Production of Stable Electrically Conductive PVDF Membranes Based on Polydopamine-Polyethyleneimine—Assisted Deposition of Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
- Method 1 (ECMs denoted as M1): CNTs were deposited on PEI crosslinked PDA-coated PVDF membranes: ECMs were prepared in a two-step process. In the first step, PVDF membranes were immersed (for 24 h) in a mixture of DA (2 mg/mL) and branched PEI (2 mg/mL, Mw 600) in a Tris buffer solution (0.1 mM, pH 8.5). In the second step, a previously prepared CNT suspension was deposited on the PDA-PEI-coated PVDF by vacuum deposition. To prepare the CNT suspension, 0.5 mg/mL CNT suspension in DI water was stirred for 5 min (600 rpm at ambient temperature) and ultrasonicated for 1 h (a microtip of 1/4″ diameter, intensity of 40%). with an interval of 2 s on and 2 s off. To facilitate the dispersion of CNTs, the suspension was mixed with 0.75 mg/mL solution of sodium dodecyl sulfate (SDS) in DI water previously stirred for 30 min (RPM 600, ambient temperature). The mixture of CNT and SDS suspensions was stirred for 15 min and ultrasonicated for 1 h at the same prob intensity and interval time as above. To deposit 5 mg of CNTs, 10 mL of 0.5 mg/mL CNT suspension was filtered under vacuum through wet PDA/PEI coated membranes placed in a glass flask under a pressure of 100 mbar.
- Method 2 (ECMs denoted as M2): PEI crosslinked CNTs were deposited on PDA-coated PVDF membranes: ECMs were prepared in a two-step process, where PVDF membranes were first coated by PDA for 24 h (2 mg/mL DA in Tris buffer solution (0.1 mM, pH 8.5)), then PEI crosslinked CNTs were vacuum filtered through the PDA coated PVDF membranes. To prepare the PEI crosslinked CNTs, the carboxylic groups of CNTs were first activated by adding 25 mg of 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and 50 mg N-hydroxysuccinimide (NHS) into a 0.5 mg/mL CNT suspension. Then, 5 mL of 2 mg/mL branched polyethyleneimine (BPEI) (Mw 600) was added to the suspension and stirred for 24 h under ambient conditions to ensure a complete reaction between the amine group and the carboxylic groups of CNTs.
- Method 3 (ECMs denoted as M3): PDA, PEI and CNTs were sequentially layer-by-layer deposited on PVDF membranes: ECMs were synthesized by a 3-step coating process. In the first step, PVDF membranes were coated with PDA (by immersion in DA 2 mg/mL in Tris buffer solution (0.1 mM, pH 8.5) for 24 h). In the second step, the membranes were dip-coated with a 2 mg/mL PEI solution for 12 h under ambient temperature. In step 3, a CNT suspension dispersed by 0.75 mg/mL SDS was vacuum filtered through the PDA-PEI coated membranes.
- Method 4 (ECMs denoted as M4): PEI crosslinked PDA was deposited on CNT-coated PVDF membranes: In this method, a CNT suspension dispersed by 0.75 mg/mL SDS was first deposited onto pristine PVDF membranes, followed by membrane coating with PEI cross-linked PDA following the exact coating procedure explained in method 1.
