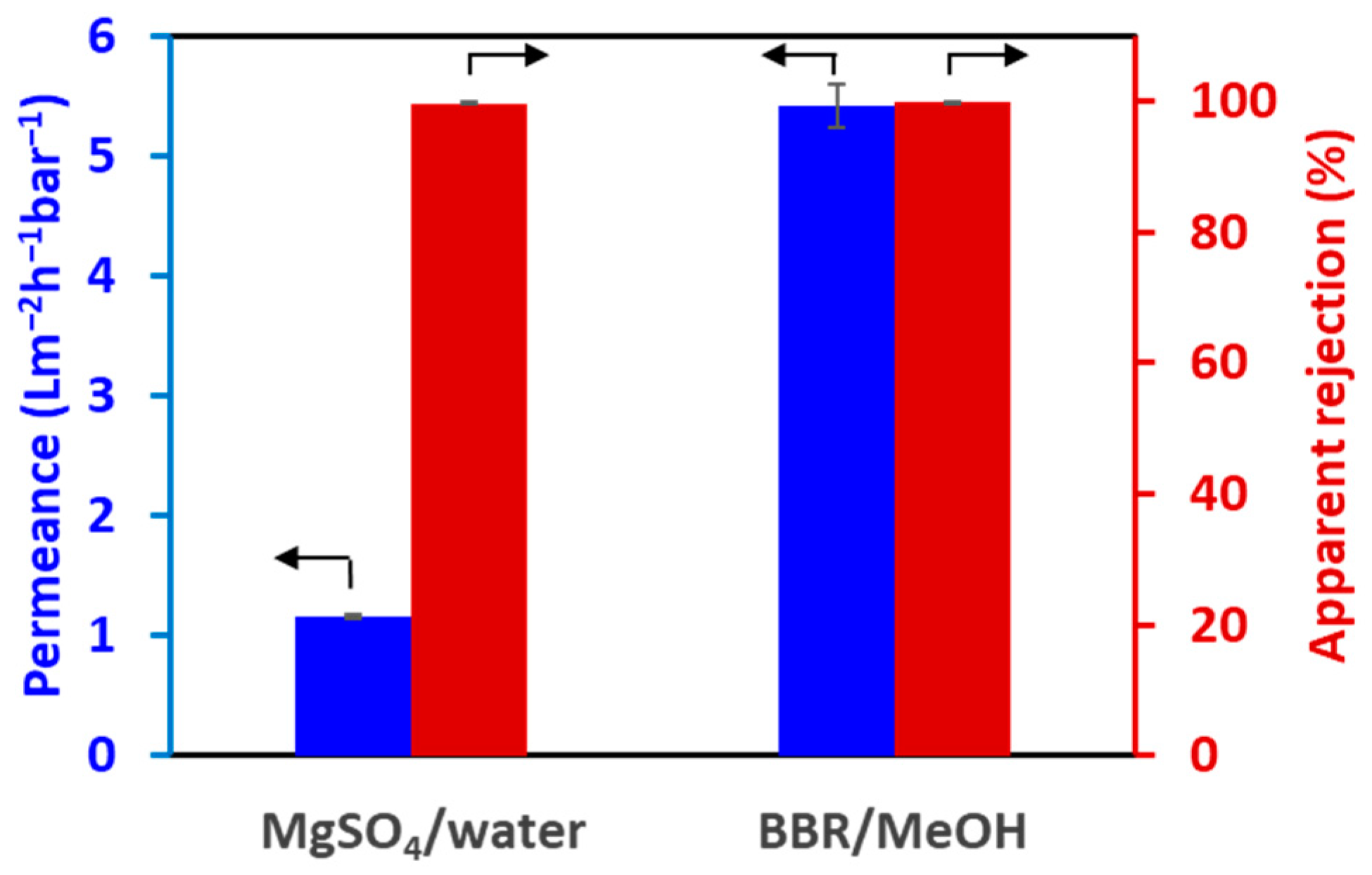
3.3. Apparent Rejection of the TFC-HF Membrane
3.4. OSFO Performance of the TFC-HF Membrane
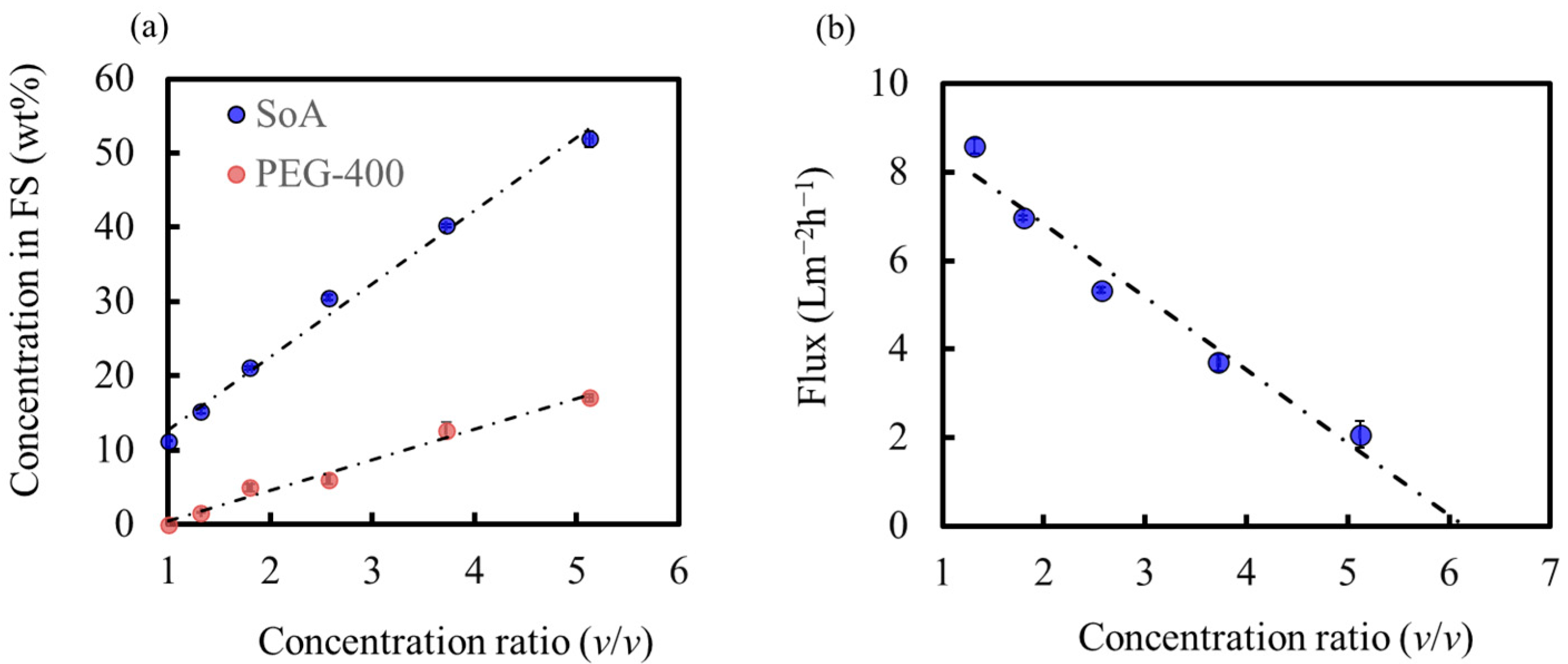
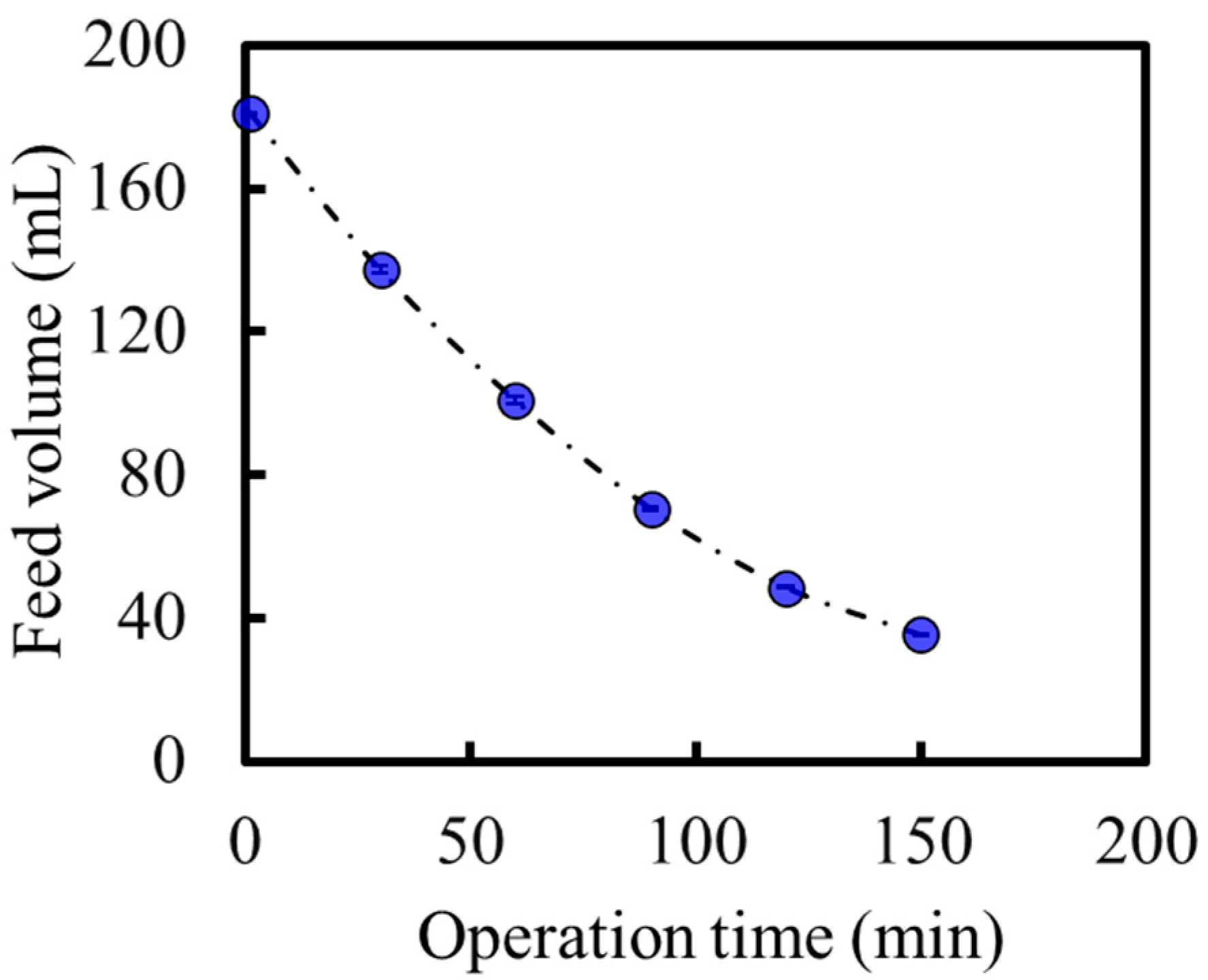
3.5. High-Degree Concentration of the Model Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mullard, A. 2021 FDA Approvals. Nat. Rev. Drug Discov. 2022, 21, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Williams, R., Jr. Six-Tenths Factor Aids in Approximating Costs. Chem. Eng. 1947, 54, 124–125. [Google Scholar]
