Membrane-Associated Ubiquitin Ligase RING Finger Protein 152 Orchestrates Melanogenesis via Tyrosinase Ubiquitination
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Antibodies and Reagents
2.2. Cell Culture
2.3. Screening for E3 Ubiquitin Ligases That Interact with Tyrosinase
2.4. Plasmid Construction and Transfection, siRNA and Transfection
2.5. Melanin Content Measurement
2.6. Immunoprecipitation (IP) and Western Blotting
2.7. Confocal Immunofluorescence Microscopy
2.8. Statistical Analyses
3. Results
3.1. Tyrosinase Ubiquitination in B16 Melanoma Cells
3.2. Identification of Tyrosinase-Specific E3 Ubiquitin Ligase
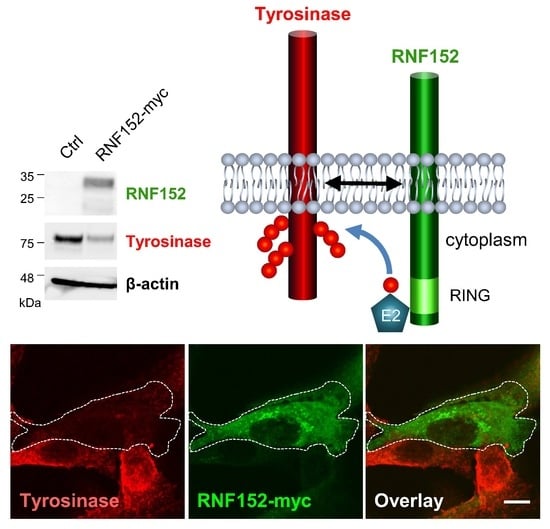
3.3. RNF152 Regulates Expression of Tyrosinase in B16 Cells
3.4. RNF152 Co-Localizes with Tyrosinase in TGN and Degrades It in Lysosomes
3.5. RNF152 Physically Associates with Tyrosinase
3.6. RNF152 Strongly Ubiquitinates Tyrosinase
3.7. RNF152 Ubiquitinates Tyrp-1 to a Lesser Degree
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Estadella, I.; Pedros-Gamez, O.; Colomer-Molera, M.; Bosch, M.; Sorkin, A.; Felipe, A. Endocytosis: A Turnover Mechanism Controlling Ion Channel Function. Cells 2020, 9, 1833. [Google Scholar] [CrossRef]
- Lecker, S.H.; Goldberg, A.L.; Mitch, W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006, 17, 1807–1819. [Google Scholar] [CrossRef]
- Apaja, P.M.; Lukacs, G.L. Protein homeostasis at the plasma membrane. Physiology 2014, 29, 265–277. [Google Scholar] [CrossRef]
- Wu, X.; Rapoport, T.A. Mechanistic insights into ER-associated protein degradation. Curr. Opin. Cell Biol. 2018, 53, 22–28. [Google Scholar] [CrossRef]
- Piper, R.C.; Luzio, J.P. Ubiquitin-dependent sorting of integral membrane proteins for degradation in lysosomes. Curr. Opin. Cell Biol. 2007, 19, 459–465. [Google Scholar] [CrossRef]
- Piper, R.C.; Dikic, I.; Lukacs, G.L. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb. Perspect. Biol. 2014, 6, a016808. [Google Scholar] [CrossRef]
- d’Azzo, A.; Bongiovanni, A.; Nastasi, T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 2005, 6, 429–441. [Google Scholar] [CrossRef]
- Bartee, E.; Mansouri, M.; Hovey Nerenberg, B.T.; Gouveia, K.; Fruh, K. Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol. 2004, 78, 1109–1120. [Google Scholar] [CrossRef]
- Fujita, H.; Iwabu, Y.; Tokunaga, K.; Tanaka, Y. Membrane-associated RING-CH (MARCH) 8 mediates the ubiquitination and lysosomal degradation of the transferrin receptor. J. Cell Sci. 2013, 126, 2798–2809. [Google Scholar] [CrossRef]
- Fukuda, H.; Nakamura, N.; Hirose, S. MARCH-III Is a novel component of endosomes with properties similar to those of MARCH-II. J. Biochem. 2006, 139, 137–145. [Google Scholar] [CrossRef]
- Tada, T.; Zhang, Y.; Fujita, H.; Tokunaga, K. MARCH8: The tie that binds to viruses. FEBS J. 2022, 289, 3642–3654. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, W.; Wu, Y.; Zheng, J.; Suo, T.; Tang, H.; Tang, J. RNF152, a novel lysosome localized E3 ligase with pro-apoptotic activities. Protein Cell 2010, 1, 656–663. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, C.; Chen, L.; Jin, J.; Wei, J.; Zhao, L.; Chen, M.; Pan, W.; Xu, Y.; Chu, H.; et al. The ubiquitination of rag A GTPase by RNF152 negatively regulates mTORC1 activation. Mol. Cell 2015, 58, 804–818. [Google Scholar] [CrossRef]
- Xiong, M.G.; Xu, Z.S.; Li, Y.H.; Wang, S.Y.; Wang, Y.Y.; Ran, Y. RNF152 positively regulates TLR/IL-1R signaling by enhancing MyD88 oligomerization. EMBO Rep. 2020, 21, e48860. [Google Scholar] [CrossRef]
