Transfer of Sodium Ion across Interface between Na+-Selective Electrode Membrane and Aqueous Electrolyte Solution: Can We Use Nernst Equation If Current Flows through Electrode?
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Potentiometric Studies
3.2. Electrochemical Impedance Studies
3.3. Chronopotentiometric Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Crespo, G.A.; Cuartero, M.; Bakker, E. Thin Layer Ionophore-Based Membrane for Multianalyte Ion Activity Detection. Anal. Chem. 2015, 87, 7729–7737. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.A.; Bakker, E. Ionophore-Based Voltammetric Ion Activity Sensing with Thin Layer Membranes. Anal. Chem. 2016, 88, 1654–1660. [Google Scholar] [CrossRef] [PubMed]
- Greenawalt, P.G.; Amemiya, S. Voltammetric Mechanism of Multiion Detection with Thin Ionophore-Based Polymeric Membrane. Anal. Chem. 2016, 88, 5827–5834. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Cuartero, M.; Crespo, G.A.; Bakker, E. Voltammetric Thin-Layer Ionophore-Based Films: Part 1. Experimental Evidence and Numerical Simulations. Anal. Chem. 2017, 89, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Cuartero, M.; Crespo, G.A.; Bakker, E. Voltammetric Thin-Layer Ionophore-Based Films: Part 2. Semi-Empirical Treatment. Anal. Chem. 2017, 89, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Crespo, G.A.; Cuartero, M. Voltammetric Ion-Selective Electrodes in Thin-Layer Samples: Absolute Detection of Ions Using Ultrathin Membranes. Anal. Chem. 2022, 94, 9140–9148. [Google Scholar] [CrossRef] [PubMed]
- Keresten, V.; Mikhelson, K. Voltammetric Ion Sensing with Ionophore-Based Ion-Selective Electrodes Containing Internal Aqueous Solution, Improving Lifetime of Sensors. Membranes 2022, 12, 1048. [Google Scholar] [CrossRef] [PubMed]
- Hupa, E.; Vanamo, U.; Bobacka, J. Novel Ion-to-Electron Transduction Principle for Solid-Contact ISEs. Electroanalysis 2015, 27, 591–594. [Google Scholar] [CrossRef]
- Vanamo, U.; Hupa, E.; Yrjänä, V.; Bobacka, J. New Signal Readout Principle for Solid-Contact Ion-Selective Electrodes. Anal. Chem. 2016, 88, 4369–4374. [Google Scholar] [CrossRef] [PubMed]
- Jarolímová, Z.; Han, T.; Mattinen, U.; Bobacka, J.; Bakker, E. Capacitive Model for Coulometric Readout of Ion-Selective Electrodes. Anal. Chem. 2018, 90, 8700–8707. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Mattinen, U.; Bobacka, J. Improving the sensitivity of solid-contact ion-selective electrodes by using coulometric signal transduction. ACS Sens. 2019, 4, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Kondratyeva, Y.O.; Tolstopjatova, E.G.; Kirsanov, D.O.; Mikhelson, K.N. Chronoamperometric and coulometric analysis with ionophore-based ionselective electrodes: A modified theory and the potassium ion assay in serum samples. Sens. Actuators B Chem. 2020, 310, 127894. [Google Scholar] [CrossRef]
- Kraikaew, P.; Sailapu, S.K.; Bakker, E. Rapid Constant Potential Capacitive Measurements with Solid-Contact Ion-Selective Electrodes Coupled to Electronic Capacitor. Anal. Chem. 2020, 92, 14174–14180. [Google Scholar] [CrossRef] [PubMed]
- Kraikaew, P.; Jeanneret, S.; Soda, Y.; Cherubini, T.; Bakker, E. Ultrasensitive Seawater pH Measurement by Capacitive Readout of Potentiometric Sensors. ACS Sens. 2020, 5, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Kraikaew, P.; Sailapu, S.K.; Bakker, E. Electronic control of constant potential capacitive readout of ion-selective electrodes for high precision sensing. Sens. Actuators B Chem. 2021, 344, 130282. [Google Scholar] [CrossRef]