- Cui, Y.; Chung, T.S. Pharmaceutical concentration using organic solvent forward osmosis for solvent recovery. Nat. Commun. 2018, 9, 1426. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.S.; Chen, Y.; Ng, D.Y.F.; Chew, J.W.; Wang, R. Organic solvent forward osmosis membranes for pharmaceutical concentration. J. Membr. Sci. 2022, 642, 119965. [Google Scholar] [CrossRef]
- Peeva, L.G.; Gibbins, E.; Luhra, S.S.; White, L.S.; Stateva, R.P.; Livingston, A.G. Effect of concentration polarisation and osmotic pressure on flux in organic solvent nanofiltration. J. Membr. Sci. 2004, 236, 121–136. [Google Scholar] [CrossRef]
- Yasukawa, M.; Tanaka, Y.; Takahashi, T.; Shibuya, M.; Mishima, S.; Matsuyama, H. Effect of Molecular Weight of Draw Solute on Water Permeation in Forward Osmosis Process. Ind. Eng. Chem. Res. 2015, 54, 8239–8246. [Google Scholar] [CrossRef]
- Alfonsi, K.; Colberg, J.; Dunn, P.J.; Fevig, T.; Jennings, S.; Johnson, T.A.; Kleine, H.P.; Knight, C.; Nagy, M.A.; Perry, D.A.; et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organization. Green Chem. 2008, 10, 31–36. [Google Scholar] [CrossRef]
- Neal, A.G. Practical Process Research and Development, 2nd ed.; Academic Press: Oxford, UK, 2012. [Google Scholar]
- JIS K 3832; Testing Methods for Bubble Point of Membrane Filters. Japanese Industrial Standards: Tokyo, Japan, 2021.