- Wan, J.; Liu, S.; Sun, W.; Yu, H.; Tang, W.; Liu, W.; Ji, J.; Liu, B. Ring finger protein 152-dependent degradation of TSPAN12 suppresses hepatocellular carcinoma progression. Cancer Cell Int. 2021, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Imaizumi, K.; Kaneko, M. The Role of Tissue-Specific Ubiquitin Ligases, RNF183, RNF186, RNF182 and RNF152, in Disease and Biological Function. Int. J. Mol. Sci. 2020, 21, 3921. [Google Scholar] [CrossRef]
- Jimbow, K.; Gomez, P.F.; Toyofuku, K.; Chang, D.; Miura, S.; Tsujiya, H.; Park, J.S. Biological role of tyrosinase related protein and its biosynthesis and transport from TGN to stage I melanosome, late endosome, through gene transfection study. Pigment Cell Res. 1997, 10, 206–213. [Google Scholar] [CrossRef]
- Marks, M.S.; Seabra, M.C. The melanosome: Membrane dynamics in black and white. Nat. Rev. Mol. Cell Biol. 2001, 2, 738–748. [Google Scholar] [CrossRef]
- Orlow, S.J.; Boissy, R.E.; Moran, D.J.; Pifko-Hirst, S. Subcellular distribution of tyrosinase and tyrosinase-related protein-1: Implications for melanosomal biogenesis. J. Investig. Dermatol. 1993, 100, 55–64. [Google Scholar] [CrossRef]
- Raposo, G.; Marks, M.S. The dark side of lysosome-related organelles: Specialization of the endocytic pathway for melanosome biogenesis. Traffic 2002, 3, 237–248. [Google Scholar] [CrossRef]
- Raposo, G.; Tenza, D.; Murphy, D.M.; Berson, J.F.; Marks, M.S. Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol. 2001, 152, 809–824. [Google Scholar] [CrossRef]
- Wang, N.; Hebert, D.N. Tyrosinase maturation through the mammalian secretory pathway: Bringing color to life. Pigment Cell Res. 2006, 19, 3–18. [Google Scholar] [CrossRef]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Niki, Y.; Adachi, N.; Fukata, M.; Fukata, Y.; Oku, S.; Makino-Okamura, C.; Takeuchi, S.; Wakamatsu, K.; Ito, S.; Declercq, L.; et al. S-Palmitoylation of Tyrosinase at Cysteine(500) Regulates Melanogenesis. J. Investig. Dermatol. 2023, 143, 317–327.e316. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Watabe, H.; Valencia, J.C.; Yasumoto, K.; Furumura, M.; Funasaka, Y.; Oka, M.; Ichihashi, M.; Hearing, V.J. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: A new aspect of ubiquitin-proteasome function. J. Biol. Chem. 2004, 279, 15427–15433. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Wen, Z.M.; Kim, H.Y.; Valencia, J.C.; Costin, G.E.; Watabe, H.; Yasumoto, K.; Niki, Y.; Kondoh, H.; Ichihashi, M.; et al. Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem. J. 2006, 394, 43–50. [Google Scholar] [CrossRef]
- Hall, A.M.; Orlow, S.J. Degradation of tyrosinase induced by phenylthiourea occurs following Golgi maturation. Pigment Cell Res. 2005, 18, 122–129. [Google Scholar] [CrossRef]
- Fujita, H.; Motokawa, T.; Katagiri, T.; Yokota, S.; Yamamoto, A.; Himeno, M.; Tanaka, Y. Inulavosin, a melanogenesis inhibitor, leads to mistargeting of tyrosinase to lysosomes and accelerates its degradation. J. Investig. Dermatol. 2009, 129, 1489–1499. [Google Scholar] [CrossRef]
- Fujita, H.; Menezes, J.C.; Santos, S.M.; Yokota, S.; Kamat, S.P.; Cavaleiro, J.A.; Motokawa, T.; Kato, T.; Mochizuki, M.; Fujiwara, T.; et al. Inulavosin and its benzo-derivatives, melanogenesis inhibitors, target the copper loading mechanism to the active site of tyrosinase. Pigment Cell Melanoma Res. 2014, 27, 376–386. [Google Scholar] [CrossRef]