- Bondar, A.V.; Keresten, V.M.; Mikhelson, K.N. Registration of small (below 1%) changes of calcium ion concentration in aqueous solutions and in serum by the constant potential coulometric method. Sens. Actuators B Chem. 2022, 354, 131231. [Google Scholar] [CrossRef]
- Bondar, A.; Mikhelson, K. Constant Potential Coulometric Measurements with Ca2+-Selective Electrode: Analysis Using Calibration Plot vs. Analysis Using the Charge Curve Fitting. Sensors 2022, 22, 1145. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, J.; Wegrzyn, K.; Michalska, A.; Maksymiuk, K. Resolution increase of ion-selective electrodes response by using a reversed amperometric setup. Electrochim. Acta 2022, 427, 140886. [Google Scholar] [CrossRef]
- Cammann, K.; Rechnitz, G.A. Exchange kinetics at ion-selective membrane electrodes. Anal. Chem. 1976, 48, 856–862. [Google Scholar] [CrossRef]
- Cammann, K. Exchange kinetics at potassium-selective liquid membrane electrodes. Anal. Chem. 1978, 50, 936–940. [Google Scholar] [CrossRef]
- Xie, S.-L.; Cammann, K.J. Apparent ion-exchange current densities at valinomycin-based potassium ion-selective PVC membranes obtained with an AC-impedance method. Electroanal. Chem. 1987, 229, 249–263. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Covington, A.K.; Evans, G.P.; Handyside, T. Exchange current determination and time-dependent effects for model liquid membrane ion-selective electrodes. Electrochim. Acta 1984, 29, 1127–1131. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Todd, M. Exchange of Li+, Na+ and K+ ions with a PVC membrane containing valinomycin and KBPh4. Electrochim. Acta 1986, 31, 1413–1415. [Google Scholar] [CrossRef]
- Armstrong, R.D. The significance of exchange currents for the PVC/water interface. J. Electroanalyt. Chem. 1988, 245, 113–116. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Senda, M. Theory of liquid ion-exchange membrane ion-selective electrodes based on the concept of the mixed ion-transfer potential. Bull. Chem. Soc. Jpn. 1984, 57, 1801–1808. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Obi, I.; Senda, M. Tetraalkylammonium Ion-selective Electrodes with Supporting Electrolytes. An Experimental Test of the Theory of Liquid Ion-exchange Membrane Ion-selective Electrodes Based on the Concept of the Mixed Ion-transfer Potential. Bull. Chem. Soc. Jpn. 1985, 58, 1636–1641. [Google Scholar] [CrossRef]
- Horvai, G.; Graf, E.; Toth, K.; Pungor, E.; Buck, R.P. Plasticized poly(vinyl chloride) properties and characteristics of valinomycin electrodes. 1. High-frequency resistances and dielectric properties. Anal. Chem. 1986, 58, 2735–2740. [Google Scholar] [CrossRef]
- Toth, K.; Graf, E.; Horvai, G.; Pungor, E.; Buck, R.P. Plasticized poly(vinyl chloride) properties and characteristics of valinomycin electrodes. 2. Low-frequency, surface-rate, and Warburg impedance characteristics. Anal. Chem. 1986, 58, 2741–2744. [Google Scholar] [CrossRef]
- Lindner, E.; Graf, E.; Niegreisz, Z.; Toth, K.; Pungor, E. Responses of site-controlled, plasticized membrane electrodes. Anal. Chem. 1988, 60, 295–301. [Google Scholar] [CrossRef]
- Senda, M. Theory of the double-layer effect on the rate of charge transfer across an electrolyte/electrolyte interface. Electrochim. Acta 1995, 40, 2993–2997. [Google Scholar] [CrossRef]
- Schmickler, W. A model for ion transfer through liquid/liquid interfaces. J. Electroanal. Chem. 1997, 426, 5–9. [Google Scholar] [CrossRef]
- Zhang, W.; Spichiger, U.E. An impedance study of Mg2+-selective membranes. Electrochim. Acta 2000, 45, 2259–2266. [Google Scholar] [CrossRef]