- Shibuya, M.; Yasukawa, M.; Mishima, S.; Tanaka, Y.; Takahashi, T.; Matsuyama, H. A thin-film composite-hollow fiber forward osmosis membrane with a polyketone hollow fiber membrane as a support. Desalination 2017, 402, 33–41. [Google Scholar] [CrossRef]
- Sato, D.; Kaneda, M.; Komatsu, T. Polyketone Porous Film. PCT International Publication WO2013/035747A1, 14 March 2013. [Google Scholar]
- Solomon, M.F.J.; Bhole, Y.; Livingston, A.G. High flux hydrophobic membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization, surface modification and solvent activation. J. Membr. Sci. 2013, 434, 193–203. [Google Scholar] [CrossRef]
- Takada, R.; Kubota, N.; Suzumura, K.; Osaki, H. Composite Hollow Fiber Membrane Module and Method for Producing Same. U.S. Patent application US2023/0264150 A1, 24 August 2023. [Google Scholar]
- Kiguchi, A.; Anan, T.; Nakamura, M.; Ohashi, T. Composite Hollow Fiber Membrane Module and Manufacturing Method Therefor. U.S. Patent US10583404 B2, 10 March 2020. [Google Scholar]
- Sun, S.P.; Chung, T.S. Outer-Selective Pressure-Retarded Osmosis Hollow Fiber Membranes from Vacuum-Assisted Interfacial Polymerization for Osmotic Power Generation. Environ. Sci. Technol. 2013, 47, 13167–13174. [Google Scholar] [CrossRef] [PubMed]
- MeOH: Safety Data Sheet of Maker. Available online: https://labchem-wako.fujifilm.com/sds/W01W0113-0182JGHEJP.pdf (accessed on 18 December 2023).
- PEG-400: Safety Data Sheet of Maker. Available online: https://labchem-wako.fujifilm.com/sds/W01W0116-0906JGHEJP.pdf (accessed on 18 December 2023).
- Yasukawa, M.; Mishima, S.; Shibuya, M.; Saeki, D.; Takahashi, T.; Miyoshi, T.; Matsuyama, H. Preparation of a forward osmosis membrane using a highly porous polyketone microfiltration membrane as a novel support. J. Membr. Sci. 2015, 487, 51–59. [Google Scholar] [CrossRef]
- Product Data Sheet of a Reverse Osmosis Membrane Element. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/RO-FilmTec-SW30XHR-440i-PDS-45-D00968-en.pdf (accessed on 18 December 2023).
- Tang, C.Y.; Kwon, Y.N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes I. FTIR and XPS characterization of polyamide and coating layer chemistry. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Shi, B.; Marchetti, P.; Peshev, D.; Zhang, S.; Livingston, A.G. Performance of spiral-wound membrane modules in organic solvent nanofiltration—Fluid dynamics and mass transfer characteristics. J. Membr. Sci. 2015, 494, 8–24. [Google Scholar] [CrossRef]
- Li, S.; Duan, L.; Zhang, H.; Li, M.; Zhao, Y.; Xing, F. Inhibition Strategies of Reverse Solute Flux in Osmotic Microbial Fuel Cells: Take Forward Osmosis as Reference. ACS EST Water 2023, 3, 2835–2848. [Google Scholar] [CrossRef]
- Repligen’s Single-Use Filters for Protein-Based Pharmaceutical Concentration. Available online: https://jp.repligen.com/products/downstream-filtration/tangenx-cassettes/tangenx-sius (accessed on 18 December 2023).
- Asahi-Kasei’s Single-Use Filters for Virus Removal of Antibody-Based Pharmaceutical. Available online: https://fluidmgmt.ak-bio.com/wp-content/uploads/2021/02/Planova-SU-VFC_Brochure.pdf (accessed on 18 December 2023).






| HF Support | |
|---|---|
| Inner diameter (μm) | 488 ± 5 |
| Outer diameter (μm) | 784 ± 3 |
| Thickness (μm) | 148 ± 3 |
| Porosity (%) | 74.3 ± 0.2 |
| Mean pore size (nm) | 110 ± 4 |
| Water permeance (L m−2 h−1 bar−1) | 2.21 ± 0.05 × 103 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, R.; Takagi, R.; Matsuyama, H. High-Degree Concentration Organic Solvent Forward Osmosis for Pharmaceutical Pre-Concentration. Membranes 2024, 14, 14. https://doi.org/10.3390/membranes14010014
Takada R, Takagi R, Matsuyama H. High-Degree Concentration Organic Solvent Forward Osmosis for Pharmaceutical Pre-Concentration. Membranes. 2024; 14(1):14. https://doi.org/10.3390/membranes14010014
Chicago/Turabian StyleTakada, Ryoichi, Ryosuke Takagi, and Hideto Matsuyama. 2024. "High-Degree Concentration Organic Solvent Forward Osmosis for Pharmaceutical Pre-Concentration" Membranes 14, no. 1: 14. https://doi.org/10.3390/membranes14010014