2.3. Membrane Characterization
3. Results and Discussion
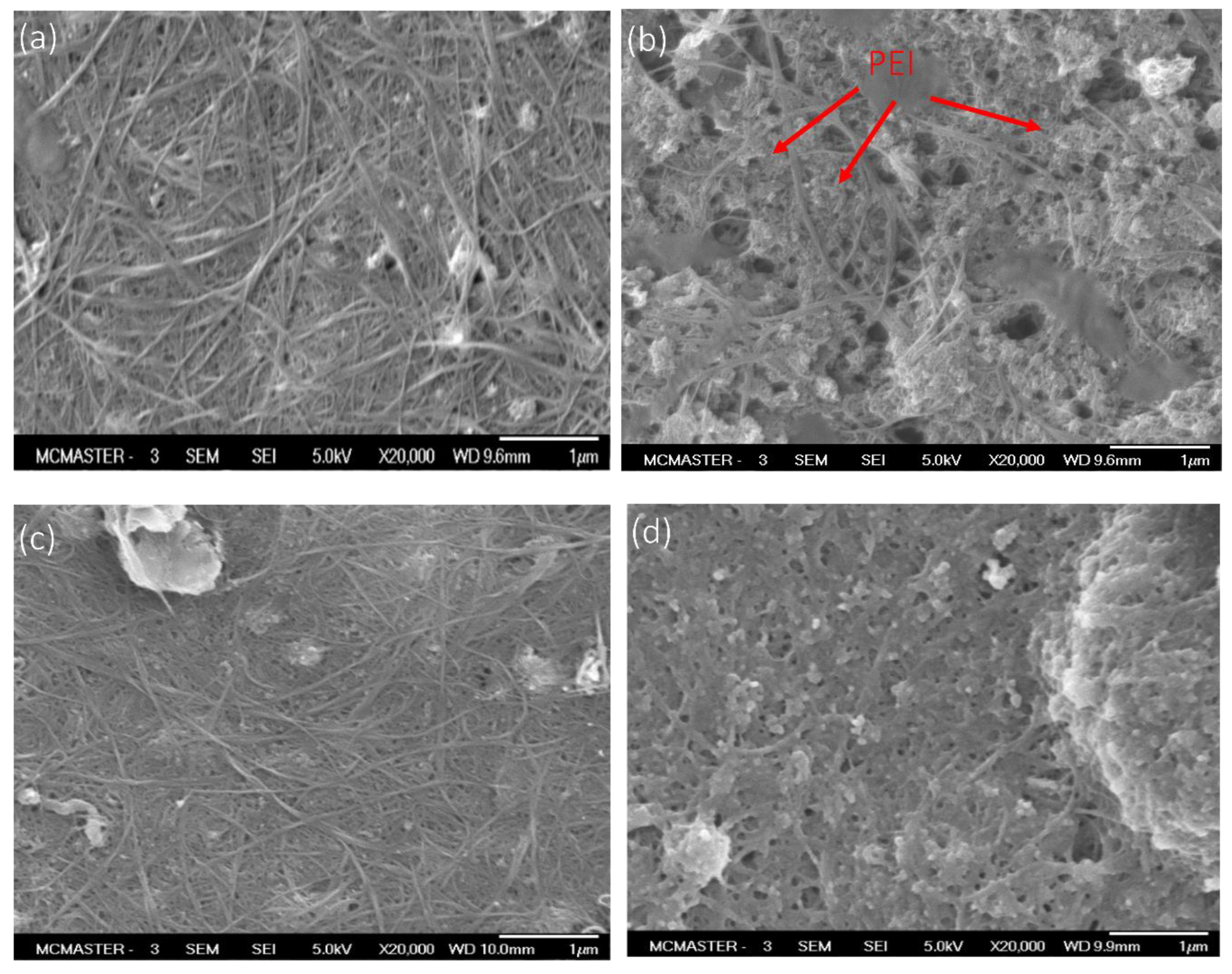
3.1. Membrane Morphology
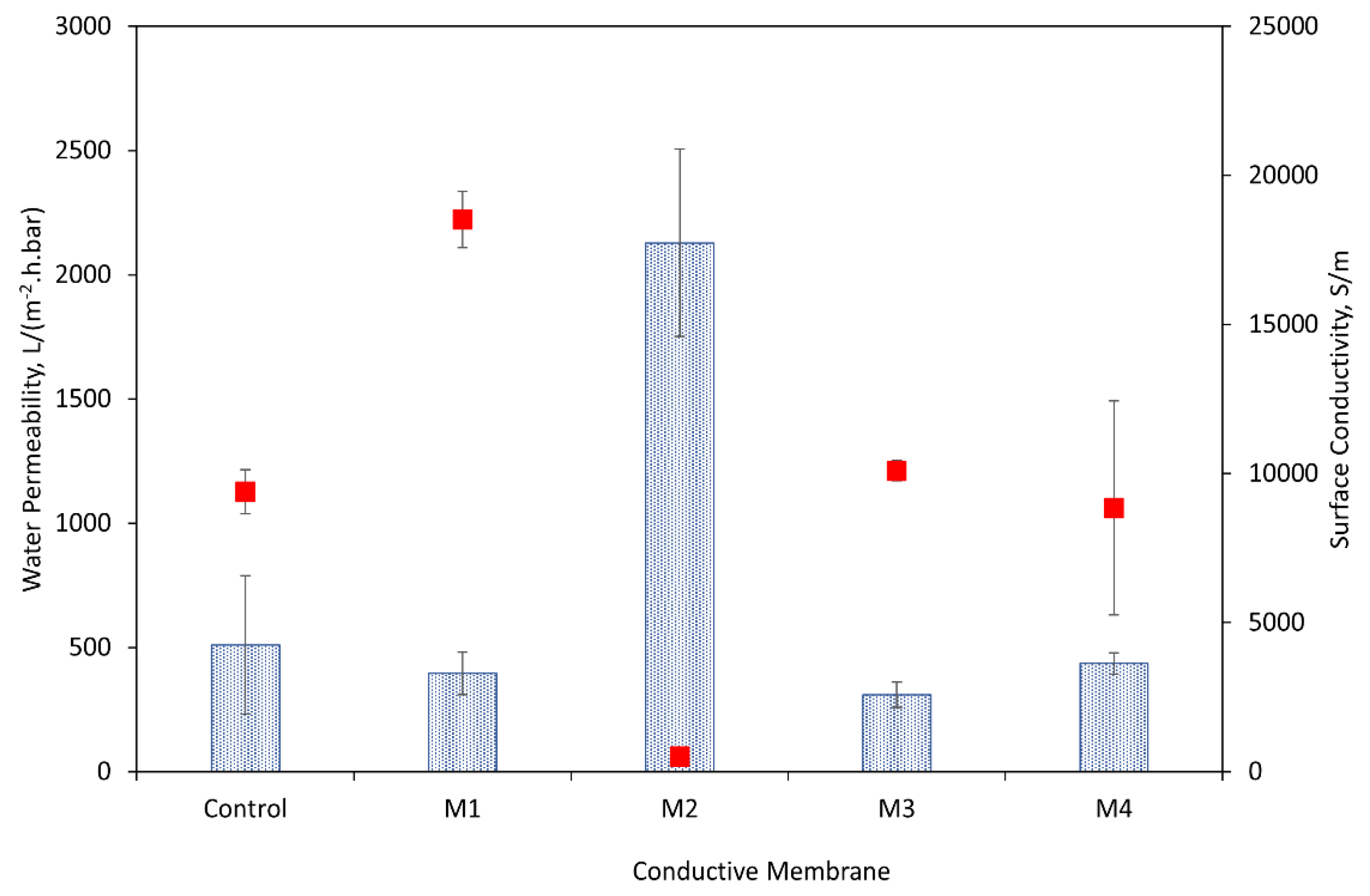
3.2. Water Permeability and Surface Conductivity
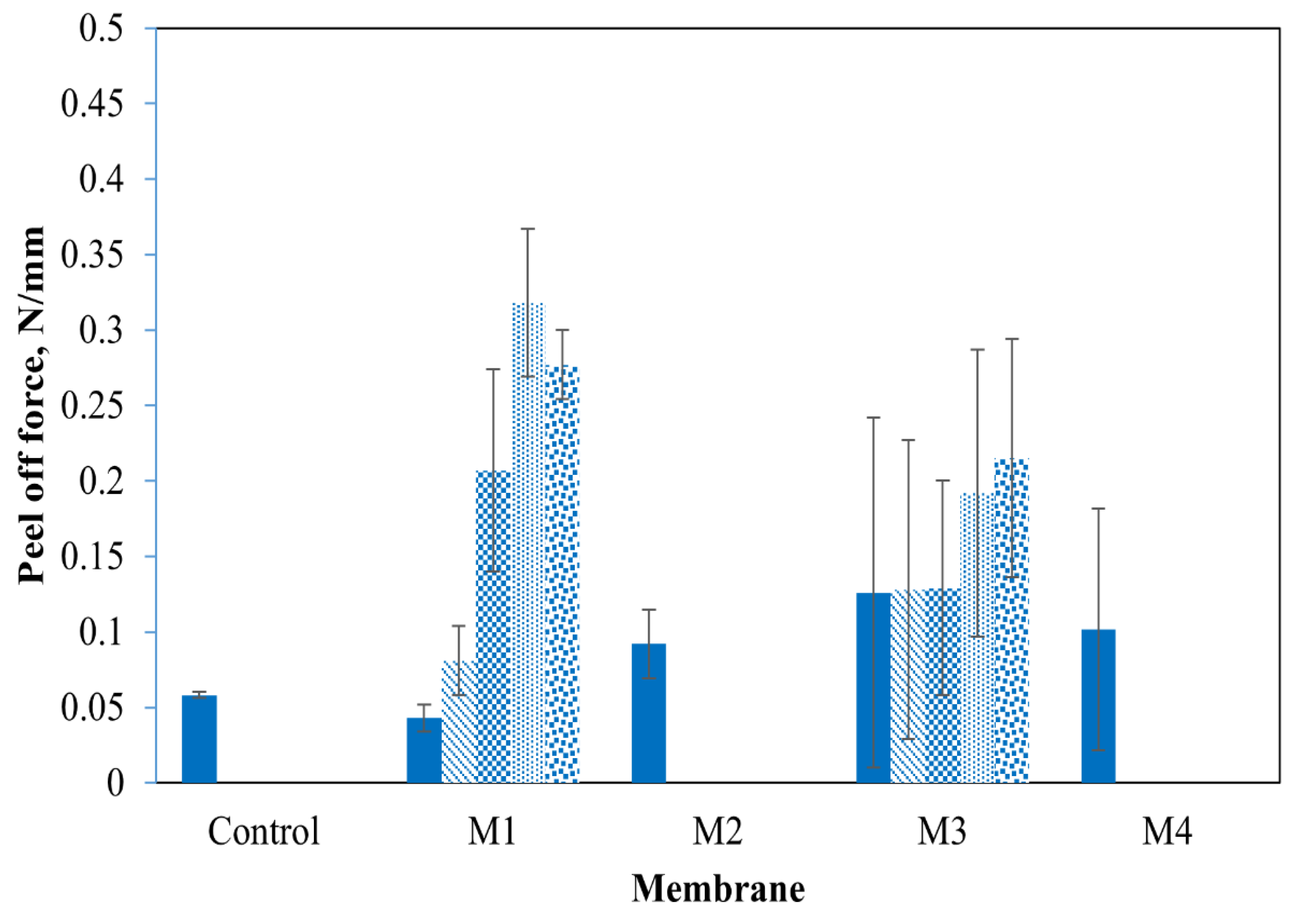
3.3. Membrane Physical Stability
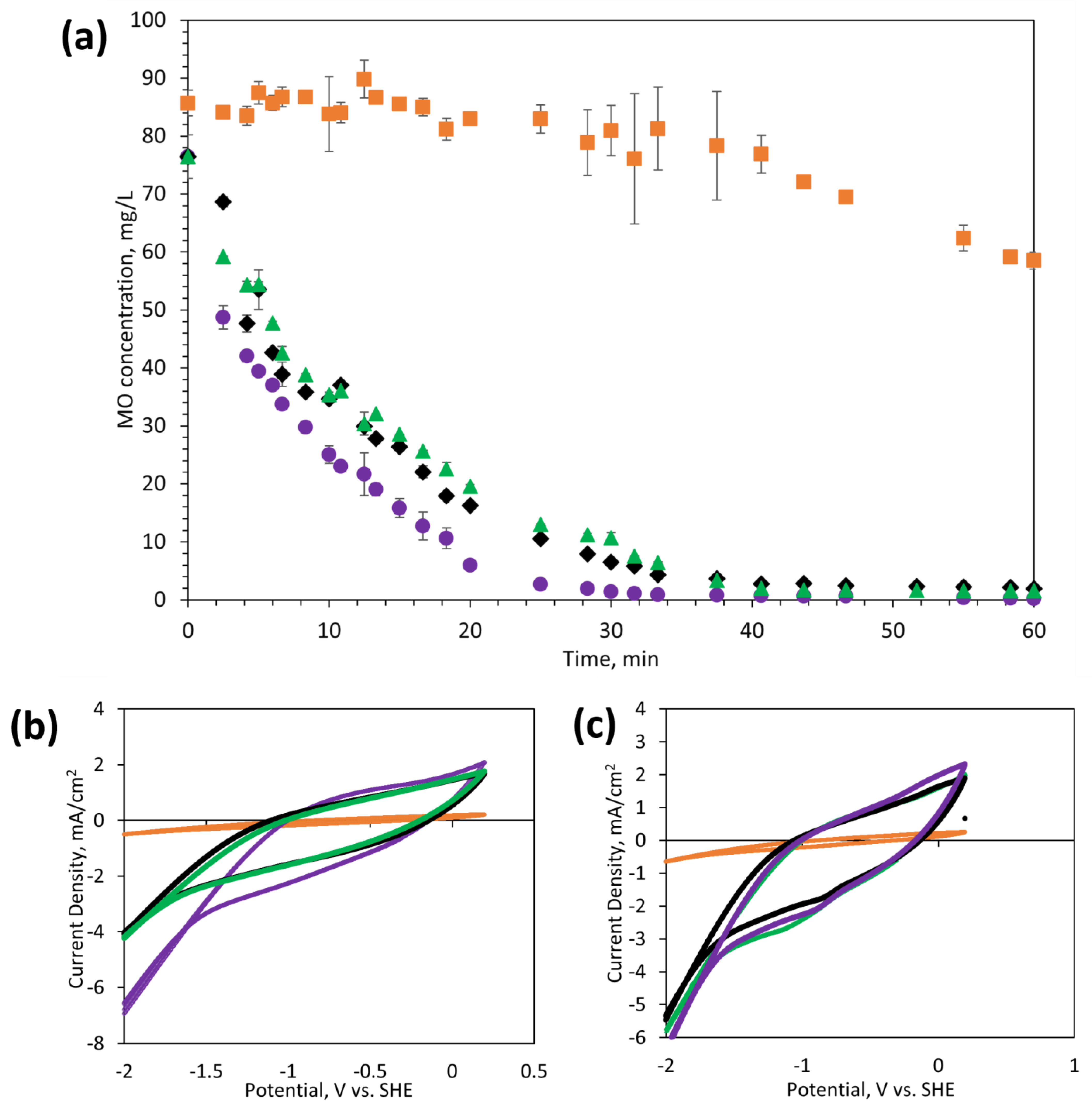
3.4. Electrochemical Activity
4. Conclusions
- Membrane structures can be controlled by coating chemistry. Dense and stable conductive membranes can be made simply by adhering non-crosslinked CNTs to PDA/PEI-coated substrates. In contrast, open porous but unstable CNT structures can be obtained through PEI crosslinking of CNTs adhered to PDA. The presence of exposed PEI at the membrane surface leads to enhanced surface hydrophilicity, but less stable CNT coating. ECMs with exposed PEI showed significantly less physical stability (i.e., adhesion forces of 0.092–0.1016 N/mm) as compared to other ECMs synthesized in this study (adhesion forces of 0.215–0.377 N/mm).
- ECMs prepared by the different methods showed variations in the water permeability and electrical conductivity, which were attributed to the membrane surface structures and hydrophilicity. ECMs prepared by deposition of PEI crosslinked CNTs resulted in more permeable but less conductive membranes as compared to the other methods.
- The use of PEI crosslinked PDA as an intermediate layer between CNTs and PVDF demonstrated the most physically stable ECMs, as confirmed by both dry peel-off (adhesion force of 0.377 N/mm) and wet adhesion tests under bath sonication.