- Mikhelson, K.N.; Bobacka, J.; Lewenstam, A.; Ivaska, A. Potentiometric performance and interfacial kinetics of neutral ionophore based ISE membranes in interfering ion solutions before and after contact with primary ions. Electroanalysis 2001, 13, 876–881. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Horvai, G. Properties of PVC based membranes used in ion-selective electrodes. Electrochim. Acta 1990, 35, 1–7. [Google Scholar] [CrossRef]
- Ye, Q.; Vincze, A.; Horvai, G.; Leermakers, F.A.M. Partial blocking of ion transport at the interface of an ion-selective liquid membrane electrode by neutral surfactants: Experiments and computer simulation. Electrochim. Acta 1998, 44, 125–132. [Google Scholar] [CrossRef]
- Horvai, G. Relationship between charge transfer resistance and exchange current density of ion transfer at the interface of two immiscible electrolyte solutions. Electroanalysis 1991, 3, 673–675. [Google Scholar] [CrossRef]
- Vanysek, P. Charge transfer processes on liquid/liquid interfaces: The first century. Electrochim. Acta 1995, 40, 2841–2847. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhelson, K. Electrochemical Properties of Nitrate-Selective Electrodes: The Dependence of Resistance on the Solution Concentration. Sensors 2018, 18, 2062. [Google Scholar] [CrossRef] [PubMed]
- Lewenstam, A. Routines and Challenges in Clinical Application of Electrochemical Ion-Sensors. Electroanalysis 2014, 26, 1171–1181. [Google Scholar] [CrossRef]
- Kielland, J. Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 1937, 59, 1675–1678. [Google Scholar] [CrossRef]
- Dvorak, J.; Koryta, J.; Bohackova, V. Elektrochemie; Academia: Praha, Czech Republic, 1975. [Google Scholar]
- Bakker, E.; Bühlmann, P.; Pretsch, E. Carrier-based ion-selective electrodes and bulk optodes. 1. General characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Bühlmann, P.; Pretsch, E.; Bakker, E. Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 1998, 98, 1593–1687. [Google Scholar] [CrossRef] [PubMed]
- Pioda, L.A.R.; Stankova, V.; Simon, W. Highly selective potassium ion responsive liquid-membrane electode. Anal. Lett. 1969, 2, 665–674. [Google Scholar] [CrossRef]
- Ammann, D.; Pretsch, E.; Simon, W. A calcium ion-selective electrode based on a neutral carrier. Anal. Lett. 1972, 5, 843–850. [Google Scholar] [CrossRef]
- Thoma, A.P.; Viviani-Nauer, A.; Arvanitis, S.; Morf, W.E.; Simon, W. Mechanism of neutral carrier mediated ion transport through ion-selective bulk membranes. Anal. Chem. 1977, 49, 1567–1572. [Google Scholar] [CrossRef]
- Van den Berg, A.; Van der Wal, P.D.; Skowronska-Ptasinska, M.; Sudholter, E.J.R.; Reinhoudt, D.; Bergveld, P. Nature of anionic sites in plasticized poly(vinyl chloride) membranes. Anal. Chem. 1987, 59, 2827–2829. [Google Scholar] [CrossRef]
- Nägele, M.; Pretsch, E. New method for determining the concentration of ionic impurities in solvent polymeric membranes. Mikrochim. Acta 1995, 121, 269–279. [Google Scholar] [CrossRef]
- Gyurcsanyi, R.E.; Lindner, E. Spectroscopic Method for the Determination of the Ionic Site Concentration in Solvent Polymeric Membranes and Membrane Plasticizers. Anal. Chem. 2002, 74, 4060–4068. [Google Scholar] [CrossRef]
- Cassaretto, F.P.; McLafferty, J.J.; Moore, C.T. Studies in the tetraarylborates: The preparation and reagent properties of sodium tetrakis(p-chlorophenyl)borate. Anal. Chim. Acta 1965, 32, 376–380. [Google Scholar] [CrossRef]
- Liu, X.; Bouchard, G.; Girault, H.H.; Testa, B.; Carrupt, P.-A. Partition Coefficients of Ionizable Compounds in o-Nitrophenyl Octyl Ether/Water Measured by the Potentiometric Method. Anal. Chem. 2003, 75, 7036–7039. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics Free Web Services. Available online: https://www.molinspiration.com (accessed on 31 January 2024).