- Chen, X.K.; Kwan, J.S.; Chang, R.C.; Ma, A.C. 1-phenyl 2-thiourea (PTU) activates autophagy in zebrafish embryos. Autophagy 2021, 17, 1222–1231. [Google Scholar] [CrossRef]
- Hwang, J.A.; Park, N.H.; Na, Y.J.; Lee, H.K.; Lee, J.H.; Kim, Y.J.; Lee, C.S. Coumestrol Down-Regulates Melanin Production in Melan-a Murine Melanocytes through Degradation of Tyrosinase. Biol. Pharm. Bull. 2017, 40, 535–539. [Google Scholar] [CrossRef]
- Cho, Y.H.; Park, J.E.; Lim, D.S.; Lee, J.S. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci. 2017, 88, 96–102. [Google Scholar] [CrossRef]
- Isogawa, K.; Asano, M.; Hayazaki, M.; Koga, K.; Watanabe, M.; Suzuki, K.; Kobayashi, T.; Kawaguchi, K.; Ishizuka, A.; Kato, S.; et al. Thioxothiazolidin derivative, 4-OST, inhibits melanogenesis by enhancing the specific recruitment of tyrosinase-containing vesicles to lysosome. J. Cell Biochem. 2021, 122, 667–678. [Google Scholar] [CrossRef]
- Watanabe, M.; Kawaguchi, K.; Nakamura, Y.; Furuta, K.; Takemori, H. GIF-2209, an Oxindole Derivative, Accelerates Melanogenesis and Melanosome Secretion via the Modification of Lysosomes in B16F10 Mouse Melanoma Cells. Molecules 2021, 27, 177. [Google Scholar] [CrossRef]
- Shi, J.; Guo, Y.; Wang, H.; Xiao, Y.; Liu, W.; Lyu, L. The ubiquitin-proteasome system in melanin metabolism. J. Cosmet. Dermatol. 2022, 21, 6661–6668. [Google Scholar] [CrossRef]
- Sawasaki, T.; Ogasawara, T.; Morishita, R.; Endo, Y. A cell-free protein synthesis system for high-throughput proteomics. Proc. Natl. Acad. Sci. USA 2002, 99, 14652–14657. [Google Scholar] [CrossRef]
- Takahashi, H.; Uematsu, A.; Yamanaka, S.; Imamura, M.; Nakajima, T.; Doi, K.; Yasuoka, S.; Takahashi, C.; Takeda, H.; Sawasaki, T. Establishment of a Wheat Cell-Free Synthesized Protein Array Containing 250 Human and Mouse E3 Ubiquitin Ligases to Identify Novel Interaction between E3 Ligases and Substrate Proteins. PLoS ONE 2016, 11, e0156718. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.Y.; Park, S.H.; Choi, Y.G.; Kwon, S.B.; Kim, M.K.; Na, J.I.; Youn, S.W.; Park, K.C. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol. Pharm. Bull. 2005, 28, 2216–2219. [Google Scholar] [CrossRef]
- Tachiyama, R.; Ishikawa, D.; Matsumoto, M.; Nakayama, K.I.; Yoshimori, T.; Yokota, S.; Himeno, M.; Tanaka, Y.; Fujita, H. Proteome of ubiquitin/MVB pathway: Possible involvement of iron-induced ubiquitylation of transferrin receptor in lysosomal degradation. Genes Cells Devoted Mol. Cell. Mech. 2011, 16, 448–466. [Google Scholar] [CrossRef]
- Yurkow, E.J.; Laskin, J.D. Purification of tyrosinase to homogeneity based on its resistance to sodium dodecyl sulfate-proteinase K digestion. Arch. Biochem. Biophys. 1989, 275, 122–129. [Google Scholar] [CrossRef]
- Shi, J.; Xiong, R.; Zhou, T.; Su, P.; Zhang, X.; Qiu, X.; Li, H.; Li, S.; Yu, C.; Wang, B.; et al. HIV-1 Nef Antagonizes SERINC5 Restriction by Downregulation of SERINC5 via the Endosome/Lysosome System. J. Virol. 2018, 92, e00196-18. [Google Scholar] [CrossRef]
- Kuchitsu, Y.; Mukai, K.; Uematsu, R.; Takaada, Y.; Shinojima, A.; Shindo, R.; Shoji, T.; Hamano, S.; Ogawa, E.; Sato, R.; et al. STING signalling is terminated through ESCRT-dependent microautophagy of vesicles originating from recycling endosomes. Nat. Cell Biol. 2023, 25, 453–466. [Google Scholar] [CrossRef]
- Allouche, J.; Rachmin, I.; Adhikari, K.; Pardo, L.M.; Lee, J.H.; McConnell, A.M.; Kato, S.; Fan, S.; Kawakami, A.; Suita, Y.; et al. NNT mediates redox-dependent pigmentation via a UVB- and MITF-independent mechanism. Cell 2021, 184, 4268–4283.e4220. [Google Scholar] [CrossRef]