- Membranes with high electrical conductivities can facilitate the electrochemical degradation of MO organic contaminants. The MO degradation mechanism was primarily attributed to direct reduction at the membrane surface.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patil, J.J.; Jana, A.; Getachew, B.A.; Bergsman, D.S.; Gariepy, Z.; Smith, B.D.; Lu, Z.; Grossman, J.C. Conductive Carbonaceous Membranes: Recent Progress and Future Opportunities. J. Mater. Chem. A 2021, 9, 3270–3289. [Google Scholar] [CrossRef]
- Mo, Y.; Zhang, L.; Zhao, X.; Li, J.; Wang, L. A Critical Review on Classifications, Characteristics, and Applications of Electrically Conductive Membranes for Toxic Pollutant Removal from Water: Comparison between Composite and Inorganic Electrically Conductive Membranes. J. Hazard. Mater. 2022, 436, 129162. [Google Scholar] [CrossRef] [PubMed]
- Anis, S.F.; Lalia, B.S.; Lesimple, A.; Hashaikeh, R.; Hilal, N. Electrically Conductive Membranes for Contemporaneous Dye Rejection and Degradation. Chem. Eng. J. 2022, 428, 131184. [Google Scholar] [CrossRef]
- Larocque, M.J.; Gelb, A.; Latulippe, D.R.; de Lannoy, C.F. Meta-Analysis of Electrically Conductive Membranes: A Comparative Review of Their Materials, Applications, and Performance. Sep. Purif. Technol. 2022, 287, 120482. [Google Scholar] [CrossRef]
- Straub, A.P.; Bergsman, D.S.; Getachew, B.A.; Leahy, L.M.; Patil, J.J.; Ferralis, N.; Grossman, J.C. Highly Conductive and Permeable Nanocomposite Ultrafiltration Membranes Using Laser-Reduced Graphene Oxide. Nano Lett. 2021, 21, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Halali, M.A.; Larocque, M.; de Lannoy, C.F. Investigating the Stability of Electrically Conductive Membranes. J. Memb. Sci. 2021, 627, 119181. [Google Scholar] [CrossRef]
- Dudchenko, A.V.; Rolf, J.; Russell, K.; Duan, W.; Jassby, D. Organic Fouling Inhibition on Electrically Conducting Carbon Nanotube-Polyvinyl Alcohol Composite Ultrafiltration Membranes. J. Memb. Sci. 2014, 468, 1–10. [Google Scholar] [CrossRef]
- Jung, H.; An, S.Y.; Lim, J.S.; Kim, D. Transparent Conductive Thin Film Synthesis Based on Single-Walled Carbon Nanotubes Dispersion Containing Polymethylmethacrylate Binder. J. Nanosci. Nanotechnol. 2011, 11, 6345–6349. [Google Scholar] [CrossRef]
- Shim, H.C.; Kwak, Y.K.; Han, C.S.; Kim, S. Enhancement of Adhesion between Carbon Nanotubes and Polymer Substrates Using Microwave Irradiation. Scr. Mater. 2009, 61, 32–35. [Google Scholar] [CrossRef]
- Pei, S.; Du, J.; Zeng, Y.; Liu, C.; Cheng, H.M. The Fabrication of a Carbon Nanotube Transparent Conductive Film by Electrophoretic Deposition and Hot-Pressing Transfer. Nanotechnology 2009, 20, 235707. [Google Scholar] [CrossRef] [PubMed]
- Sanahuja, O.; Diez, J.L.; Benito, A.M.; Santidria, A.; Maser, W.K.; Mun, E.; Anso, A. Chemical Postdeposition Treatments to Improve the Adhesion of Carbon Nanotube Films on Plastic Substrates’. ACS Omega 2019, 4, 2804–2811. [Google Scholar] [CrossRef]
- Larocque, M.J.; Latulippe, D.R.; de Lannoy, C.F. Formation of Electrically Conductive Hollow Fiber Membranes via Crossflow Deposition of Carbon Nanotubes—Addressing the Conductivity/Permeability Trade-Off. J. Memb. Sci. 2021, 620, 118859. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Winter, L.R.; Zhao, Y.; Ma, W.; Hedtke, T.; Kim, J.-H.; Elimelech, M. Electrified Membranes for Water Treatment Applications. ACS ES T Eng. 2021, 1, 725–752. [Google Scholar] [CrossRef]