- Keresten, V.M.; Bykov, A.G.; Gofman, I.V.; Solovyeva, E.V.; Vlasov, A.Y.; Mikhelson, K.N. Non-constancy of the bulk resistance of ionophore-based ion-selective membranes within the Nernstian response range: A semi-quantitative explanation. J. Membr. Sci. 2023, 683, 121830. [Google Scholar] [CrossRef]
- Harrison, J.D.; Li, X. Measurement of concentration profiles inside a nitrite ion selective electrode membrane. Anal. Chem. 1991, 63, 2168–2174. [Google Scholar] [CrossRef]
- Chan, A.D.C.; Li, X.; Harrison, J.D. Evidence for a water-rich surface region in poli(vinyl chloride)-based ion-selective electrode membranes. Anal. Chem. 1992, 64, 2512–2517. [Google Scholar] [CrossRef]
- Solovyeva, E.V.; Lu, H.; Khripoun, G.A.; Mikhelson, K.N.; Kazarian, S.G. In situ ATR-FTIR spectroscopic imaging of PVC, plasticizer and water in solvent-polymeric ion-selective membrane containing Cd2+-selective neutral ionophore. J. Membr. Sci. 2020, 619, 118798. [Google Scholar] [CrossRef]
- Bard, L.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA; Chichester, UK; Weinheim, Germany; Brisbane, Australia; Singapore; Toronto, ON, Canada, 2001. [Google Scholar]


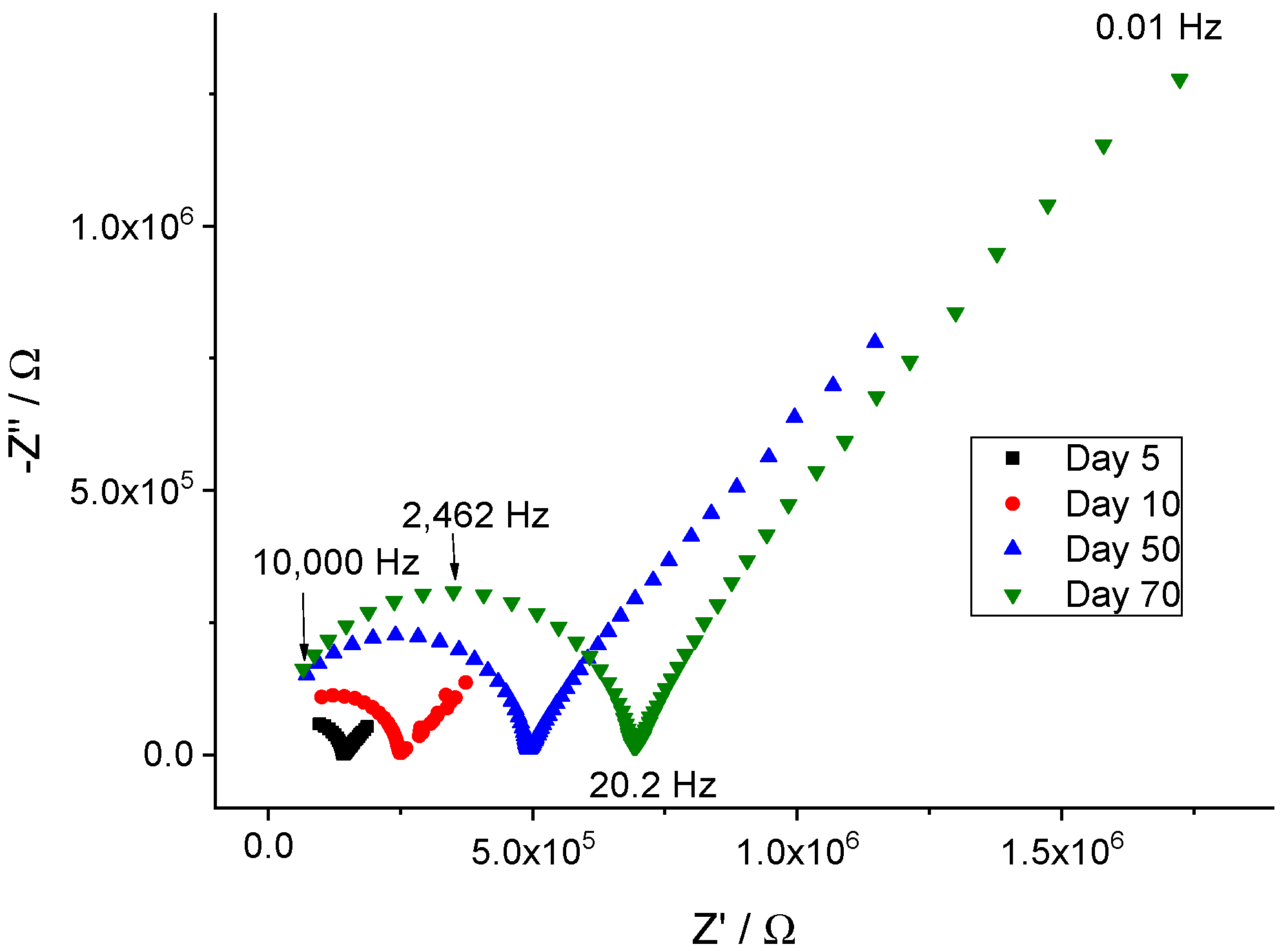
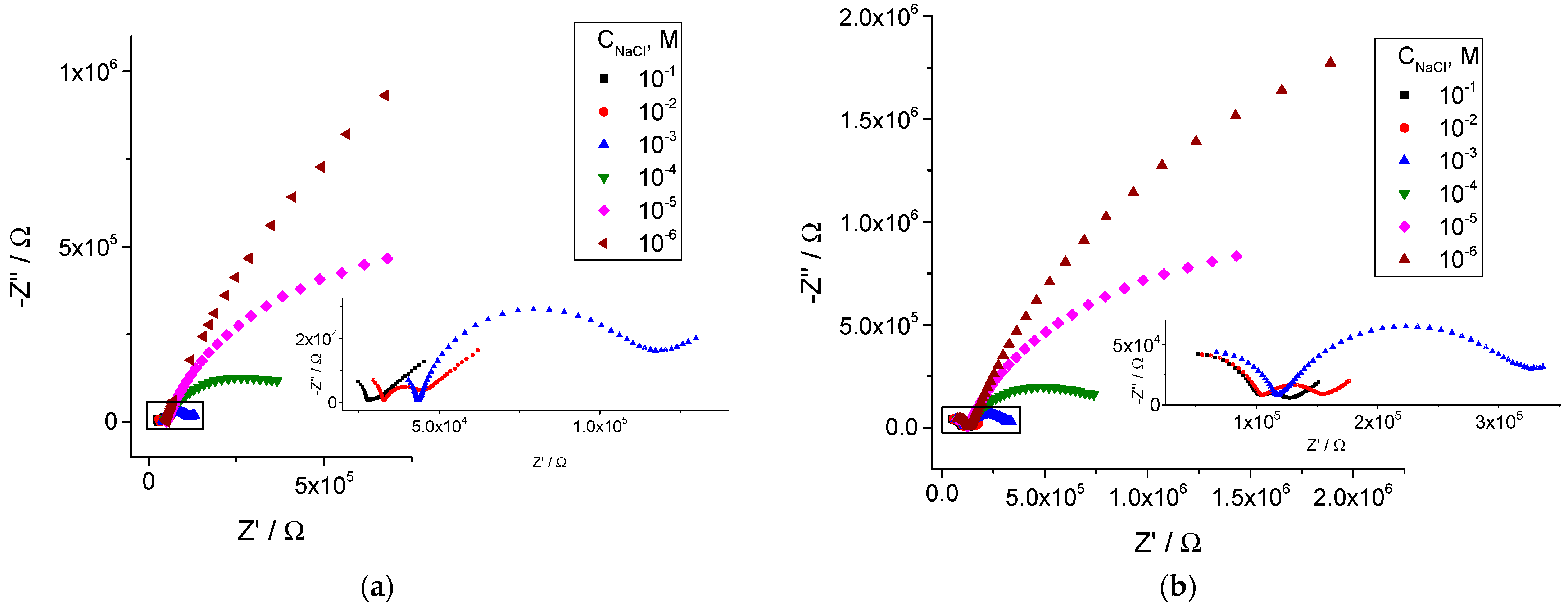

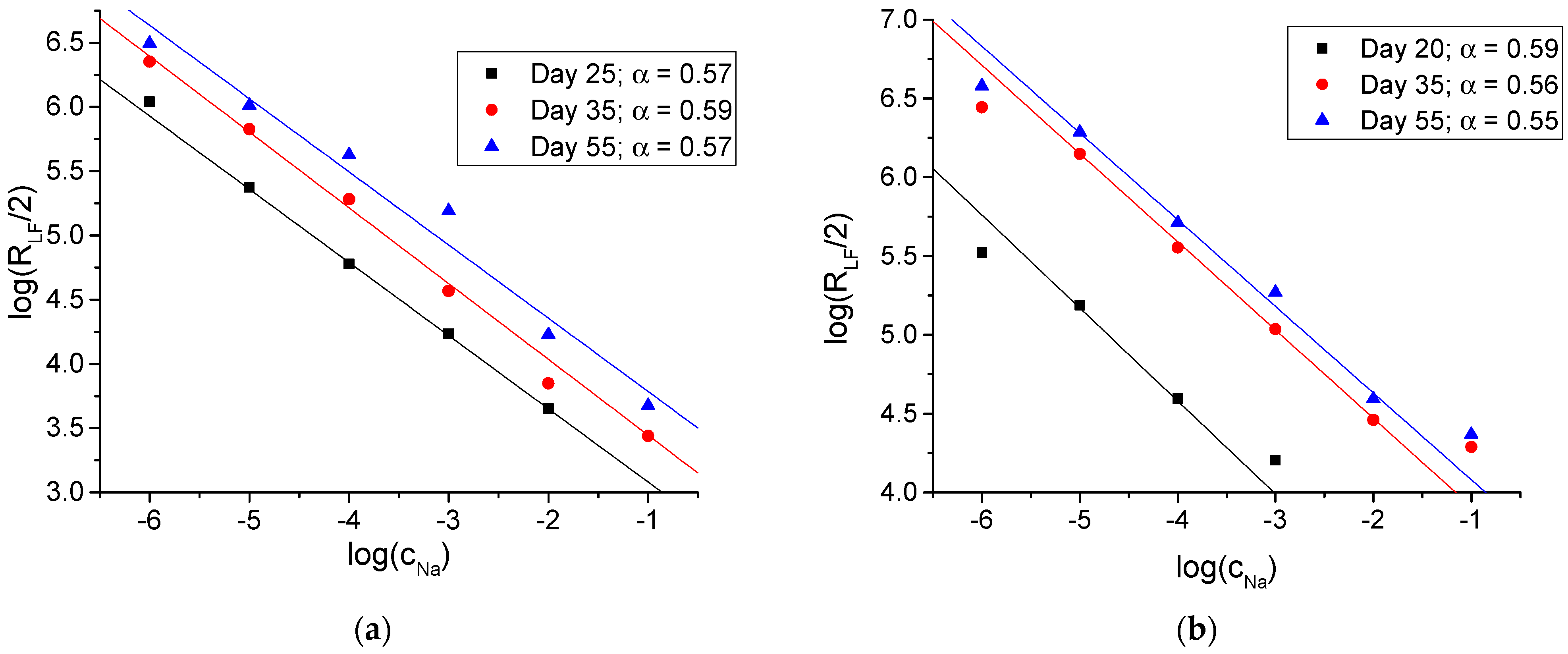


| Membrane | Na X | KClTPB | ETH 500 | oNPOE | PVC |
|---|---|---|---|---|---|
| M1 | 15 mmol 1.0% | 7.5 mmol 0.2% | 7.5 mmol 0.6% | 65.5% | 32.7% |
| M2 | 15 mmol 1.0% | 7.5 mmol 0.2% | - | 65.9% | 32.9% |
| M3 | - | 7.5 mmol 0.2% | - | 66.5% | 33.3% |
| M4 | 15 mmol 1.0% | - | - | 66.0% | 33.0% |
| Membrane, Day | , kΩ | , µA/cm2 | ||
|---|---|---|---|---|
| M1, day 25 | 2.51 | −0.57 | 0.324 | 30.0 |
| M1, day 35 | 2.86 | −0.59 | 0.724 | 13.6 |
| M1, day 55 | 3.22 | −0.57 | 1.66 | 5.95 |
| M2, day 20 | 2.22 | −0.59 | 0.166 | 59.5 |
| M2, day 35 | 3.35 | −0.56 | 2.24 | 4.41 |
| M2, day 55 | 3.53 | −0.55 | 3.39 | 2.91 |
| M2, day 70 | 3.51 | −0.56 | 3.24 | 3.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keresten, V.; Lazarev, F.; Mikhelson, K. Transfer of Sodium Ion across Interface between Na+-Selective Electrode Membrane and Aqueous Electrolyte Solution: Can We Use Nernst Equation If Current Flows through Electrode? Membranes 2024, 14, 74. https://doi.org/10.3390/membranes14040074
Keresten V, Lazarev F, Mikhelson K. Transfer of Sodium Ion across Interface between Na+-Selective Electrode Membrane and Aqueous Electrolyte Solution: Can We Use Nernst Equation If Current Flows through Electrode? Membranes. 2024; 14(4):74. https://doi.org/10.3390/membranes14040074
Chicago/Turabian StyleKeresten, Valentina, Fedor Lazarev, and Konstantin Mikhelson. 2024. "Transfer of Sodium Ion across Interface between Na+-Selective Electrode Membrane and Aqueous Electrolyte Solution: Can We Use Nernst Equation If Current Flows through Electrode?" Membranes 14, no. 4: 74. https://doi.org/10.3390/membranes14040074