- Kobayashi, T.; Hearing, V.J. Direct interaction of tyrosinase with Tyrp1 to form heterodimeric complexes in vivo. J. Cell Sci. 2007, 120, 4261–4268. [Google Scholar] [CrossRef]
- Halaban, R.; Cheng, E.; Hebert, D.N. Coexpression of wild-type tyrosinase enhances maturation of temperature-sensitive tyrosinase mutants. J. Investig. Dermatol. 2002, 119, 481–488. [Google Scholar] [CrossRef]
- Winder, A.J.; Wittbjer, A.; Rosengren, E.; Rorsman, H. The mouse brown (b) locus protein has dopachrome tautomerase activity and is located in lysosomes in transfected fibroblasts. J. Cell Sci. 1993, 106 Pt 1, 153–166. [Google Scholar] [CrossRef]
- Calvo, P.A.; Frank, D.W.; Bieler, B.M.; Berson, J.F.; Marks, M.S. A cytoplasmic sequence in human tyrosinase defines a second class of di-leucine-based sorting signals for late endosomal and lysosomal delivery. J. Biol. Chem. 1999, 274, 12780–12789. [Google Scholar] [CrossRef]
- Simmen, T.; Schmidt, A.; Hunziker, W.; Beermann, F. The tyrosinase tail mediates sorting to the lysosomal compartment in MDCK cells via a di-leucine and a tyrosine-based signal. J. Cell Sci. 1999, 112 Pt 1, 45–53. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Chen, L.; Liu, Y.Y.; Venkatarangan, V.; Reist, L.; Hanson, P.; Xu, H.; Wang, Y.; Li, M. A conserved ubiquitin- and ESCRT-dependent pathway internalizes human lysosomal membrane proteins for degradation. PLoS Biol. 2021, 19, e3001361. [Google Scholar] [CrossRef]
- Goto, E.; Ishido, S.; Sato, Y.; Ohgimoto, S.; Ohgimoto, K.; Nagano-Fujii, M.; Hotta, H. c-MIR, a human E3 ubiquitin ligase, is a functional homolog of herpesvirus proteins MIR1 and MIR2 and has similar activity. J. Biol. Chem. 2003, 278, 14657–14668. [Google Scholar] [CrossRef] [PubMed]







| E3 Binding Assay Result to V5-Tyrosinase | Predicted Localization * | Predicted Number of Transmembrane Domains * | ||||
|---|---|---|---|---|---|---|
| Sample/Mock | Inulavosin/DMSO | |||||
| Symbol | Rank | DMSO | Inulavosin | |||
| RNF152 | 1 | 16.65 | 22.13 | 1.33 | Lysosome | 1 |
| VPS41 | 2 | 17.99 | 20.97 | 1.17 | Cytosol, lysosome, Golgi, endosome | 0 |
| RNF41 | 3 | 17.84 | 20.55 | 1.15 | Cytosol | 0 |
| ZNF598 | 4 | 16.43 | 18.52 | 1.13 | Cytosol | 0 |
| TRIM21 | 5 | 19.82 | 21.73 | 1.10 | Cytosol, nucleus | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueda, R.; Hashimoto, R.; Fujii, Y.; Menezes, J.C.J.M.D.S.; Takahashi, H.; Takeda, H.; Sawasaki, T.; Motokawa, T.; Tokunaga, K.; Fujita, H. Membrane-Associated Ubiquitin Ligase RING Finger Protein 152 Orchestrates Melanogenesis via Tyrosinase Ubiquitination. Membranes 2024, 14, 43. https://doi.org/10.3390/membranes14020043
Ueda R, Hashimoto R, Fujii Y, Menezes JCJMDS, Takahashi H, Takeda H, Sawasaki T, Motokawa T, Tokunaga K, Fujita H. Membrane-Associated Ubiquitin Ligase RING Finger Protein 152 Orchestrates Melanogenesis via Tyrosinase Ubiquitination. Membranes. 2024; 14(2):43. https://doi.org/10.3390/membranes14020043
Chicago/Turabian StyleUeda, Ryota, Rina Hashimoto, Yuki Fujii, José C. J. M. D. S. Menezes, Hirotaka Takahashi, Hiroyuki Takeda, Tatsuya Sawasaki, Tomonori Motokawa, Kenzo Tokunaga, and Hideaki Fujita. 2024. "Membrane-Associated Ubiquitin Ligase RING Finger Protein 152 Orchestrates Melanogenesis via Tyrosinase Ubiquitination" Membranes 14, no. 2: 43. https://doi.org/10.3390/membranes14020043