- Ji, Y.L.; Ang, M.B.M.Y.; Hung, H.C.; Huang, S.H.; An, Q.F.; Lee, K.R.; Lai, J.Y. Bio-Inspired Deposition of Polydopamine on PVDF Followed by Interfacial Cross-Linking with Trimesoyl Chloride as Means of Preparing Composite Membranes for Isopropanol Dehydration. J. Memb. Sci. 2018, 557, 58–66. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Supporting Online Material Mussel-Inspired Surface Chemistry for Multifunctional Coatings. science 2007, 426, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zuo, F.; Liao, Z.; Qin, Z.; Du, S.; Zhao, Z. Mussel-Inspired One-Pot Synthesis of a Fluorescent and Water-Soluble Polydopamine – Polyethyleneimine Copolymer. Macromol 2015, 36, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, S.J.; Du, Y.; Yang, H.C.; Xu, Z.K. Co-Deposition Kinetics of Polydopamine/Polyethyleneimine Coatings: Effects of Solution Composition and Substrate Surface. Langmuir 2018, 34, 13123–13131. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, M.; Li, Y.; Chen, Y.; Wan, L.; Xu, Z. Effects of Polyethyleneimine Molecular Weight and Proportion on the Membrane Hydrophilization by Codepositing with Dopamine. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Deng, S.; Liu, X.; Liao, J.; Lin, H.; Liu, F. PEI Modified Multiwalled Carbon Nanotube as a Novel Additive in PAN Nanofiber Membrane for Enhanced Removal of Heavy Metal Ions. Chem. Eng. J. 2019, 375, 122086. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, I.; Chen, C.; Hsu, Y.; Chang, C.; Lee, M. Delivery of Small Interfering RNAs in Human Cervical Cancer Cells by Polyethylenimine-Functionalized Carbon Nanotubes. Nanoscale Res. Lett. 2013, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Yue, R.; Huang, Y. Talanta Polyethylenimine-Carbon Nanotubes Composite as an Electrochemical Sensing Platform for Silver Nanoparticles. Talanta 2016, 160, 607–613. [Google Scholar] [CrossRef] [PubMed]



| Membrane | Rationale | Process Steps |
|---|---|---|
| M1 | PEI and PDA were cross-linked together to assess the impact of PEI on PDA aggregates. | Step 1: PVDF coated with PEI cross-linked-PDA Step 2: CNT coating |
| M2 | CNTs and PEI were cross-linked to assess the impact of PEI on CNT dispersion. | Step 1: PVDF coated with PDA Step 2: CNTs cross-linked with PEI Step 3: PEI cross-linked CNTs coating |
| M3 | Each coating was deposited independently as a baseline. PEI is coated on PDA and crosslinked to CNTs | Step 1: PVDF coated with PDA Step 2: PVDF-PDA coated with PEI Step 3: CNT coating |
| M4 | The order of PEI-PDA cross-linked coating was the inverse of M1, to assess the impact on CNT stability. | Step 1: PVDF coated with CNTs Step 2: PEI crosslinked PDA coating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, A.M.; de Lannoy, C.-F. Production of Stable Electrically Conductive PVDF Membranes Based on Polydopamine-Polyethyleneimine—Assisted Deposition of Carbon Nanotubes. Membranes 2024, 14, 94. https://doi.org/10.3390/membranes14040094
Awad AM, de Lannoy C-F. Production of Stable Electrically Conductive PVDF Membranes Based on Polydopamine-Polyethyleneimine—Assisted Deposition of Carbon Nanotubes. Membranes. 2024; 14(4):94. https://doi.org/10.3390/membranes14040094
Chicago/Turabian StyleAwad, Abdelrahman M., and Charles-François de Lannoy. 2024. "Production of Stable Electrically Conductive PVDF Membranes Based on Polydopamine-Polyethyleneimine—Assisted Deposition of Carbon Nanotubes" Membranes 14, no. 4: 94. https://doi.org/10.3390/membranes14040094






